Abstract
Thermodynamic assessments have been performed for the Ni-Ti binary and Ni-Cr-Ti ternary system by the CALPHAD method. Combining the experimental and ab initio calculated data on the enthalpies of formation of Ni-Ti compounds, a better description for Ni-Ti phases has been obtained. Based on the new assessments of the binary sub-systems and the recent experimental data on phase equilibria, a reassessment of the Ni-Cr-Ti system was carried out. Apart from the thermodynamic assessments, the interdiffusion coefficients for the fcc phase of the binary Ni-Ti were re-optimized and the ternary Ni-Cr-Ti system were determined experimentally over a temperature range from 1123 to 1273 K employing the diffusion-couple technique. Subsequently, atomic mobility data for the fcc phase of the Ni-Cr-Ti system were assessed and most diffusivity data were satisfactorily described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Knowledge of phase equilibria of the Ni-Ti-Cr system is of fundamental interest in developing new Ni-based and Ti-based high-temperature structural materials. Addition of Ti into Ni-Cr alloys enhances their high-temperature corrosion resistance and physical and/or mechanical properties. The Ni-based superalloys containing Cr and Ti such as 617, 625, 718, X750, 751, MA754, are widely used to manufacture parts of aircraft engines and land-based turbines.[1,2] Their application in combination with Ti-based alloys may lead to a significant reduction of the specific weight of various structural components.[3]
As well known, reliable information about the ternary and its sub-systems is a guarantee for predicting the thermodynamic properties and phase diagrams for multicomponent systems. Consequently, thermodynamic and mobility assessments for ternary Ni-Cr-Ti system should be carried out. Isomäki et al.[4] have thermodynamically assessed the Ni-Cr-Ti system and paid attention to several phase boundaries determination. They utilized the assessments for the Ni-Cr system by Lee,[5] the Ni-Ti system by Tokunaga et al.[6] and the Cr–Ti system by Ghosh.[7] However, new evaluations of the binary sub-systems have been published. Keyzer et al.,[8] Pavlů et al.[9] and Zhu et al.[10] re-optimized the Ni-Ti, Cr–Ti and Ni-Cr systems, respectively. Therefore the Ni-Cr-Ti ternary system should be updated.
The atomic mobilities for the fcc phase of the Ni-Cr-Ti ternary system have not yet been assessed. In the present work, the diffusion couple method was used to investigate interdiffusion coefficients in the fcc phase of the Ni-Cr-Ti system at 1123 and 1273 K in order to evaluate atomic mobilities for the ternary system.
The purpose of the present work is to obtain a better evaluation of the Ni-Cr-Ti ternary system, including a thermodynamic description of the entire system and the atomic mobilities for the fcc phase. The paper is organized as follows: section 2 introduces the thermodynamic models for all phases in the Ni-Cr-Ti system and the mobility model used for the fcc phase. Then the experimental procedure of the diffusion couple technique is described in section 3. Subsequently, the thermodynamic assessments of the binary Ni-Ti, Cr–Ti and ternary Ni-Cr-Ti systems by the CALPHAD method are presented using the latest reliable experimental data in the literature. Based on the thermodynamic results and experimental diffusivities for the fcc phase, the determination of binary and ternary interdiffusion coefficients from the experimental composition profiles of the diffusion couples is discussed in section 4. In addition, the atomic mobilities of Ni, Cr and Ti in fcc Ni-Cr-Ti alloys are also evaluated. Finally, a summary is given in section 5.
2 Models
2.1 Thermodynamic Models
In the present work all the thermodynamic calculations and optimizations were performed with the Thermo-Calc software.[11] All the stable phases presenting in the Ni-Cr-Ti system are listed in Table 1. The Gibbs energies for the pure elements were obtained from Dinsdale.[12]
The Gibbs energy of the liquid, fcc, bcc, and hcp phases are described with the substitutional solution model as,
where \(x_{i}\) is the mole fraction of Ni, Cr and Ti, respectively. R is the gas constant and T is the absolute temperature. \({}^{0}G_{i}^{\phi }\) is the molar Gibbs energy of the pure component \(i\). The standard element reference (SER) state at 298.15 K and 1 bar[12] is used as the reference state of Gibbs energy. \({}^{ex}G_{m}^{\phi }\) is the molar excess Gibbs energy, given by
\(L_{i,j}^{\phi }\) are binary interaction parameters described by Redlich–Kister polynomials
where \({}^{n}L_{i,j}^{\phi } = a + bT\).
