Abstract
The isothermal section of the Zr-Sn-Cu ternary system at 700 °C was investigated by using x-ray diffraction, scanning electron microscope and energy dispersive spectroscopy. A new ternary compound τ (Zr25.3Cu66.1Sn8.6) was observed in the Cu-rich corner of this system. The previous known ZrCuSn and ZrCuSn2 ternary compound were confirmed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Copper alloys are widely used in electrical industries because of their excellent electrical and thermal conductivities. Additional of Zr and other alloying elements improve the strength of copper alloys.[1,2] Zirconium alloys are widely used as cladding and core structure materials in fuel reactions, due to their low neutron absorbtion cross section, perfect corrosion and creep resistance properties in high pressure and high temperature environment.[3] Tin is a common alloying element in commercial zirconium alloys, since the addition of tin offsets the deterioration of corrosion resistance by nitrogen and improves the creep resistance of alloys.
Development of the HANA serial alloys (Zr-Nb-Cu alloys) indicates that copper has a positive effect on the corrosion resistance of commercial zirconium alloys.[4,5,6,7] Since the development of the HANA serial alloys, dilute copper additions have been added to experimental Zr-based alloys to improve their corrosion resistance.[8,9,10,11,12,13,14] Among those experimental alloys, Zr-Sn-Cu is an important subsystem. Though no Sn-containing compound has been reported in commercial zirconium alloys, the interaction of tin with Zr and other alloying elements is still vital for understanding phase transformations in commercial Zr-based alloys. For example, the interaction of tin and other alloying elements will change the phase boundary between α-Zr and β-Zr as well as those between α-Zr, β-Zr, and precipitates. Temperatures associated with these boundaries are very important for thermal fabrication.
Most commercial Zr-based alloys are multicomponent. To understand phase transitions in multicomponent alloys, having a thermodynamic description of the alloys by using CALPHAD techniques is both convenient and powerful.[15] Experimental work on binary and ternary phase diagrams provide basic data for the thermodynamic optimization of databases. To benefit the establishment of a thermodynamic description of the Zr-based alloys, this work aims to determine phase equilibria in the Zr-Sn-Cu system.
Subsytems for the Zr-Sn-Cu alloy are the Zr-Cu, Zr-Sn, and Cu-Sn binary systems. In 1990, Aria and Abriata assessed the phase diagram of the Zr-Cu system.[16] According to Aria and Abriata, there are 8 compounds existing in the Zr-Cu system, i.e., Cu9Zr2, CuZr, Cu10Zr7, Cu24Zr13, Cu2Zr, Cu8Zr3, Cu51Zr14, and Cu5Zr. Later, Zeng et al.[17] optimized this system. Zeng et al.[17] pointed out that Cu5Zr rather than Cu9Zr2 exists in this system. According to Zeng et al.,[17] the stable phases at 700 °C for the Zr-Cu system are CuZr2, Cu10Zr7, Cu8Zr3, Cu51Zr14, and Cu5Zr. In 2010, a thermodynamic assessment of the Zr-Cu system has been carried out by Kang and Jung.[18] Kang and Jung[18] reported that the stable compounds in the Zr-Cu system were Cu9Zr2, Cu51Zr14, Cu8Zr3, Cu2Zr, C10Zr7, CuZr, Cu5Zr8, and CuZr2. Recently, a new experiment work and thermodynamic assessment on the Zr-Cu system were conducted by Liu et al.[19] Experiment work from Liu et al. revealed that Cu2Zr and Cu5Zr8 were not the stable phases. This result is consistent with that in Ref 17. As discussed by Zeng et al.[17], the existence of Cu5Zr was supported by XRD results on the extracted precipitate,[20] and electron diffraction results on the precipitate in spray-cast Cu-Zr alloys.[21] Recently, results from electron diffraction confirmed that Cu5Zr was the stable compound in the Zr-Cu system too.[22,23] It shows that the modeling work of the Zr-Cu system by Liu et al.[19] is more reliable than that by Kang and Jung.[18] Summarily, the stable compounds in Zr-Cu system are Cu5Zr, Cu51Zr14, Cu8Zr3, C10Zr7, CuZr, and CuZr2. CuZr is a high temperature phase, which decomposes through eutectoid reaction CuZr ↔ Cu10Zr7 + CuZr2 at 717.6 °C.[19]
The Cu-Sn phase diagram was experimentally re-investigated by Fütauer et al.[24] A new thermodynamic assessment of this system was carried out by Li et al.[25] too. According to Fütauer et al.[24] and Li et al.,[25] solid solution (Cu), Cu17Sn3, Cu3Sn and liquid are stable at 700 °C in the Cu-Sn system. Cu17Sn3 and Cu3Sn (ht), designated as β and γ in Ref 24 respectively, presenting order–disorder transition with composition change, are stable in the composition range of 15.0-27.5 Sn at.% at 700 °C respectively. Cu17Sn3 has the bcc W prototype, and Cu3Sn (γ) adopts the fcc BiF3 prototype.
