Abstract
The isothermal section of the Mo-Ni-Zr system at 900 °C was investigated by characterization of eighteen equilibrium alloys. Electron probe microanalysis (EPMA) and x-ray diffraction (XRD) were used to identify the phases and obtain their compositions. The existence of two ternary compounds, Zr65Mo18−x Ni16.5+x (τ1, cF96-Ti2Ni) and Zr65Mo27.3Ni7.7 (τ2, hP28-Hf9Mo4B), was confirmed in the Zr-rich corner, and the compositions of the two phases were determined. The isothermal section of the Mo-Ni-Zr system at 900 °C consists of 15 three-phase regions and 29 two-phase regions. The following three-phase equilibria were well established: (1) (Ni) + Ni7Zr2 + Ni5Zr, (2) MoNi + MoNi3 + Ni7Zr2, (3) Ni7Zr2 + MoNi + (Mo), (4) (Mo) + Ni7Zr2 + Ni3Zr, (5) (Mo) + Ni3Zr + Ni21Zr8, (6) (Mo) + Ni21Zr8 + Ni10Zr7, (7) (Mo) + Ni10Zr7 + NiZr, (8) (Mo) + Mo2Zr + NiZr, (9) NiZr2 + Mo2Zr + τ1, (10) τ1 + Mo2Zr + τ2, (11) τ2 + Mo2Zr + (Zr)ht, (12) NiZr2 + τ1 + (Zr)ht and (13) τ1 + τ2 + (Zr)ht. Several binary phases, such as MoNi3, Ni7Zr2 and Mo2Zr, dissolve appreciable amount of the third component.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ti(C,N)-based cermets have been widely used as cutting tool materials owing to their excellent wear resistance, high-temperature hardness, well chemical stability, low friction coefficient to metals, and superior thermal deformation resistance.[1,2] The addition of Mo or Mo2C into cermets can improve the wettability between ceramic phase and binder phase, and the cermets with finer microstructure and better mechanical properties can be obtained.[3] The addition of ZrC into cermets can improve its toughness and thermal shock resistance,[4,5] while Ni is an important binder metal for Ti(C,N)-based cermets. In addition, the Ni-Zr binary system has been widely investigated as an important metallic glass system. Recently, Yang et al.[6] found that the appropriate addition of Mo can stabilize an ordered structure of metallic glass with a higher atomic packing density and lower energy state, and it can also improve the glass formation ability of Mo-Ni-Zr alloys.
In this paper, to facilitate reading, the symbols to denote the stable phases in the Mo-Ni-Zr ternary system are summarized in Table 1.[7–9]
The binary phase diagrams used in the present work are briefly described as follows. The Ni-Zr binary system was recently assessed by Tokunaga et al.[10] and it shows the existence of eight intermediate phases: Ni5Zr, Ni7Zr2, Ni3Zr, Ni21Zr8, Ni10Zr7, Ni11Zr9, NiZr and NiZr2. Except for Ni3Zr phase, which is formed through a peritectoid reaction Ni7Zr2 + Ni21Zr8 ↔ Ni3Zr, the formation of the other phases is associated with the liquid phase. The Ni7Zr2, NiZr and NiZr2 phases melt congruently, while the Ni5Zr, Ni21Zr8, Ni11Zr9 and Ni10Zr7 phases melt peritectically. The Ni-Zr phase diagram assessed by Tokunaga et al.[10] is shown in Fig. 1(a). The Mo-Ni binary system assessed by Santhy et al.[11] shows the presence of three intermetallic compounds: MoNi, MoNi3 and MoNi4. The MoNi phase is formed through a peritectic reaction L + (Mo) ↔ MoNi and the other two phases are formed through peritectoid reactions MoNi + (Ni) ↔ MoNi3 and MoNi3 + (Ni) ↔ MoNi4. The eutectic reaction L ↔ MoNi + (Ni) occurs in the Ni-rich part of this binary system. The Mo-Ni phase diagram assessed by Santhy et al.[11] is shown in Fig. 1(b). The stable compound in the Mo-Zr system is Mo2Zr, which melts peritectically.[12] The Mo-Zr phase diagram assessed by Jerlerud et al.[12] is shown in Fig. 1(c).
