Abstract
We have determined the thermodynamic activity of Pb and Sb in the Pb-Sb binary system. For the experiments, we used a galvanic cell in which the measuring electrode was made of Pb-Sb alloys with different chemical compositions. The reference electrode was manufactured from pure lead, and a mixture of salts (35% KCl, 17% NaCl and 48% PbCl2) was used as an electrolyte, which had a melting temperature of 399 °C (672 K). Tungsten wires were used for the contact conductors of the electrodes, and platinum wires were used for the electrical contacts between the measurement instrument and the contact conductors. The variations of activity coefficients depending on its atomic fraction were calculated for the Pb and Sb in the Pb-Sb binary alloy system. The experimental measurements were performed at a temperature of 650 °C (923 K). The obtained data are reliable and consistent, and now, based on these, thermodynamic functions can be obtained that can help the scientific community to correctly describe the behavior of the Pb-Sb binary alloy system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Thermodynamic information concerning the components of a system is of great importance in the development, design, planning and operation processes in the chemical industry and metallurgy.[1,2] The importance of knowing the values of thermodynamic measurements (Gibbs free energy, enthalpy, entropy) of system components and their variation to the temperature is justified by the significant number of scientific papers which have appeared over time in the specialized literature.[3,4,5,6,7,8,9,10,11] In recent works,[8,9,12,13,14] the study of various binary alloy systems have had a different form of presentation and a superior accuracy compared to previous publications (period 1960–1970).
Given the importance of knowing the thermodynamic measurement values for the system components[15,16] and their variation with temperature, in the present work we aim to achieve a broader and more precise thermodynamic study of Pb-Sb binary alloy systems. The Pb-Sb system has great importance in different domains such as the electrical industry or the automotive industry (as anti-friction alloys), or in applications such as soldering alloys, fusible alloys, cable sheathing alloys, and typography alloys.
After analyzing the main experimental methods for determining the thermodynamic activities of the elements in metallic smelt, we have focused on the measurements of electromotive voltage for defining the thermodynamic activities in Pb-Sb binary alloys. Therefore, in this article, we present our experimental research regarding the thermodynamic activities in the Pb-Sb binary system by analyzing the results of measurements of the electromotive voltage which we obtained using a galvanic cell. The choice of this method was made due to its high precision and good reproducibility as proven in other research concerning the thermodynamics of metallurgical melts.
The element of originality and novelty of the paper consists in the working temperature used for the experimental measurements of the thermodynamic activities which were performed at 650 °C (923 K) for the Pb-Sb binary system and which are not mentioned in the specialized literature. Based on these measurements, the calculations of the most important thermodynamic measurements of this system (partial or integral molar properties) were effected for the first time.[17,18,19]
2 Materials and Methods
The method used in our experimental research was based on the measurements of the electromotive voltage in a galvanic cell which has a reversible concentration with a liquid or solid electrolyte. In order to determine thermodynamic activities of the Pb and Sb from Pb-Sb binary system we used galvanic cells in which the measuring electrode was prepared from the Pb-Sb alloy (we used Sb and Pb of 99.99% purity) with different chemical compositions. The reference electrode was prepared from pure lead, and the electrolyte used was a mixture of salts from the system KCl-NaCl-PbCl2 whose chemical composition, in mole percent, was 35% KCl, 17% NaCl and 48% PbCl2. The melting temperature of the electrolyte was 399 °C (672 K).
The electrolyte was prepared from pure chlorides of potassium and lead which were previously dried in an oven at 70 °C for about 2 h. Successive weighings of the electrolyte were made and introduced into the oven, and the drying process ended when the measured value of each weighed electrolyte was constant. The contact conductors for the electrodes were made of tungsten wires, a metal which is insoluble in lead or in the Pb-Sb binary alloy, and platinum wires were used for the electrical contacts between the two tungsten wires and the instrument measuring the electromotive voltage.
The galvanic cell was built of quartz which is not attacked by the Pb-Sb alloy, the pure lead or the mixture of electrolytic salts. Since the tungsten wires penetrate into the cell from the top through the electrolyte layer, quartz sheaths prevent contact with it. The measurement cell was placed in the reaction chamber of an electric oven with thermal resistances made of Kanthal and provided with an automated system for adjusting and maintaining a constant temperature. The temperature inside the cell was measured by placing a thermocouple of chromel–alumel (with a ceramic sheath) directly into the electrolyte.
After the introduction in the solid state of the electrolytic components (pure lead, the Pb-Sb alloy, and the electrolyte) into the cell compartments, they were heated slowly to the preset temperature of 650 °C (923 K) and the temperature was kept constant for the whole duration of the experiments. After reaching the working temperature were introduced the tungsten wires and, after stabilization of the temperature, were coupled to the electronic millivoltmeter for measuring the electromotive voltage of the cell.