The Zr-Sn system was experimentally updated and assessed by Pérez et al.[26] Four binary compounds were found to exist at 700 °C, i.e., ZrSn2, Zr5Sn4, Zr5Sn3, and Zr4Sn in this system.[26] Zr5Sn3 (Mn5Si3 prototype structure) and Zr5Sn4 (Ti5Si4 prototype structure) have close related crystallographic structures.[27] These two phases are stable up to 1100 °C, and merge into a single phase Zr5Sn3+x, with 0 < x < 1, at higher temperature, due to their structural similarities.[27] The Zr5Sn3+x phase was referred as η by Pérez et al.[26]
Two isothermal sections of the Zr-Sn-Cu system at 397 and 497 °C were experimentally determined by Romaka et al. across the whole composition range.[28] Two new ternary compounds, ZrCuSn and ZrCuSn2, were identified. The ZrCuSn compound adopts the TiNiSi structure type with cell parameters of a = 0.66279(1) nm, b = 0.43679(9) nm, c = 0.76791(2) nm. The ZrCuSn2 compound has the HfCuSi2 structure type with cell parameters of a = 0.41350(7) nm, c = 0.9225(3) nm. Most commercial zirconium-based alloys are thermally fabricated, for example, by forging and extruding in the temperature range from 700 to 800 °C. In this work, the isothermal section of this system at 700 °C was investigated.
2 Experimental Procedures
Nuclear grade sponge Zr, pure Cu (99.95 wt.%), and Sn (99.95 wt.%) were used as starting materials. Ingots (4 g) were arc melted under the environment of pure argon (99.99%). To reduce contamination by oxygen, a titanium getter was melted prior to melting. The resultant buttons were sealed in vacuum quartz tubes. The alloy buttons were directly annealed at 700 °C for 60 days followed by water quench.
The alloys were subject to x-ray diffraction (XRD). The XRD was carried out on the XD-3 diffractometer with CuKa radiation and graphite monochromator. The metallographic specimens were prepared by mechanical grinding and polishing, but without etching. Microstructural observation and compositional analysis were carried out on a Scanning Electron Microscope (SEM, EVO 18) coupled with energy dispersive spectrometer (EDS, Bruke). Compositions of the alloys and those of the identified phases were obtained in area scan mode and dot scan mode, respectively, with ZAF corrections. No standard was used for the EDS compositional analysis. Chemical composition for each phase is the average of at least three measurements.
3 Results and Discussions
Results of the investigated alloys by combining use of SEM/EDS and XRD are presented in Table 1. Measured compositions for the investigated alloys and the identified phases are included. It is noted that phase identification is mainly based on composition, because of the overlap of diffraction peak in the Zr-Cu compounds.