The ternary interaction parameter \(L_{Ni,Cr,Ti}^{\phi }\) is given by the following equation:
Intermetallic phases, such as NiTi2, Ni3Ti and NiTi, are modeled using the Compound Energy Formalism (CEF) as described by Hillert et al.[13] The component elements are assumed to occupy and possibly mix in two or more sublattices related by a simple formula, e.g. (A,B)m(C,D)n meaning that A and B mix on the first sub-lattice while C and D mix on the second one.
2.2 Atomic Mobility Model for fcc Phase
Based on the new thermodynamic description of the Ni-Cr-Ti system, the atomic mobilities for the fcc phase were optimized by the DICTRA software.[14] According to absolute rate theory, the atomic mobility is defined as follows,[15]
where \(Q_{i}\) is the activation energy, \(M_{i}^{0}\) is the frequency factor, R is the gas constant and T is the absolute temperature. \(\varPhi_{i}\) is a composition and temperature dependent property which can be expressed by Redlich–Kister polynomials,
where \(x_{p}\) is the mole fraction of species \(p\), \(\varPhi_{i}^{p}\) is the value of \(\varPhi_{i}\) for \(i\) in species \(p\), \({}^{r}\varPhi_{i}^{p,q}\) and \({}^{s}\varPhi_{i}^{p,q,v}\) are binary and ternary interaction parameters, respectively.
All diffusion coefficients can be calculated from the atomic mobilities. The tracer diffusion coefficient \(D_{i}^{ * }\) is correlated with the corresponding mobility from
And the interdiffusion coefficient can be computed from
where \(\delta_{ip}\) is the Kronecker delta (\(\delta_{ip} = 1\) when \(i = p\), otherwise \(\delta_{ip} = 0\)) and \(\mu_{i}\) is the chemical potential of species \(i\).
3 Experiments
The ternary alloys listed in Table 2 for diffusion couples were prepared by arce melting in an Ar atmosphere using the WK-II non-consumption vacuum melting furnace. The raw materials used were 99.99 pct nickel, 99.99 pct chromium and 99.99 pct titanium. In order to obtain good homogeneity, the ingots were re-melted five times and homogenized at 1473 K for 168 h. After homogenization, blocks with a size of 6 × 6×6 mm3 were cut from the ingots using wire-electrode cutting. The diffusion surface of each sample was ground, polished and bound to the other part of the diffusion couple. The prepared diffusion couple was sealed into an argon protective quartz capsule and then annealed at certain temperatures and times listed in Table 2, followed by quenching in water. After polishing of the surface along the diffusion direction, the composition profiles of the diffusion couple were measured by an Electron Probe Micro-Analyser (EPMA, JEOL JXA 8900) and the compositions of both ends of the couples were also determined by EPMA. The method to determine ternary interdiffusion coefficients was described in our previous work[16,17,18] and will not be repeated here.
4 Results and Discussion
4.1 Thermodynamic Assessments of the Sub-binaries
Murray[19] assessed thermochemical data of the Ni-Ti system, including Gibbs energies of formation for the different compounds and enthalpy of mixing for the liquid phase. However Ni3Ti had no homogeneity range in her evaluation. This binary was later thermodynamically evaluated by Kaufman and Nesor,[20] Bellen et al.,[21] and Tang et al.[22] In the work by Kaufman and Nesor, all the intermediate phases were described as stoichiometric phases, while Bellen et al. and Tang et al. used sublattice formalism to account for the homogeneity range of the compounds. In their work, the formula (Ni, Ti)0.75(Ni, Ti)0.25 was used for the Ni3Ti phase. Tokunaga et al.[6] also critically assessed the stable phase diagram. However, for the NiTi phase (a CsCl-type B2 superstructure), none of the above models can describe the bcc A2/B2 transformation considering the structure relationship between the disordered A2 and ordered B2. On the basis of the assessment by Bellen et al., Keyzer et al.[8] partially reassessed the Ni-Ti system handling successfully the A2/B2 order–disorder transformation, which were adopted in the present work.
