Abstract
Based on the available literature experimental phase equilibria, the Ti-RE (RE = Ce, Er, Tm, Y) binary systems have been critically reviewed and modeled by means of the CALPHAD (CALculation of PHAse Diagram) method. No compound exists in these binary systems. The solution phases, i.e., liquid, bcc(βTi, δCe, βY), hcp(αTi, Er, Tm, αY) and fcc(γCe, γY), are described by the substitutional solution model. A set of self-consistent thermodynamic parameters is finally obtained for each of these binary systems. Comparisons between the calculated and measured phase diagrams show that most of the experimental data are satisfactorily accounted for by the present thermodynamic descriptions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ti alloys have been widely used for aviation, aerospace, automotive, biomedical applications, etc., due to their high specific intensity, good corrosion resistance, excellent toughness and good biocompatibility.[1] The rare earth (RE) elements are important modifier of multi-component commercial Ti alloys. For example, the addition of Ce and Y to Ti alloys can deoxidize the oxygen content of the titanium alloy and improve the oxidation resistance.[2,3] In addition, RE-transition metals are used as permanent magnets which derive their exceptional magnetic properties from the favourable combination of rare earth metals with transition metals,[4] such as Ti-, Zr-based multi-component alloys with rare earth element. The design of compositions of Ti-RE based novel materials requires information about the phase equilibria of the Ti-RE systems. The Ti-RE (RE = Gd, Nd, La) systems have been modeled by Mattern et al.[5,6,7] The present work is devoted to thermodynamic description of the Ti-RE (RE = Ce, Er, Tm, Y) systems.
The Ti-RE (RE = Ce, Er, Tm, Y) systems are subsystems of many multi-component Ti alloys. In order to provide a reliable basis for thermodynamic extrapolations and calculations in related ternary and higher-order systems, accurate thermodynamic assessment of the Ti-RE (RE = Ce, Er, Tm, Y) systems is essential. Thermodynamic description for the Ti-RE (RE = Ce, Er, Tm, Y) systems is part of our efforts[1,8,9] to establish thermodynamic databases of multi-component Ti based alloys. However, to the best of our knowledge, so far the thermodynamic parameters of these binary systems are not available. The purposes of the present work are to critically evaluate the measured phase diagram data available in the literature and to obtain a self-consistent set of thermodynamic parameters for these binary systems by means of the CALPHAD approach.[10,11]
2 Evaluation of Experimental Data
2.1 The Ti-Ce System
The Ti-Ce system has been experimentally investigated by two groups of authors Taylor[12] and Savitskii and Burkhanov.[13,14] No binary phase has been found and a miscibility gap exists in the liquid phase. Based on the microscopy and x-ray diffraction, Taylor[12] examined the Ti-rich alloys containing up to 7.2 at.% Ce. The solubility of Ce in (αTi) and (βTi) was less than 0.4 at.% Ce in the range of 750-980 °C. By means of microscopy and thermal analysis, Savitskii and Burkhanov[13] studied the diagram in the range of 0-7.2 at.% Ce and reported two invariant reactions Liquid#2 ↔ Liquid#1 + (βTi) and Liquid#1 + (βTi) ↔ (αTi) located at 1450 and 915 °C, respectively. Later, Savitskii and Burkhanov[14] studied in detail the phase equilibria of the Ti-Ce system by means of thermal analysis and microscopy. Four invariant reactions, i.e., Liquid#2 ↔ Liquid#1 + (βTi), Liquid#1 + (βTi) ↔ (αTi), Liquid#1 ↔ (δCe) + (αTi) and (δCe) ↔ (γCe) + (αTi) were reported located at 1450 ± 25, 910 ± 10, 790 ± 20 and 710 ± 20 °C, respectively. Murray[15] reviewed the Ti-Ce system based on the above experimental work[12,13,14] and constructed the Ti-Ce phase diagram.
2.2 The Ti-Er System
For the Ti-Er system, limited experimental phase equilibria data are available in the literature. This system is characterized by the absence of intermetallic compounds and limited mutual solubilities in the solid state. Love[16] determined the solid-liquid equilibria of the Ti-Er system using metallography, x-ray diffraction and differential thermal analysis. Two invariant reactions were reported by Love,[16] i.e., the one eutectic reaction Liquid ↔ (Er) + (βTi) at 1320 ± 20 °C and 26 at.% Ti, and the other peritectoid reaction (Er) + (βTi) ↔ (αTi) at 890 °C and 99.7 at.% Ti. Beck[17] reported some miscibility gaps in the liquid phase by metallography. However, thermodynamic calculations by Murray[18] suggest that the miscibility gap is metastable in this system although the miscibility gap lies close to the liquidus. Wang[19] examined the splat-cooled Ti-Er alloys for evidence of metastable extension of the solid solubility of Ti in (Er) using transmission electron microscopy and found the maximum solubility was about 5 at.%, representing a possible slight (~ 1 at.%) extension from the equilibrium value. Based on the experimental information of the Ti-Er system, Murray[18] constructed an assessed Ti-Er phase diagram.