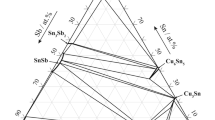
The Zr-Sn-Cu phase diagram at 700 °C is given in Fig. 1. A new ternary compound, designated as τ, was found in this work. The previously reported ternary compounds, ZrCuSn and ZrCuSn2,[28] were designated as τ1 and τ2 in Fig. 1, respectively. Tie-triangles with dash lines were deduced by the adjacent phase relationships and Gibbs’ phase rule. The end-points of these tie-triangles were tentatively determined, too. Cu17Sn3 (β) and Cu3Sn (γ) experience order–disorder transition with the composition change.[24,25] A dash line was drawn to separate two-phase region of [β + ZrCuSn] and [γ + ZrCuSn]. For the convenience of the reader, the composition points for the investigated alloys were marked in Fig. 1. It is noted that there are several composition points out of the tie-triangles defining by the alloys. As an evidence to support the existence of the related tie-triangles, results from these alloys are reserved in Table 1 too.
Figure 2 is the backscattered electron (BSE) image of alloy 18. It is clear that the alloy is composed of three phases. Composition of the white grey phase is Zr55.3Cu12.5Sn32.2 (at.%), which indicates this phase is Zr5Sn4. The deeply grey phase Zr22.9Cu76.6Sn0.6 was identified as Cu51Zr14. The composition of the grey phase is Zr24.8Cu67.6Sn7.6 (at.%), designated as τ.
Alloy 21 consists of Zr5Sn4, Cu51Zr14, and τ phase too, as shown in Fig. 3. Results from alloys 18 and 21 indicate that the tie-triangle of (Zr5Sn4 + Cu51Zr14 + τ) is in this system. The τ phase with very similar composition was observed in alloys 10, 15, 19, and 23, see Table 1. The average composition of τ is calculated to be Zr25.3Cu66.1Sn8.6. Co-existence of τ and Cu51Zr14 in alloys 18 and 21 reveals that τ and Cu51Zr14 are different. This τ phase is identified as a new ternary compound rather than a ternary phase derived from the nearby Zr-Cu compounds. Further work needs to be done to clarify the nature of this new ternary compound.
The tie-triangle of [(Cu) + τ + ZrCuSn] is supported by phase identification in alloy 19, as shown in Fig. 4. The dark phase with composition of Cu99.3Sn0.7 is (Cu). The grey phase with composition of Zr35.2Cu34.2Sn30.6 is ZrCuSn. The white grey phase with composition of Zr24.4Cu66.7Sn8.9 is the new ternary compound τ. The color contract in the BSE image is related to the average atomic number of the phase. In this alloy, (Cu) with lowest average atomic number is the darkest.
X-ray diffraction (XRD) observed major CuZr2 and Zr5Sn4, and minor Cu10Zr7 in alloy 7, as shown in Fig. 5(a). Figure 5(b) is the BSE image for microstructure of this alloy. As shown in Fig. 5(b), the white grey phase with composition of Zr58.5Cu11.2Sn30.3 is Zr5Sn4, which is confirmed by XRD. Phases between the island Zr5Sn4 have little color contract in the BSE image. However with the help of EDS, the deeply grey phase is CuZr with a measured composition to be Zr50.5Cu49.2Sn0.3 and the grey phase is CuZr2 with a composition of Zr66.7Cu33.1Sn0.2. The boundary between CuZr and CuZr2 is dim, which imply that CuZr is in the diffusion processing. The composition of Cu10Zr7 was not detected. Similar results were found in alloy 9, where Zr5Sn4 and Cu10Zr7 and CuZr2 were detected by XRD. However, EDS detected only the composition of Zr5Sn4 and Cu10Zr7 and CuZr. The boundary between CuZr and Cu10Zr7 is not clear too. The CuZr phase decomposes via. eutectoid reaction CuZr ↔ Cu10Zr7 + CuZr2 at 717.6 °C.[19] The dim boundary of CuZr phase and Cu2Zr in alloy 7, and Cu10Zr7 in alloy 8 suggested that CuZr is in diffusion decomposition processing. Thus, CuZr phase detected in alloys 6-9 is considered to be metastable. Results in alloys 7 and 8 define the tie-triangle of [Cu10Zr7 + Zr5Sn4 + CuZr2].