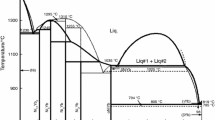
Besides, there are many researches on the enthalpy of formation of Ni3Ti experimentally and theoretically.[6,23,24,25,26,27,28,29,30] However, the experiment data and calculated data are inconsistent with the assessment by Keyzer et al. Therefore the thermodynamic parameters of fcc, bcc and Ni3Ti were re-optimized. The formation enthalpy of Ni3Ti is shown in Fig. 1 and 2 shows the evaluated Ni-Ti phase diagram together with experimental data.[19,31,32,33,34,35,36,37,38]
Calculated enthalpy of formation of the compounds at 298.15 K in comparison with the ab initio calculations and experimental data in the Ni-Ti binary system Solid line: the present work; dotted line: Keyzer et al.[8]
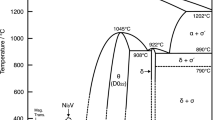
For the Cr–Ti system, Pavlů et al.[9] performed ab initio calculations for the Laves phase,and obtained a better description for the Cr–Ti binary system. Figure 3 shows the calculated phase diagrams of Cr–Ti binary system together with the experimental data.[39,40,41,42,43,44,45,46,47] It is clear that the calculated phase diagram by Pavlů et al.[9] better agrees with the experimental data.
The Ni-Cr system was reviewed and critically assessed by Zhu et al.[10] Their assessment agrees well with the experimental data and the model parameters were adopted in the present work.
4.2 Thermodynamic Assessment of the Ni-Cr-Ti System
Recently, Isomäki et al.[4] comprehensively assessed the Ni-Cr-Ti system and the optimized thermodynamic database can well reproduce the phase equilibrium of the ternary Ni-Cr-Ti alloys. However, during the evaluating the Ni-Ti binary system, Isomäki et al.[4] directly adopted the thermodynamic parameters from Tokunaga et al.[6] in which the calculated results cannot agree well with the experimental data in the whole temperature range, implying that the thermodynamic parameters of the Ni-Cr-Ti ternary system need to be reassessed in the present work.
Figure 4(a) and (b) respectively represent the calculated isothermal sections of Ni-Cr-Ti ternary system at 1023 and 1273 K comparing with the experimental data.[3,33,48] The calculated results in the present work agree well with the experimental data. It indicates that the reassessed thermodynamic parameters of Ni-Cr-Ti ternary system in the present work, which are listed in Table 3, are reliable and can be adopted as the thermodynamic basis to further evaluate the atomic mobilities for the fcc Ni-Cr-Ti phase. And Table 4 shows the calculated invariant phase equilibria.
4.3 Determination of Ternary Interdiffusion Coefficients
The determined ternary interdiffusion coefficients at the intersecting points of the diffusion paths and the corresponding compositions are listed in Table 5. It can be seen that the main interdiffusion coefficients, \(\tilde{D}_{CrCr}^{Ni}\) and \(\tilde{D}_{TiTi}^{Ni}\), is positive. As expected, the cross interdiffusion coefficients are smaller than the main interdiffusion coefficients. This indicates that the diffusion fluxes of Cr and Ti are mainly governed by their own concentration gradients and the influence of the gradient of the other element is small and positive. The extracted interdiffusion coefficients were checked and all fulfill the constraints[49] based on the thermodynamic stability condition. Thereafter the extracted ternary interdiffusion coefficients were employed for the assessment of atomic mobilities.