2.3 The Ti-Tm System
For the Ti-Tm system, an experimentally determined phase diagram is not available in the literature. A tentative Ti-Tm phase diagram has been reported by Kubaschewski-von Goldbeck[20] according to the general characteristic of the Ti-RE system. Similar with the Ti-Er system, there is no binary phase and liquid miscibility gap in the Ti-Tm system. The solubilities of Tm in (αTi) and (βTi) and Ti in (Tm) are limited. Two invariant reactions were reported by Kubaschewski-von Goldbeck,[20] i.e., the one eutectic reaction Liquid ↔ (Tm) + (βTi) at 1330 °C and 26.4 at.% Ti, and the other peritectoid reaction (Tm) + (βTi) ↔ (αTi) at 890 °C and 99.2 at.% Ti. The Ti-Tm phase diagram has been redrawn by Massalski.[21]
2.4 The Ti-Y System
The phase equilibria of the Ti-Y system have been investigated by several groups of researchers.[14,16,22,23,24] Love[16] determined invariant reactions temperatures by differential thermal analysis and estimated liquidus temperatures by optical pyrometry. However, the experimental results were scattered. The solid solubilities, invariant melting temperatures and liquidus compositions were measured by Bare and Carlson[22] using the microscopy, incipient melting technique and chemical analysis, respectively. By means of metallography, Lundin and Klodt[23] determined the reaction type of (βY) ↔ (αY) with the liquid phase to be the catatectic one and the solubilities of Y in (αTi) and (βTi) to be about 0.1 and 0.2 at.% at the eutectoid temperature 870 °C. Savitskii and Burkhanov[14] studied in detail the phase equilibria of the Ti-rich part of the Ti-Y system by means of thermal analysis and microscopy. Two invariant reactions Liquid#1 ↔ Liquid#2 + (βTi) and (βTi) ↔ (αTi) + (αY), located at 1400 and 890 °C, respectively, were reported and the phase diagram of Ti-20 wt.% Y was constructed by Savitskii and Burkhanov.[14] Beaudry[24] verified the catatectic reaction (βY) ↔ Liquid + (αY) at 1440 ± 5 °C and constructed the Y-rich part of the phase diagram up to 22 at.% Ti by thermal analysis. The Ti-Y system has been thermodynamic optimized by Gong et al.[25] based on the reported experimental data in the literature. However, the catatectic reaction (βY) ↔ Liquid + (αY) was not been considered, and the thermodynamic parameters of the Ti-Y system were not provided in the work of Gong et al.[25] Therefore, it is necessary to reassess the Ti-Y system based on the available experimental data.
3 Thermodynamic Modeling
3.1 Unary Phase
The Gibbs energy of the pure element i (i = Ti, Ce, Er, Tm, Y) in the phase φ is expressed by the following equation:
in which \({}^{\text{o}}G_{i}^{\varphi }\) is the Gibbs free energy of the pure element, H SER i is the molar enthalpy of the element i at 298.15 K and 1 bar in its standard element reference (SER) state, T is the absolute temperature, and the coefficients a ~ h are constant. In the present work, the Gibbs energies of Ti, Ce, Er, Tm and Y are taken from the SGTE compilation by Dinsdale.[26]
3.2 Solution Phase
The Gibbs energies of the solution phases liquid, bcc(βTi, δCe, βY), hcp(αTi, Er, Tm, αY) and fcc(γCe, γY) are described by the Redlich–Kister polynomial.[27] The molar Gibbs energy of the solution phase φ (φ = liquid, bcc(βTi, δCe, βY), hcp(αTi, Er, Tm, αY) or fcc(γCe, γY)) is expressed as follows:
where H SER is the abbreviation of x Ti · H SERTi + x i · H SER i , x Ti and x i are the mole fractions of Ti and component i (i = Ce, Er, Tm, Y), respectively. \(x_{\text{Ti}} \cdot {}^{\text{o}}G_{{{\text{T}}i}}^{\varphi } + x_{i} \cdot {}^{\text{o}}G_{i}^{\varphi }\) denotes the weighted sum of the Gibbs energies of the pure Ti and component i (i = Ce, Er, Tm, Y) in the state of φ phase. RT(x Ti ln x Ti + x i ln x i ) correspond to the contribution of the ideal entropy of mixing to the Gibbs energy. \(x_{Ti} \cdot x_{i} \cdot \sum\nolimits_{j = 0}^{nj} {L_{{{\text{Ti}},i}}^{\varphi } \left( {x_{\text{Ti}} - x_{i} } \right)^{j} }\) denotes the excess Gibbs energy. \(^{j} L_{{{\text{Ti}},i}}^{\varphi }\) is the jth interaction parameter of phase φ, and it is equal to j L φ = a j + b j · T. The coefficients a j and b j (j = 0, 1, 2) are to be optimized in the present work according to the experimental data.