The tie-triangle of (Zr5Sn4 + Cu10Zr7 + Cu8Zr3) is supported by alloy 16 as seen in the Fig. 6. The white grey phase Zr54.9Cu13.1Sn32.0 is Zr5Sn4, and the white light grey phase Zr29.6Cu70.3Sn0.1 is Cu8Zr3, while the deeply grey phase Zr45.2Cu54.0Sn0.8 is Cu10Zr7.
Figure 7 is the BSE image of alloy 15. The BSE image shows that the alloy consists of three phases. These phases were identified to be Zr5Sn4, τ, and ZrCuSn by considering the results from XRD and SEM/EDS analysis. The composition of Zr5Sn4 is Zr56.9Cu9.4Sn33.8, and of τ and ZrCuSn are Zr24.4Cu67.0Sn8.7 and Zr34.9Cu31.5Sn33.6. Co-existence of Zr5Sn4, τ, and ZrCuSn was observed in alloy 23 too. Results from alloys 15 and 23 confirmed the tie-triangle of (Zr5Sn4 + τ + ZrCuSn).
At 700 °C, liquid was present in the alloys close to the Cu-Sn side. In this work, liquid phase was observed in alloys 12 and 13. Figure 8 is the BSE image of alloy 12. The large island phase is the ZrCuSn ternary compound. The grey phase is Cu3Sn (γ) at 700 °C. As shown in Fig. 8, the light grey phase is Cu6Sn5, which is the product of liquid during quenching. After the precipitate of Cu6Sn5, the retained liquid solidified into a mixture Cu6Sn5 and (Sn). These two phases could not be distinguished in the current image resolution. Results from alloy 12 define a tie-triangle [Cu3Sn + ZrCuSn + Liquid]. Since Zr has a small solubility (5 at.% at 700 °C) in liquid Sn,[26] it is believed that Zr has small solubility in Cu-Sn liquid too. Thus, the liquid point in the tie-triangle [Cu3Sn + ZrCuSn + Liquid] is assigned to be the point in liquidus of the Cu-Sn system at 700 °C. Figure 9 is the BSE image of alloy 13. Liquid and ZrCuSn were confirmed to exist together at 700 °C. No alloys contained [ZrCuSn + ZrCuSn2 + Liquid] and [Zr2Sn + ZrCuSn2 + Liquid] were found in this work. Tie-triangles [ZrCuSn + ZrCuSn2 + Liquid] and [Zr2Sn + ZrCuSn2 + Liquid] are determined based on the adjacent phase relationships. The liquid points in the these two tie-triangles are arbitrary tentative.
The ZrCuSn2 phase was observed to co-exist with ZrSn2 in alloy 22. This result shows that ZrCuSn2 is stable at 700 °C. Taking into account the data listed in Table 1, it is noted that the measured content of Sn in the Zr-Cu side binary compounds is very small. This fact means that the solubility of Sn in the Zr-Cu binary compounds is negligible.
4 Conclusions
-
1.
A new ternary compound, designated as τ with composition of about Zr25.3Cu66.1Sn8.6 was identified. The previously reported ternary compound ZrCuSn and ZrCuSn2 are confirmed to be stable at 700 °C.
-
2.
Phase relationships in the Zr-rich corner, of interested in nuclear industrial applications, are (Zr5Sn3 + αZr + CuZr2) and (Zr5Sn3 + Zr4Sn + αZr).