4.4 Evaluation of Atomic Mobilities for the fcc Ni-Cr-Ti Alloys
According to the published diffusivities including interdiffusion, tracer, impurity and intrinsic diffusivities, Zhu et al.[10] evaluated the mobilities of Ni and Cr while evaluating the diffusivities and atomic mobilities for disordered fcc Ni-Cr-Mo phases, showing a very good consistency between the calculated results and experimental data. Hence, the mobility parameters of Ni-Cr evaluated by Zhu et al.[10] were accepted in the present work. Liu et al.[50] recently reassessed the atomic mobilities for fcc Ni-Ti alloys using the revised thermodynamic description based on Keyzer et al.,[8] showing a very good consistency between the calculated results and experimental data. Due to the update of the thermodynamic parameters of the Ni-Ti system, the corresponding atomic mobilities were revised based on the experimental data of the interdiffusion coefficients.[51,52,53,54] Since the fcc Ti does not exist, diffusivities of Ti and Ni in fcc Ti are impossible to obtain experimentally. The impurity diffusivity of Ni in the fcc Ti phase was assumed to be equal to the self-diffusivity of Ti in fcc Ti (\(\varPhi_{Ni}^{Ti} = \varPhi_{Ti}^{Ti}\)).[11]
Figure 5 presents the comparison between the calculated interdiffusion coefficients and experimental data in the fcc Ni-Ti alloys at a temperature range of 1173–1648 K. It can be observed that the calculated results with present mobilities better fit to June et al.’s[53] experimental data in the whole temperature range, while the calculated results by Liu et al.[50] are better fit to Komai et al.’s[54] experimental data at 1273, 1373 and 1473 K. Figure 6 shows the calculated composition profiles of the Ni/Ni-8.0 at.%Ti diffusion couples annealed at 1173 K for 4 and 336 h, along with the corresponding experimental data. However, as the fcc phase is unstable in the Cr–Ti binary system, no diffusion experimental data is available. The mobility parameter corresponding to impurity diffusion in unstable fcc-Ti was assumed to be equal to the self-diffusivity of Ti in fcc Ti (\(\varPhi_{Cr}^{Ti} = \varPhi_{Ti}^{Ti}\)).
The atomic mobilities for the fcc Ni-Cr-Ti alloys were assessed by means of the PARROT module of the DICTRA software based on all the experimentally determined interdiffusion coefficients, as listed in Table 6. The thermodynamic description for the fcc Ni-Cr-Ti system was adopted from the present work.
Figure 7 shows the comparison of logarithmic values of the interdiffusion coefficients calculated using presently assessed mobility parameters and experimental data. The dashed lines refer to the diffusion coefficients with a factor of 2 or 0.5. It can be concluded that the present atomic mobilities can describe most experimental interdiffusivities satisfactorily, especially the main interdiffusion coefficients, \(\tilde{D}_{{CrC{\text{r}}}}^{Ni}\) and \(\tilde{D}_{TiTi}^{Ni}\).
To further explore the relationship between the interdiffusion coefficients and compositions in the fcc Ni-Cr-Ti alloys, the compositions were marked together with the corresponding interdiffusion coefficients determined by the present calculations and measurements, as shown in Fig. 8. In the Fig. 8(a), \(\tilde{D}_{{CrC{\text{r}}}}^{Ni}\) increases with increasing the Ti and Cr content, when the Cr content is low. \(\tilde{D}_{{CrC{\text{r}}}}^{Ni}\) decreases with increasing Cr when the Cr content is higher than 15 at.%. In Fig. 8(b), \(\tilde{D}_{TiTi}^{Ni}\) increases with increasing Ti and decreases with increasing Cr, and almost keeps constant when the Cr content is higher than 10 at.%. Both \(\tilde{D}_{{CrC{\text{r}}}}^{Ni}\) and \(\tilde{D}_{TiTi}^{Ni}\) increase sharply when the Ti content is close to 10 at.% and the Cr content is low. One can therefore perceive the larger influence of the Ti content than Cr on the interdiffusion coefficients in fcc Ni-Cr-Ti alloys.