The binary Pb-Sb alloy system is a well-known and well-researched system. Noticeable research on the Pb-Sb binary alloy system has been made by many researchers at different temperatures ranges and different atomic fractions of elements,[20,21,22,23,24] but none of these studies have used the temperature of 650 °C (923 K) at which we performed the measurements in our research. In recent research[25] on the Pb-Sb binary system the enthalpy of the mixture was measured at the temperature of 700 °C (973 K) with an atomic fraction of Sb ranging from 0.2 to 0.6 using direct liquid–liquid reaction calorimetry (DLLRC).
Thermodynamic activities measurements at the temperature of 650 °C (923 K) were performed for the first time in this experimental research. For a thorough check, we compared the results obtained by us with the results obtained[20] at the temperature of 627 °C (900 K). Unfortunately, measurements of the electromotive voltage were not conducted in that research.
2.1 Principle of the Method
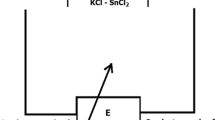
The electrochemical systems, in which the electrolytes differ only by the (concentration) of elements, are called galvanic concentration cells. The source of the energy in this type of cell is represented by the transfer energy from the substance with the higher thermodynamic activity to the substance with lower activity. The electrochemical chain of a galvanic concentration cell can be illustrated as shown in Fig. 1:
The transfer of an atoms-gram of metal Me from the reference electrode to the measuring electrode determines a variation of the free energy of the system (partial molar free energy of the mixture) between the two electrodes that are in equilibrium, at a constant temperature or pressure, is equal to the electric activity, as per the formula:
where \(\Delta \overline{G}\) is the variation of the partial molar free energy of mixture in the measuring electrode at the transfer between the two electrodes of one atoms-gram of the element Me with the charge of the z ion, in the j/atom-gram; F is the Faraday constant (96485.34 C/atom-g); and E is the electromotive equilibrium intensity of the cell, in V.
The partial molar propriety of a substance in a mixture (\(\Delta \overline{Y}_{i}\)), represents the difference between the extensive partial molar property adequate for the substance in the solution (\(\overline{Y}_{i}\)) and the extensive partial molar property of the pure substance (\(Y_{i}^{0}\)). As a result, for the analyzed cell, the variation of the partial molar free energy of the mixture (\(\Delta \overline{G}\)) represents the difference between the partial molar free energy of the Me metal from the measuring electrode (\(\overline{G}_{\text{Me}}\)) and, respectively, the partial molar free energy of the pure metal from the reference electrode (\(G_{\text{Me}}^{0}\)):
The partial molar free energy of the pure metal corresponds to the following transformation:
And it is given by the relationship:\(G_{\text{Me}}^{0} = RT\ln p_{\text{Me}}^{0}\), where \(p_{\text{Me}}^{0}\) is the vapor pressure of the metal Me in the pure state. The partial molar free energy of the Me metal in the Me–Me solution corresponds to the following transformation:
And is given by the relationship: \(\overline{G}_{\text{Me}} = RT\ln p_{\text{Me}}\), where m and n represent the number of moles of the metals Me and Me′ in the solution Me–Me′, \(p_{\text{Me}}\) is the vapor intensity of the metal Me in the solution Me–Me′.
Replacing the expression of the free energy from the relationship 2 and taking into account the definition of thermodynamic activity as being the ratio of the vapor pressure of an element in the solution and the vapor pressure in the pure state, (\(a_{\text{Me}} = p_{\text{Me}} /p_{\text{Me}}^{0}\)) and thus results in:
From the formula 1, we obtain:
or
Based on the relationship 3, through the measurement of the equilibrium electromotive voltage of a type (I) galvanic cell, the thermodynamic pressure of the metal (\(a_{\text{Me}}\)) can be determined in the alloy Me–Me′.
Regarding the binary Me-Me′ system, if the activity of the metal Me depending on the concentration is known, the activity of the other element (\(a_{{{\text{Me}}'}}\)) at a constant temperature can be determined using the Gibbs-Duhem relationship:
From the relationships:
where \(\gamma_{\text{Me}}\) and \(\gamma_{{M{\text{e}}'}}\) are the activity coefficients of the two elements, resulting in:
Also, considering that \(x_{\text{Me}} + x_{{{\text{Me}}'}} = 1\) and \({\text{d}}x_{\text{Me}} = - {\text{d}}x_{{{\text{Me}}'}}\), result in the relationships:
Based on these mathematical relationships, the thermodynamic activity or the activity coefficient of the Me metal is determined by varying the activity coefficient of the Me metal with its atomic fraction.