The Mo-Ni-Zr system was previously reviewed by Gupta[13] and the phase equilibria determinations have been carried out mainly by Virkar et al.[14] and Prima et al.[15–17] Their investigations[14–17] on the Mo-Ni-Zr system were performed in the entire composition region at 900 °C. Nevertheless, there are several contradictions among their work: (1) Virkar et al.[14] reported the presence of three ternary intermediate phases, Zr4MoNi, Zr9Mo4Ni and Zr33.3Mo3Ni63.7. However, only the Zr4MoNi ternary phase was found by Prima et al.[15–17] Thus the existence of the ternary phases Zr9Mo4Ni and Zr33.3Mo3Ni63.7 was questionable and required further confirmation; (2) in the Ni-rich corner, the MoNi3 phase was found to be in equilibrium with the Ni5Zr and Ni7Zr2 by Virkar et al.,[14] while the equilibrium suggested by Prima et al.[15–17] was (Ni), Ni5Zr and Ni7Zr2. Thus, the phase relationship among (Ni), MoNi3, Ni5Zr and Ni7Zr2 was controversial; (3) the homogeneous range of the (Mo) phase was different among their work.[14–17] A large extension of the (Mo) phase region was suggested by Virkar et al.[14] This feature seems to be incorrect and needs further investigation; and (4) the binary phase Ni3Zr, which was stable in the assessed binary system,[10] was not found in their work at 900 °C.[14–17]
To clarify the above contradictions on the phase equilibria in the Mo-Ni-Zr system and provide new phase diagram data for a future thermodynamic modeling, the present work is intended to investigate the phase equilibria of the Mo-Ni-Zr system at 900 °C experimentally.
Experimental Procedure
The pure Mo rods (99.99 wt.%), Ni granules (99.99 wt.%) and Zr rods (99.99 wt.%) were used as starting materials. Eighteen Mo-Ni-Zr samples covering the whole composition range were prepared. The alloy samples with the mass of about 2 g were prepared by arc-melting in an arc furnace under high purity argon atmosphere (WKDHL-1, Opto-electronics Co, Ltd., Beijing, China). The large difference of melting temperature among Mo, Zr and Ni makes it difficult to prepare homogeneous alloys by direct melting these three components. Thus, Mo and Zr pure metals were melted firstly to prepare master alloys and then melted with Ni. Each alloy was remelted at least four times to improve their homogeneity. The weight loss after melting was less than 0.5 wt.%, indicating that the real compositions of alloys are close to their nominal compositions. The prepared samples were encapsulated in an evacuated silica capsules under vacuum and then annealed at 900 °C in a high-precision diffusion furnace (L4514, Qingdao Institute & Equipment Co. Ltd., China) for 40 days followed by quenching in cold water.
Phase identification was performed by x-ray diffraction (XRD, Bruker D8 Advance, Germany) using a monochromatic Cu Kα (λ = 1.54056 Å) with a LYNXEYE_XE detector at 40 kV and 40 mA. High purity Si (99.999 wt.%) powder was used as an internal standard for the calculations of lattice parameters. Most of the prepared Mo-Ni-Zr alloys are ductile, so the samples had to be filed into fine powders and re-annealed at the previous heat-treatment temperature for 12 h in order to remove the stress of powders and obtain sharp diffraction patterns. Lattice parameters for the identified phases were calculated by using Jade software.[18] The metallographic alloy samples were examined by optical microscopy (Leica DMLP, Germany) and then analyzed by electron probe microanalysis (EPMA, JXA-8230, JEOL, Japan) for microstructure observation and phase composition measurement.
Results and Discussion
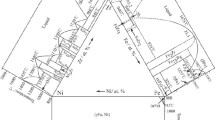
The nominal compositions of the prepared ternary alloys are listed in Table 2, in which the identified phases, calculated lattice parameters and phase compositions are also presented. The lattice parameters cannot be obtained for a few Ni-Zr binary phases due to their small quantity and poor quality of diffraction patterns. The typical Back-scattering electron (BSE) images of Mo-Ni-Zr samples are shown in Fig. 2(a)-(f), while the selected XRD patterns for Mo-Ni-Zr alloys are shown in Fig. 3(a)-(f). Based on the present experimental work, the isothermal section of the Mo-Ni-Zr system at 900 °C is constructed and presented in Fig. 4, together with the nominal compositions of the prepared samples.
According to the XRD and EPMA results, samples 1, 3-9 and 11-15 are located in three-phase regions. Thirteen three-phase regions were observed in the present work: (1) (Ni) + Ni7Zr2 + Ni5Zr, (2) MoNi + MoNi3 + Ni7Zr2, (3) Ni7Zr2 + MoNi + (Mo), (4) (Mo) + Ni7Zr2 + Ni3Zr, (5) (Mo) + Ni3Zr + Ni21Zr8, (6) (Mo) + Ni21Zr8 + Ni10Zr7, (7) (Mo) + Ni10Zr7 + NiZr, (8) (Mo) + Mo2Zr + NiZr, (9) NiZr2 + Mo2Zr + τ1, (10) τ1 + Mo2Zr + τ2, (11) τ2 + Mo2Zr + (Zr)ht, (12) NiZr2 + τ1 + (Zr)ht and (13) τ1 + τ2 + (Zr)ht.