4.5 Validation of Presently Assessed Atomic Mobilities
In order to check the reliability of the mobilities assessed in the present work, calculations were performed to predict the composition profiles and diffusion paths of the ternary diffusion couples. As shown in Fig. 9(a–h), the calculated composition curves based on the assessed atomic mobilities agree well with the experimental data for diffusion couples annealed at 1123 K for 336 h and 1273 K for 168 h.
The calculated isothermal section at 1123 and 1273 K based on the thermodynamic assessment in the present work and the predicted diffusion paths for all the eight diffusion couples are presented in Fig. 10, together with the experimental values. The S-shaped diffusion paths, which are due to the differences in diffusion coefficients, are observed. There is a satisfactory agreement between experimental and predicted profiles.
4.6 Effects of Alloying Elements on the Interdiffusion in the fcc Ni-Cr Alloys
To further explore the effects of alloying elements on the interdiffusion in fcc Ni-Cr alloys, the main interdiffusivities at 1273 K in various fcc ternary alloys were calculated based on the atomic mobilities reported in the literature[10,55] and the present work. As mentioned above, Campbell[55] verified the reliability of the atomic mobilities of the fcc Ni-Cr–Al alloys based on the experimental data. In addition, Zhu et al.[10] evaluated the atomic mobilities of the fcc Ni-Cr-Mo alloys. It is thus feasible to calculate the difference of Cr (i.e. \(Log_{10} \tilde{D}_{CrCr}^{Ni}\)) in the Ni-5.0 at.%Cr and Ni-5.0 at.%Cr-X (X = Al, Mo and Ti) alloys respectively. Difference of calculated \(Log_{{_{10} }} \tilde{D}_{CrCr}^{Ni}\) in Ni-5.0 at.%Cr alloys with and without additions of alloying elements at 1273 K is show in Fig. 11.
As shown in Fig. 11, \(\tilde{D}_{CrCr}^{Ni}\) varies differently with additions of different alloying elements. Obviously, additions of Al and Ti enhance \(\tilde{D}_{CrCr}^{Ni}\) whereas addition of Mo reduces \(\tilde{D}_{CrCr}^{Ni}\), indicating that the diffusion of Cr in the Ni-5.0 at.% Cr alloy is promoted by Al and Ti but retarded by Mo.
5 Summary
The thermodynamic description of the Ni-Cr-Ti ternary system has been reassessed in the present work. Based on the composition profiles in the fcc Ni-Cr-Ti diffusion couples annealed at 1123 K for 336 h and 1273 K for 168 h, the interdiffusion coefficients were extracted at intersections of every two diffusion paths using the Whittle-Green method. Further combined with the thermodynamic description the atomic mobilities of Ni, Cr and Ti were assessed by using DICTRA software. The presently assessed atomic mobilities can satisfactorily predict the interdiffusion coefficients, composition profiles and diffusion paths of the fcc Ni-Cr-Ti alloys annealed at 1123 and 1273 K. By analyzing the relationship between interdiffusion coefficients and compositions, the conclusions are presented as follows:
-
(a)
\(\tilde{D}_{{CrC{\text{r}}}}^{Ni}\) increases with increasing Ti and Cr content, when the Cr content is low, but decreases with increasing Cr when the Cr content is higher than 15 at.%.
-
(b)
\(\tilde{D}_{TiTi}^{Ni}\) increases with increasing Ti and decreases with increasing Cr, and almost keeps constant when the Cr content is higher than 10 at.%.
-
(c)
Both \(\tilde{D}_{{CrC{\text{r}}}}^{Ni}\) and \(\tilde{D}_{TiTi}^{Ni}\) increase sharply when the Ti content is close to 10 at.% and the Cr content is low.
-
(d)
Ti promotes the diffusion of Cr in fcc Ni-based alloys.