4 Results and Discussion
The optimization of the thermodynamic parameters in the Ti-RE (RE = Ce, Er, Tm, Y) systems was performed with the PARROT module of the Thermo-calc software,[28] which works by minimizing the square sum of the differences between experimental and computed values. The step-by-step optimization procedure described by Du et al.[29] was utilized in the present assessment. In the optimization, each piece of experimental information was given a certain weight based on uncertainties of the data.
Taking the Ti-Ce system as an example, the optimization began with the invariant reaction Liquid#2 ↔ Liquid#1 + (βTi) at 1450 ± 25 °C. The parameters \({}^{0}L_{{}}^{{L{\text{iquid}}}}\), \({}^{1}L_{{}}^{{L{\text{iquid}}}},\) and \({}^{2}L_{{}}^{{L{\text{iquid}}}}\) in Eq 2 of the liquid phase were introduced in order to give an adequate description of the liquid phase due to the liquid miscibility gap. In addition, the parameters 0 L (βTi) and 1 L (βTi) in Eq 2 of the (βTi) phase were also included for reproducing the invariant reaction Liquid#2 ↔ Liquid#1 + (βTi). Then, similar to the invariant reaction Liquid#2 ↔ Liquid#1 + (βTi), the other invariant reactions Liquid#1 + (βTi) ↔ (αTi), Liquid#1 ↔ (δCe) + (αTi) and (δCe) ↔ (γCe) + (αTi) were introduced in the modeling one by one. Each time when the invariant reaction was calculated, the parameters of the involved phases in the reaction were optimized. Finally, all the parameters were simultaneously optimized by taking into account all of the phase diagram experimental data. The procedures of optimization for the Ti-RE (RE = Er, Tm and Y) systems were analogous with that for the Ti-Ce system.
The optimized thermodynamic parameters for the Ti-RE (RE = Ce, Er, Tm and Y) systems are listed in Table 1, and the calculated compositions and temperatures of the invariant reactions for the Ti-RE (RE = Ce, Er, Tm and Y) systems along with the literature data are listed in Table 2. It can be seen that the calculated temperatures and compositions of invariant reactions agree well with the literature data.
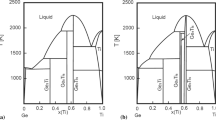
Figure 1 is the calculated Ti-Ce phase diagram. Figure 1(a) is the one with the whole composition and Fig. 1(b) is the one at the Ti-rich part along with the experimental data from the work of Savitskii and Burkhanov.[14] As can be seen from Fig. 1 and Table 2, the experimental data are reproduced well by the present calculation. The calculated liquid miscibility gap is 51 at.% Ti at 1589 °C. The monotectic reaction Liquid#2 ↔ Liquid#1 + (βTi) experimentally observed is well reproduced by this work. The temperatures of peritectic reaction Liquid#1 + (βTi) ↔ (αTi) and eutectic reaction Liquid#1 ↔ (δCe) + (αTi) are calculated to be 906 and 787 °C, which are consistent with the experimental ones 910 ± 10 and 790 ± 20 °C, respectively. The calculated temperature of the eutectoid reaction (δCe) ↔ (γCe) + (αTi) is to be 718 °C, which also agree well with the experimental one 710 ± 20 °C.