References
A.J. Perry, The Properties of Directionally-Solidified Eutectic and Hypo-eutectic Copper-Zirconium Alloys, J. Mater. Sci., 1973, 8, p 443-450
J.A. Juarea-Islas, R. Perez, L.A. Alabarran, V. Rivera, and L. Martinz, Development of High-Strength, High-Conductivity Copper Alloys by Rapid Solidification, J. Mater. Sci. Lett., 1992, 11, p 1104-1106
A.T. Motta, Waterside Corrosion in Zirconium Alloys, JOM, 2011, 63(8), p 63-67
J.M. Kim and Y.H. Jeong, Influence of Thermomechanical Treatment on the Corrosion Behavior of Zr-1Nb-0.2 Cu Alloys, J. Nucl. Mater., 1999, 275(1), p 74-80
H.S. Hong, J.S. Moon, S.J. Kim, and K.S. Lee, Investigation on the Oxidation Characteristics of Copper-Added Modified Zircaloy-4 Alloys in Pressurized Water at 360 °C, J. Nucl. Mater., 2001, 297(2), p 113-119
J.-Y. Park, B.-K. Choi, S.J. Yoo, and Y.H. Jeong, Corrosion Behavior and Oxide Properties of Zr-1.1 wt.% Nb-0.05 wt.% Cu Alloy, J. Nucl. Mater., 2006, 359(1–2), p 59-68
Y.H. Jeong, S.-Y. Park, M.H. Lee, B.-K. Choi, J.-H. Baek, J.-Y. Park, J.H. Kim, and H.G. Kim, Out-of-Pile and In-Pile Perfomance of Advanded Zirconium Alloys (HANA) for High Burn-Up Fuel, J. Nucl. Sci. Technol., 2006, 43(9), p 977-983
S. Li, M. Yao, X. Zhang, J. Geng, J. Peng, and B. Zhou, Effect of Adding Cu on the Corrosion Resistance of M5 Alloy in Superheated Steam at 500 °C, Acta Metall. Sin., 2011, 47(2), p 163-168
M.Y. Yao, Y. Zhang, S.L. Li, X. Zhang, J. Zhou, and B.X. Zhou, Effect of Cu Content on the Corrosion Resistance of Zr-0.80Sn-0.34Nb-0.39Fe-0.10Cr-xCu Alloy in Superheated Steam at 500 °C, Acta Metall. Sin., 2011, 47(7), p 872-876
X. Zhang, M.Y. Yao, Z.K. Li, J. Zhou, Q. Li, and B.X. Zhou, Corrosion Resistance of Zr-0.80Sn-0.4Nb-0.4Fe-0.10Cr-xCu Alloys in Super-Heated Steam at 400 °C, Rare Met. Mater. Eng., 2013, 42(6), p 1210-1214
L.M. Tu, J.L. Zhang, Q.D. Xu, M.Y. Yao, and B.X. Zhou, Corrosion Resistance of Zr-0.7Sn-1Nb-0.03Fe-xCu-xGe (x = 0, 0.05, 0.2) Alloys in Superheated Steam at 400 °C/10.3 MPa, Rare Met. Mater. Eng., 2015, 44(6), p 1391-1396
L. Chen, Q. Zeng, J. Li, J. Lu, Y. Zhang, L.-C. Zhang, X. Qin, W. Lu, L. Zhang, and L. Wang, Effect of Microstructure on Corrosion Behavior of a Zr-Sn-Nb-Fe-Cu-O Alloy, Mater. Des., 2016, 92, p 888-896
L. Chai, B. Luan, J. Chen, J. Zhou, and Q. Liu, Effect of Cooling Rate on β → α Transformation During Quenching of a Zr–0.85Sn–0.4Nb–0.4Fe–0.1Cr–0.05Cu Alloy, Sci. China Ser. E, 2012, 55(10), p 2960-2964
L.J. Chai, B.F. Luan, S.S. Gao, J.W. Chen, and Q. Liu, Study of Precipitate Evolution and Recrystallization of Beta-Quenched Zr-Sn-Nb-Fe-Cr-Cu Alloy During Aging, J. Nucl. Mater., 2012, 427(1–3), p 274-281