References
Y.H. Tan and Y. Du, Isothermal Section at 927 C of Cr-Ni-Ti System, Trans. Nonferrous Met. Soc., 2007, 17, p 711-714 (in Chinese)
J.R. Davis, Properties and Selection Nonferrous Alloys and Special Purpose Materials. Materials Handbook, ASM International, 1990, p 436-441
J.A. van Beek, A.A. Kodentsov, and F.J.J. van Loo, ChemInform Abstract: Phase Equilibria in the Ni-Cr-Ti System at 850°C, Alloys Compd., 1998, 279, p 218-223
I. Isomäki, M. Hämäläinen, and M. Gasik, Thermodynamic Assessment of the Ternary Ni-Ti-Cr System, J. Alloy. Compd., 2012, 543, p 12-18
B.J. Lee, On the Stabiliity of Cr Carbides, CALPHAD, 1992, 16(2), p 121-149
T. Tokunaga, K. Hashima, H. Ohtani, and M. Hasebe, Thermodynamic Analysis of the Ni-Si-Ti System Using Thermochemical Properties Determined from Ab Initio, Calculations, Materials Transactions, 2004, 45(5), p 1507-1514
G. Ghosh, Thermodynamic and Kinetic Modeling of the Cr-Ti-V System, Journal of Phase Equilibria, 2002, 23(4), p 310
J.D. Keyzer, G. Caccismani, N. Dupin, and P. Wollants, Thermodynamic Modeling and Optimization of the Fe-Ni-Ti System, CALPHAD, 2009, 33(1), p 109-123
J. Pavlů, J. Vreat’ál, and M. Šob, Thermodynamic Modeling of Laves Phases in the Cr-Hf and Cr-Ti Systems: Reassessment Using First-Principles Results, CALPHAD, 2010, 34(2), p 215-221
N.Q. Zhu, J.C. Li, X.G. Lu, and Y.L. He, Experimental and Computational Study of Diffusion Mobilities for fcc Ni-Cr-Mo Alloys, Metall. Mater. Trans. A, 2015, 46(11), p 5444-5455
J.O. Andersson, T. Helander, L. Höglund, P. Shi, and B. Sundman, Thermo-Calc & DICTRA, Computational Tools for Materials Science, CALPHAD, 2002, 26, p 273-312
A.T. Dinsdale, SGTE Data for Pure Elements, CALPHAD, 1991, 15, p 317-425
M. Hillert, Phase Relations in the ZrO2 -Nd2O3 -Y2O3 System: Experimental Study and Advanced Thermodynamic Modeling, J. Alloys Compounds, 2001, 320, p 161-176
A. Borgenstam, A. Engström, L. Höglund, and J. Ågren, DICTRA, A Tool for Simulation of Diffusional Transformations in Alloys, J. Phase Equilib. Diffus., 2000, 21, p 269-280
J.O. Andersson and J. Ågren, Models for Numerical Treatment of Multicomponent Diffusion in Simple Phases, J. Appl. Phys., 1992, 72, p 1350-1355
Y. Wang, N.Q. Zhu, H. Wang, and X.G. Lu, Interdiffusion and Diffusion Mobility for fcc Ni-Co-Al Alloys, Metall. Mater. Trans. A, 2017, 48, p 943-947
J.F. Wang, Y. Wang, N.Q. Zhu, and X.G. Lu, Experimental and Computational Study of Interdiffusion for fcc Ni-Co-W Alloys, J. Phase Equilib. Diffus., 2017, 38, p 37-50
Y. Wang and X.G. Lu, Interdiffusion and Diffusion Mobility for Fcc Ni-Co-Mo Alloys, J. Phase Equilib. Diffus., 2017, 38, p 656-664