2.2 Experimental Method
To determine the thermodynamic activities of the Pb and Sb in the Pb-Sb binary system, we have used a galvanic concentration cell of type (II) (Fig. 2) in which the measuring electrode was prepared from Pb-Sb alloys with different chemical compositions, the reference electrode was prepared from pure lead, and as an electrolyte, we have used a mixed of salts from the system KCl-NaCl-PbCl2 as we previously described in Section 2.
The scheme of the experimental appliance used in this paper is shown in Fig. 3.
The measurements of temperature in the galvanic cell were carried out through inserting the thermocouple directly into the electrolyte. For the measurements of electromotive voltage, we used a Hewlett-Packard electron multimeter having impedance measurements with a coverage range between 1 V and 1010 Ω.
3 Results and Discussion
The research was realized in the concentration area of \({\chi}_{\text{Sb}} \in \left( {0.1} \right)\). For all alloys, two measurements have been made and, using the average values, we have defined the thermodynamic activity of the lead with relationship 4, which in the case of the Pb-Sb system at the temperature of 650 °C (923 K), becomes as follows:
The results of the experimental measurements of the electromotive voltage and the activity coefficient at the temperature of 650 °C (923 K) are shown in Tables 1 and 2.
The variation of the activity coefficients of the Pb depending on the molar fraction in comparison with the Raoult line are shown in Fig. 4.
To obtain the maximum activity coefficient of the Pb \(\gamma_{\text{Pb}}^{0}\) in the Pb-Sb alloy, defined as the limit of the rapport \(\frac{{a_{\text{Pb}} }}{{x_{\text{Pb}} }}\) when \(x_{\text{Pb}} \to 0\).1 (Fig. 5) we represent the variation of the lead activity coefficient depending on its atomic fraction and we extrapolate until the source of the \(x_{\text{Pb}} = 0\), which results in the value \(\gamma_{\text{Pb}}^{0} = 0.881\).
The thermodynamic activity of Sb and the activity coefficient depending on its molar fraction were determined through the analytical method using the Gibbs–Duhem relationship (Eq 5-7), which in the case of the Pb-Sb system at the temperature of 923 K, becomes as follows:
Relationships 10 and 11 have been resolved by using the MathCAD software, and the obtained results, regarding the thermodynamic activity and the activity coefficient, are set out in Table 3.
The variation of the activity coefficients of Sb depending on its molar fraction in comparison with the Raoult line are shown in Fig. 6.
The variations of the activity coefficients depending on its molar fraction present a negative deviation from the Raoult line, both for Pb (Fig. 4) and for Sb (Fig. 6). The experimental results and also those estimated from the activity coefficients of Pb and Sb are under the Raoult line (negative deviation from the Raoult law), but close to the unit considering the concentration area, and this indicates a closeness to the ideal solutions.
Because we have not found any results for the Pb-Sb system at the temperature of 650 °C (923 K) in the scientific literature, we have compared our experimental results of measurements of the electromotive voltage with the results of other researchers[20] acquired at the temperature of 627 °C (900 K). The comparative results of the thermodynamic activity and of the activity coefficients for Pb and Sb in the Pb-Sb binary alloy are shown in Table 4 and Fig. 7 and 8.
According to Fig. 7 and 8, it can be observed that our experimental results are in good agreement with the scientific results. In these circumstances, the results can be considered as a reference point for further mathematical adaptation and modeling in order to obtain thermodynamic functions which can correctly describe the thermodynamic activity of the Pb-Sb binary alloy system.
4 Conclusions
The thermodynamic activities and also the activity coefficients of the Pb and Sb in the Pb-Sb binary alloys systems were calculated by measuring the electromotive voltage at 923 K, highlighting the fact that, because experimental results obtained at this temperature for the Pb-Sb binary system cannot befound in the specialized literature, this is a novelty in this field. The variation of the activity coefficients depending on its molar fraction presents a negative deviation from the Raoult line both for Pb and for Sb, which indicates the closeness to the ideal solutions.
The results obtained through the method of measuring the electromotive voltage at the temperature of 923 K were tabulated and compared to those obtained at the temperature of 900 K by others researchers. The experimental results are in good agreement with the scientific results and represent a benchmark for further research on this system of alloys. Using an appropriate thermodynamic model, the thermodynamic functions that can help in the correct description of the thermodynamic behavior of the Pb-Sb binary alloy systems can be obtained.