Y. Du, S. Liu, L. Zhang, H. Xu, D. Zhao, A. Wang, and L. Zhou, An Overview on Phase Equilibria and Thermodynamic Modeling in Multicomponent Al Alloys: Focusing on the Al-Cu-Fe-Mg-Mn-Ni-Si-Zn System, CALPHAD, 2011, 35(3), p 427-445
D. Arias and J.P. Abriata, Cu-Zr (Copper-Zirconium), Bull. Alloys Phase Diagr., 1990, 11, p 452-459
K.J. Zeng, M. Hamalainen, and H.L. Lukas, A New Thermodynamic Description of the Cu-Zr System, J. Phase Equilib., 1994, 15, p 577-586
D.H. Kang and I.H. Jung, Critical Thermodynamic Evaluation and Optimization of the Ag-Zr, Cu-Zr and Ag-Cu-Zr Systems and its Applications to Amorphous Cu-Zr-Ag Alloys, Intermetallics, 2010, 18, p 815-833
Y. Liu, S. Liu, C. Zhang, Y. Du, J. Wang, and Y. Li, Experimental Investigation and Thermodynamic Description of the Cu-Zr System, J. Phase Equilib. Diffus., 2017, 38, p 121-134
M.Y.W. Lou and N.J. Grant, Identification of Cu5Zr Phase in Cu-Zr Alloys, Metall. Trans. A, 1984, 15, p 1491-1493
R.P. Singh, A. Lawley, S. Friedman, and Y.V. Murty, Microstructure and Properties of Spray Cast CuZr Alloys, Mater. Sci. Eng. A, 1991, 145, p 243-255
M.A. Turchanin, P.G. Agraval, and A.R. Abdulo, Electron Microscopic Investigation of Aging in the Cu-0.06% Zr Alloy, Phys. Met. Metall., 2016, 117, p 710-718
L.J. Peng, X.J. Mi, B.Q. Xiong, H.F. Xie, and G.J. Huang, Microstructure of Phases in a Cu-Zr Alloy, Rare Met., 2015, 34(10), p 706-709
S. Fürtauer, D. Li, D. Cupid, and H. Flandorfer, The Cu-Sn Phase Diagram, Part I: New Experimental Results, Intermetallics, 2013, 34, p 142-147
D. Li, P. Franke, S. Fürtauer, D. Cupid, and H. Flandorfer, The Cu-Sn Phase Diagram Part II: New Thermodynamic Assessment, Intermetallics, 2013, 34, p 148-158
R.J. Pérez, C. Toffolon-Masclet, J.M. Joubert, and B. Sundman, The Zr-Sn Binary System: New Experimental Results and Thermodynamic Assessment, CALPHAD, 2008, 32, p 593-601
Y.-U. Kwon and J.D. Corbett, The Zirconium-Tin System, with Particular Attention to the Zr5Sn3-Zr5Sn4 Region and Zr4Sn, Chem. Mater., 1990, 2, p 27-33
L. Romaka, N. Koblyuk, Y.V. Stadnyk, D. Frankevych, and R. Skolozdra, Phase Equilibria in the Zr-Cu-Sn System and Crystal Structure of ZrCuSn and ZrCuSn2, Pol. J. Chem., 1998, 72(7), p 1154-1159
Acknowledgments
Financial support from the Natural Science Foundation of China (51301045, 51071033), and State Nuclear Bao Ti Zirconium Industry Company are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, G., Luo, W., Ouyang, Y. et al. The Isothermal Section of the Zr-Sn-Cu Ternary System at 700 °C. J. Phase Equilib. Diffus. 39, 196–203 (2018). https://doi.org/10.1007/s11669-018-0621-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-018-0621-8