J.L. Murray, Phase Diagrams of Binary Titanium Alloys, ASM International, Materials Park, 1987, OH, p. 197–211, 59–68
L. Kaufman and H. Nesor, Coupled Phase Diagrams and Themochemical Data for Transition Metal Binary System-II, CALPHAD, 1978, 2(1), p 81-108
P. Bellen, K.C.H. Kumar, and P. Wollants, Thermodynamic Assessment of the Ni-Ti Phase Diagram, Z. Metallkd., 1996, 87, p 972-978
W. Tang, B. Sundman, R. Sandström, and C. Qiu, New Modelling of the B2 Phase and its Associated Martensitic Transformation in the Ti-Ni System, Acta material, 1999, 47(12), p 3457-3468
A. Pastural, C. Colinet, M.D. Nguyen, A.T. Paxton, and M. van Schilfgaarde, Electronic Structure and Phase Stability Study in the Ni-Ti System, Phys. Rev., 1995, B52, p 15176-15190
K. Santhy and K.C. Hari Kumar, Thermodynamic Assessment of Mo-Ni-Ti Ternary System by Coupling First-Principle Calculations with CALPHAD Approach, Intermetallics, 2010, 18(9), p 1713-1721
O. Kubaschewski, H. Villa, and W.A. Dench, The Reaction of Titanium Tetrachloride with Hydrogen in Contact with Various Refractories, Trans. Faraday Soc., 1956, 52, p 214-222
J.C. Gachon, M. Notin, and J. Hertz, The Enthalpy of Mixing of the Intermediate Phases in the Systems FeTi, CoTi, and NiTi by Direct Reaction Calorimetry, Thermochim. Acta, 1981, 48, p 155-164
G.A. Levshin and V.I. Alekseev, Thermodynamic Properties of Nickel-Titanium System Alloys, Russ. J. Phys. Chem., 1979, 53, p 437-439
P.A. Gomozov, Y.V. Zasypalov, and B.M. Mogutnov, Enthalpies of Formation of Intermetallic Compounds with the CsCl Structure (CoTi, CoZr, CoAl, NiTi), Russ. J. Phys. Chem., 1986, 60, p 1122-1124
H.C. Yi and J.J. Moore, Combustion Synthesis of TiNi Intermetallic Compounds Part 1: Determination of Heat of Fusion of TiNi and Heat Capacity of Liquid TiNi, J. Mater. Sci. Lett., 1989, 24, p 3449-3455
Q.T. Guo and O.J. Kleppa, Standard Enthalpies of Formation of Some Alloys Formed Between Group IV Elements and Group VIII, Elements, Determined by Hightemperature Direct Synthesis Calorimetry II. Alloys of (Ti, Zr, Hf) with (Co, Ni), J. Alloys. Compd., 1998, 269, p 181-186
C. C. Jia, K. Ishida and T. Nishizawa, Experimental Methods of Phase Diagram Determination, TMS. Publisher, 1994, PA, p. 31–38
D.M. Poole and W. Hume-Rothery, The Equilibrium Diagram of the System Nickel-Titanium, J. Inst. Metals, 1954, 83, p 473-480
A. Taylor and R.W. Floyd, The Constitution of Nickel-Rich Alloys of the Nickel-Chromium-Aluminium System, Inst. Metals, 1951, 80, p 577-587
Y.A. Bagariatskii and Y.D. Tyapkin, On the Atomic Structure of Solid Solutions of Chromium in Nickel, Z. Neorg Khim, 1958, p. 151–158.