Calculated phase diagram of the Ti-Ce system according to the present work: (a) the whole composition, and (b) the Ti-rich side along with the experimental data[14]
Figure 2 shows the calculated phase diagram of the Ti-Er system. As can be seen from Fig. 2 and Table 2, the calculated eutectic point of reaction Liquid ↔ (Er) + (βTi) lies at 1314 °C and 29.0 at.% Ti and the calculated peritectoid point of reaction (Er) + (βTi) ↔ (αTi) is located at 888 °C and 0.4 at.% Ti, which agree well with the literature's reported ones.[16] However, the curvature of the liquidus on the Ti-rich side is a little strange. Due to the limited experimental data from the literature, the invariant reaction Liquid ↔ (Er) + (βTi) at 1320 ± 20 °C and 26 at.% Ti reported by Love[16] is considered to be reliable in this work. During the optimization, the parameters of the liquid, (Er) and (βTi) phases are used in order to reproduce the temperature and composition of this invariant reaction. Based on the good agreement with this piece of experimental datum,[16] the miscibility gap of the liquid phase appears on the Ti-rich side, or a little strange curvature of the liquidus always arises whatever parameters are used. However, thermodynamic calculations by Murrry[18] suggest that the miscibility gap of the liquid phase is metastable in this system. Thus, the result with a little strange curvature of the liquidus is accepted in the present work. The calculated results are consistent with the assessed phase diagram of the Ti-Er system by Murrry.[18] For the strange curvature of the liquidus, more experiments are necessary to determine it in our future work.
The calculated Ti-Tm phase diagram is shown in Fig. 3. It can be seen from the Fig. 3 and Table 2, the calculated eutectic reaction Liquid ↔ (Tm) + (βTi) at 1332 °C and 26.4 at.% Ti and the peritectoid reaction (Tm) + (βTi) ↔ (αTi) at 887 °C and 0.5 at.% Ti agree well with the predicted ones 1330 °C and 26.4 at.% Ti, and 890 °C and 1.0 at.% Ti, respectively. The curvature of the liquidus in the Ti-rich side of the Ti-Tm system is also a little strange. The characteristic of the Ti-Tm system is similar to the Ti-Er system.
Figure 4 presents the calculated Ti-Y phase diagram according to the present work, in which Fig. 4(a) is the whole composition, Fig. 4(b) is the Ti-rich side in composition range of 86-100 at.% Ti along with the experimental data,[14] and Fig. 4(c) is the Y-rich side in the composition range of 0-25 at.% Ti along with the experimental data.[22,24] As can be seen from this figure, most of the experimental data are well accounted for by the present modeling. The calculated liquid miscibility gap exists at 1502 °C and 41 at.% Ti.
5 Conclusions
All the experimental phase diagram data in the literature for the Ti-RE (RE = Ce, Er, Tm, Y) binary systems were critically evaluated. A set of self-consistent thermodynamic parameters is obtained for the Ti-RE (RE = Ce, Er, Tm, Y) systems by using the CALPHAD method. The calculated phase diagrams and thermodynamic properties agree well with the experimental data in the literature.
References
B. Hu, J. Wang, C. Wang, Y. Du, and J.B. Zhu, CALPHAD-Type Thermodynamic Assessment of the Ti-Mo-Cr-V Quaternary System, CALPHAD, 2016, 55, p 103-112
W. Xiong, Y. Du, X.G. Lu, J.C. Schuster, and H.L. Chen, Reassessment of the Ce-Ni Binary System Supported by Key Experiments and ab Initio Calculations, Intermetallics, 2007, 15(11), p 1401-1408
Y.F. Yang, S.D. Luo, G.B. Schaffer, and M. Qian, Impurity Scavenging, Microstructural Refinement and Mechanical Properties of Powder Metallurgy Titanium and Titanium Alloys by a Small Addition of Cerium Silicide, Mater. Sci. Eng. A, 2013, 573(20), p 166-174
D. Goll and H. Kronmüller, High-Performance Permanent Magnets, Naturwiss, 2000, 87(10), p 423-438
N. Mattern, J.H. Han, O. Fabrichnaya, M. Zinkevich, W. Löser, J. Werner, R. Nowak, I. Kaban, O. Shuleshova, D. Holland-Moritz, J. Bednarĉik, N. Sobczak, and J. Eckert, Experimental and Thermodynamic Assessment of the Gd-Ti System, CALPHAD, 2013, 42, p 19-26