References
H.L. Lukas, S.G. Fries, and B. Sundman, Computational Thermodynamics: The Calphad Method, Cambridge University Press, Cambridge, 2007
T.B. Massalski, H. Okamoto, P.R. Subramanian, and L. Kacprzak, Ed., Binary Alloy Phase Diagrams, ASM International, Metals Park, 1990
R. Hultgren, P. Desai, D. Hawkins, M. Gleiser, K. Kelley, and D. Wagman, Selected Values of the Thermodynamic Properties of the Elements, American Society for Metals (ASM), Metals Park, 1973
R. Hultgren, P.D. Desai, D.T. Hawkins, M. Gleiser, and K.K. Kelly, Selected Values of Thermodynamic Properties of Binary Alloys, American Society for Metals (ASM), Metals Park, 1973
O. Kubaschewski and E.L. Evans, Metallurgical Thermochemistry, 3rd ed., Pergamon, Oxford, 1958
O. Kubaschewski and C.B. Alcock, Metallurgical Thermochemistry, 5th ed., Pergamon, Oxford, 1979
M. Kaufman, Principles of Thermodynamics, CRC , Boca Raton, 2002
I. Barin, Thermochemical Data of Pure Substances, 3rd ed., VCH, Weinheim, 1989
I. Barin, Thermochemical Data of Pure Substances, 2nd ed., VCH, Weinheim, 1993
H. Okamoto, M.E. Schlesinger, E.M. Mueller (eds.), ASM Handbook Volume 3—Alloy Phase Diagrams (American Society for Metals (ASM), Metals Park, 1990)
P.B. Landolt-Börnstein, Group IV: Physical Chemistry, vol. 5: Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys, Subvol. H (Springer, Heidelberg, 1998), pp 143–144.
J. Wang, H.S. Liu, L.P. Liu, and Z.P. Jin, Interfacial Reaction Between Sn-Ag Alloys and Ni Substrate, J. Alloys Compd., 2008, 455, p 159-163. doi:10.1016/j.jallcom.2007.01.024
Y. Liu, L. Zhang, and D. Yu, Diffusion Mobilities in fcc Cu-Au and fcc Cu-Pt Alloys, J. Phase Equilib. Diffus., 2009, 30, p 136. doi:10.1007/s11669-009-9469-2
V.L. Stolyarova, S.I. Lopatin, S.M. Shugurov, and A.L. Shilov, Thermodynamic Properties of the System MgO-B2O3 Melts, Russ. J. Gen. Chem., 2010, 80, p 689-694. doi:10.1134/S1070363210040018
W. Gierlotka, C. Lee, P. Chumpanaya, M.A. Rahman, and T.N. Ko, Thermodynamic Re-Optimization of the Binary Pb-Sb System, J. Phase Equilib. Differ., 2013, 34, p 421-424. doi:10.1007/s11669-013-0252-z
P.A. Arkhipov, S.I. Kumkov, YuR Khalimullina, and A.S. Kholkina, Estimation of the Activity of Lead in the Binary Pb-Sb and Pb-Bi Systems, Russ. Metall., 2013, 2, p 115-122. doi:10.1134/S0036029513020043
F. Niculescu, D.F. Marcu, I. Constantin, and I.D.S. Heica, Thermodynamic Measures Calculation for Pb-Sb Binary Alloy System Using Redlich-Kister Theoretical Model, U.P.B. Sci. Bull Ser. B, 2016, 78, p 239-245
F. Niculescu, I. Constantin, M. Buzatu, D.F. Marcu, and I. Csaki, The Thermodynamic Properties of the Pb-Sb System, U.P.B. Sci. Bull Ser. B, 2016, 78, p 215-224
F. Pereteanu, D. Taloi, and I. Constantin, Thermodynamic Study of Binary Pb-Sb System Using the Poss Model, U.P.B. Sci. Bull Ser. B, 2010, 72, p 167-174
H. Seltz and B.J. Dewitt, A Thermodynamic Study of the Lead-Antimony System, J. Am. Chem. Soc., 1939, 61, p 2594-2597
J.F. Elliott and J. Chipman, The Thermodynamic Properties of Liquid Ternary Cadmium Solutions, J. Am. Chem. Soc., 1951, 73, p 2682-2693
V.N. Eremenko and O.M. Eremenko, Ukr. Khim. Zh., 1952, 18, p 232-238
L.W. Diller, R.L. Orr, and R. Hultgren, Thermodynamics of the Lead—Antimony System, J. Phys. Chem., 1960, 64, p 1736-1738. doi:10.1021/j100840a032
E. Schurmann and H. Trager, Die Empfindlichkeit und die Wiederholbarkeit von Messungen mit dem Kleinkalorimeter (The Sensitivity and Reproducibility of Measurements with Microcalorimeter), Arch. Eisen \(\ddot{h}\) uttenwes., 1961, 32, p 397-498
S. Hassam, D. Boya, Y. Fouque, K.P. Kotchi, and J. Rogez, Thermodynamic Investigation of the Pb-Sb System, J. Alloys Compd., 2009, 479, p 74-78
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niculescu, F., Marcu, D., Burciu, Ş. et al. Experimental Researches Regarding the Thermodynamic Activities in the Binary Pb-Sb Alloy System. J. Phase Equilib. Diffus. 38, 700–706 (2017). https://doi.org/10.1007/s11669-017-0555-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-017-0555-6