G.R. Purdy and J.G. Parr, A Study of the Titanium-Nickel System Between Ti2Ni and TiNi, Trans. AIME, 1961, 221, p 636-639
G.F. Bastin and G.D. Rieck, Diffusion in the Titanium-Nickel System: I. Occurrence and Growth of the Various Intermetallic Compounds, Metall. Trans, 1974, 5, p 1817-1826
R. Vogel and H.S. Wallbaum, Das System Eisen‐Nickel‐Nickeltitanid Ni3Ti‐Eisentitanid Fe2 Ti, Arch. Eisenhüttenwes, 1938, 12, p 299-304
H. Margolin, E. Ence, and J.P. Nielsen, The Titanium-nickel Phase Diagram, Trans. AIME, 1953, 197, p 243-247
M.K. McQuillan, A Provisional Constitutional Diagram of the Chromium Titanium System, J. Inst. Met., 1951, 80, p 379-390
F.B. Cuff, N.J. Grant, and C.F. Floe, Titanium Chromium Phase Diagram, J. Met., 1952, 4, p 848-853
R.J. Van Thyne, H.D. Kessler, and M. Hansen, The Systems Titanium Chromium and Titanium-Iron, Trans. Am. Soc. Met., 1952, 44, p 974-989
F. Ermanis, P.A. Farrar, and H. Margolin, A Reinvestigation of the Systems Ti-Cr and Ti-V, Trans. Metall. Soc. AIME, 1961, 221, p 904-908
V.S. Mikheyev and T.S. Chernova, Solubility of Chromium in a-Titanium and Mechanical Properties of the Binary System Titanium-Chromium, in Titan I Ego Splavy AN SSSR Inst., 1962, p. 68–73
V.N. Svechnikov and Y.A. Kocherzhinsky, Consitution Diagram of Chromium Titanium, Probl. Phys. Met. Metall., 1962, 32, p 132-135
P.A. Farrar and H. Margolin, A Re-Investigation of the Chromium Rich Region of the Titanium Chromium System, Trans Metall Soc AIME, 1963, 227, p 1342-1345
V.N. Svechnikov, M.Y. Teslyuk, A.Y. Kocherzhinsky, V.V. Petkov, and E.V. Dabizha, Three Modifications of TiCr2, Dopov Akad Nauk Ukr RSR, 1970, 32, p 837-842
S.A. Minaeva, B.P. Budberg and A.L. Gavze, Phase Structure of Ti-Cr Alloys, in Izv Akad Nauk SSSR Met, 1971
A. Taylor, Constitution of Nickel-Rich Quaternary Alloys of the Ni-Cr-Ti-Al System, Trans. AIME, 1956, 206, p 1356-1362
J.S. Kirkaldy and D.J. Young, Diffusion in the condensed state, in the Institute of Metals, 1987, London, p. 83–87
M. Liu, L.J. Zhang, W.M. Chen, and J.H. Xin, Diffusivities and Atomic Mobilities in fcc_A1 Ni–X (X = Ge, Ti and V) Alloys, CALPHAD, 2013, 41, p 108-118
D. Bergner, Zur diffusion von Hf und Ti in Ni, Kristall Technik, 1972, 7, p 651-656
R.A. Swalin and A. Martin, Solute Diffusion in Nickel-Base Substitutional Solid Solutions, Trans. AIME, 1956, 206, p 567-572
S.B. Jung, T. Yamane, Y. Minamino, K. Hirao, H. Araki, and S. SajI, Interdiffusion and Its Size Effect in Nickel Solid Solutions of Ni-Co, Ni-Cr and Ni-Ti Systems, J. Mater. Lett., 1992, 11, p 1333-1337
N. Komai, M. Watanabe, Z. Horita, T. Sano, and M. Nemoto, Analytical Electron Microscopy Study of Ni/Ni–8 mol% Ti Diffusion Couples, Acta Mater., 1998, 46, p 4443-4451
C.E. Campbell, Assessment of the Diffusion Mobilites in the γ’ and B2 Phases in the Ni-Al-Cr System, Acta Mater., 2008, 56(16), p 4277-4290
Acknowledgments
The authors gratefully acknowledge the financial support from the National Key R&D Program of China (Grant Number: 2017YFB0701502).
Author information
Authors and Affiliations
Corresponding author
Additional information
This invited article is part of a special issue of the Journal of Phase Equilibria and Diffusion in honor of Prof. Zhanpeng Jin’s 80th birthday. The special issue was organized by Prof. Ji-Cheng (JC) Zhao, The Ohio State University; Dr. Qing Chen, Thermo-Calc Software AB; and Prof. Yong Du, Central South University.
Rights and permissions
About this article
Cite this article
Huang, J., Wang, Y., Wang, J. et al. Thermodynamic Assessments of the Ni-Cr-Ti System and Atomic Mobility of Its fcc Phase. J. Phase Equilib. Diffus. 39, 597–609 (2018). https://doi.org/10.1007/s11669-018-0650-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-018-0650-3