N. Mattern, Y. Yokoyama, A. Mizuno, J.H. Han, O. Fabrichnaya, T. Harada, S. Kohara, and J. Eckert, Experimental and Thermodynamic Assessment of the Nd-Ti System, CALPHAD, 2014, 47, p 136-143
N. Mattern, Y. Yokoyama, A. Mizuno, J.H. Han, O. Fabrichnaya, M. Richter, and S. Kohara, Experimental and Thermodynamic Assessment of the La-Ti and La-Zr Systems, CALPHAD, 2016, 52, p 8-20
B. Hu, X.M. Yuan, Y. Du, J. Wang, and Z.-K. Liu, Thermodynamic Reassessment of the Ni-Si-Ti System Using a Four-Sublattice Model for Ordered/Disordered fcc Phases Supported by First-Principles Calculations, J. Alloys Compd., 2017, 693, p 344-356
B. Hu, B. Yao, J. Wang, J.-R. Zhao, F.-F. Min, and Y. Du, Thermodynamic Assessment of the Al-Mo-V Ternary System, J. Min. Metall. Sect. B Metall., 2017, 53(3), p 95-106
H.L. Lukas, S.G. Fries, and B. Sundman, Computational Thermodynamics: The CALPHAD Method, Cambridge University Press, Cambridge, 2007
B. Hu, Y. Du, J.-J. Yuan, Z.-F. Liu, and Q.-P. Wang, Thermodynamic Reassessment of the Mn-Ni-Si System, J. Min. Metall. Sect. B Metall., 2015, 51(2), p 125-132
J.L. Taylor, Preliminary Investigation of the Ti-Ce System, Trans. AIME, 1957, 209, p 94-96
E.M. Savitskii and G.S. Burkhanov, Diagramy Sostoyaniya Splavov Titan-Lantan I, Titan-Tserii, Zh. Neorg. Khim., 1957, 2(11), p 2609-2616
E.M. Savitskii and G.S. Burkhanov, Phasen-Diagramme Von Titan-Legierungen Mit Seltenen Erdmetallen, Less-Common Met., 1962, 4(4), p 301-314
J.L. Murray, Binary Alloy Phase Diagrams, T.B. Massalski, Ed., American Society for Metals, Metals Park, 1986,
B. Love, WADD Technical Report 60-74, Part I (1960)
R. Beck, U.S. Atomic Energy Commission LAR-10, 93 (1960)
J.L. Murray, Binary Alloy Phase Diagrams, Vol 1, 2nd ed., T.B. Massalski, Ed., Materials Information Society, Materials Park, 1990,
R. Wang, Solubility and Stability of Liquid-Quenched Metastable hcp Solid Solutions, Mater. Sci. Eng., 1976, 7(3), p 135-140
O. Kubaschewski-von Goldbeck, Titanium: Physico-Chemical Properties of Its Compounds and Alloys. Special Issue No. 9, Atomic Energy Review, K.L. Komarek, Ed., IAEA, Vienna, 1983,
T.B. Massalski, Binary Alloy Phase Diagrams, Vol 3, 2nd ed., T.B. Massalski, Ed., Materials Information Society, Materials Park, 1990,
D.W. Bare and O.N. Carlson, Phase Equilibria and Properties of Yttrium-Titanium Alloys, Trans. ASM, 1961, 53, p 1-11
C.E. Lundin and D.T. Klodt, Phase Equilibria of the Group IVA Metals with Yttrium, Trans. Met. Soc. AIME, 1962, 224, p 367-372
B.J. Beaudry, The Effect of Titanium on the Melting Point and Transition Temperature of Yttrium, J. Less-Common Met., 1968, 14(3), p 370-372
W.P. Gong, T.F. Chen, D.J. Li, and Y. Liu, Thermodynamic Investigation of Fe-Ti-Y Ternary System, Trans. Nonferrous Met. Soc. China, 2009, 19(1), p 199-204
A.T. Dinsdale, SGTE Data for Pure Elements, CALPHAD, 1991, 15(4), p 317-425
O. Redlich and A.T. Kister, Algebraic Representation of Thermodynamic Properties and the Classification of Solutions, Ind. Eng. Chem., 1948, 40(2), p 345-348
B. Sundman, B. Jansson, and J.-O. Andersson, The Thermo-Calc Databank System, CALPHAD, 1985, 9(2), p 153-190
Y. Du, R. Schmid-Fetzer, and H. Ohtani, Thermodynamic Assessment of the V-N System, Z. Metallkd., 1997, 88(7), p 545-556
Acknowledgments
The financial support from the National Natural Science Foundation of China (Nos. 51501002 and 51601228), the China and Anhui Province Postdoctoral Science Foundation (Nos. 2015M581972 and 2017B210), and the Graduate Innovation Foundation of Anhui University of Science and Technology (No. 2017CX2102) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, B., Hu, B., Zhang, A. et al. Thermodynamic Assessment of the Ti-RE (RE = Ce, Er, Tm, Y) Binary Systems. J. Phase Equilib. Diffus. 39, 44–50 (2018). https://doi.org/10.1007/s11669-017-0605-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-017-0605-0