In Ni-rich corner, the exact compositions of Ni7Zr2 phase in the three-phase equilibria (Ni) + MoNi3 + Ni7Zr2 were not determined, and thus the tie-triangle in Fig. 4 is presented as dotted line. The reason is due to the small quantity of Ni7Zr2 phase in sample 2. We proposed the tie-triangle containing (Ni), MoNi3 and Ni7Zr2 to be stable at 900°C mainly on the basis of the three phases observed for alloys 1,3 and 4 (see Fig. 2a).
Among the Ni-Zr binary phases, Ni3Zr shows instability at a slightly higher temperature above 900 °C.[10] The XRD patterns of samples 5 and 6 show the very weak peaks of Ni3Zr phase and the equilibria of those regions were mainly constructed based on EPMA results (see Fig. 2b).
In sample 7, a metastable binary phase Ni2Zr was found, which was reported as a ternary phase Zr33.3Mo3Ni63.7 by Virkar et al.[14] However, Bsenko[19] confirmed it to be an oxygen stabilized product. In this work, the metastable binary phase was detected by XRD, but the results of EPMA show that only three phases, (Mo), Ni21Zr8 and Ni10Zr7, were observed in sample 7 (see Fig. 2c). Furthermore, the binary phases Ni21Zr8 and Ni10Zr7 were found in bulk x-ray diffraction (see Fig. 3b) but not in the powder x-ray diffraction. The results from XRD pattern of bulk sample is preferable due to the short time exposed on air. Based on the above discussions, the tie-triangle of (Mo) + Ni21Zr8 + Ni10Zr7 was established.
In the present work, for alloys whose compositions lie in the ternary triangle region edged by Mo2Zr, NiZr2 and NiZr often show the coexistence of more than three phases (see sample 10, Fig. 2d). The NiZr2 metastable phase with Fd-3 m structure was found in the XRD pattern of sample 10 (see sample 10, Fig. 3e), which is not a stable phase in the Ni-Zr binary side. According to XRD and EPMA results, the phase relationship of sample 10 with four phases, Mo2Zr, NiZr, NiZr2 with Fd-3 m structure, and NiZr2 with I4/mcm structure, was confirmed. It was found that these two NiZr2 phase dissolve different amounts of Mo which are 13.58 and 1.15 at.% respectively and contain same quantity of Zr. Based on the results of other samples (i.e. sample 11), the large solubility of Mo in NiZr2 with Fd-3 m structure is abnormal. It is worth noting that the τ 1 ternary phase has the same structure and the analogous composition as metastable NiZr2 phases, which are Zr65Mo18−x Ni16.5+x and Zr65Mo13.58Ni21.42 correspond to τ1 and metastable NiZr2 phase, respectively. Besides, the influence of oxygen and other impurities (such as B) on the formation of the metastable NiZr2 was confirmed by Altounianet al.[20] However, several investigations reported by Cacciamani et al.[21,22] in Ni-Zr and B-Ni-Zr system did not indicate any ternary phases corresponding to the metastable NiZr2 on the Zr-rich corner. Moreover, three complementary alloys, which have the same composition as sample 10, were prepared and annealed at 900 °C for different annealing times (30, 40 and 50 days). However, the identical phase relationship was found as in sample 10. The above conclusions indicating that the existence of the metastable NiZr2 phase is probably caused by oxygen impurity and the existence of Mo. Nevertheless, the essential reason for the formation of the metastable NiZr2 phase is still unclear and needs further investigations.
In the presently investigated Mo-Ni-Zr isothermal section, a few binary phases dissolve appreciable amounts of the third component. For example, the maximum solubility of Zr in MoNi3 was determined to be 2.01 at.% Zr. The solubilities of Ni in Mo2Zr and Mo in Ni7Zr2 were measured to be 3.98 at.% Ni and 2.48 at.% Mo, respectively.
In the Zr-rich corner, two ternary compounds (τ 1 and τ 2) were detected, together with their crystal structure, cF96-Ti2Ni and hP28-Hf9Mo4B, respectively. Based on the EPMA results on several alloys containing the τ 1 phase (see also sample 14, Fig. 2e), a homogeneity region extending along the ~65 at.% Zr concentration line was established, leading to the general formula Zr65Mo18−x Ni16.5+x (0 ≤ x≤2.5) for τ 1. The lattice parameter calculated for x = 2.5 is lower than that for x = 0 (see Table 2), which is consistent with the feature that the atomic radius of Ni is smaller than that of Mo. The solubility range of τ2 is negligible, and the stoichiometric composition of τ 2 was determined to be Zr65Mo27.3Ni7.7.
In the Ni-rich corner, a two-phase region constructed by (Ni) and Ni5Zr was confirmed (Fig. 2f). The solubility of Zr in (Ni) is low, and it was measured to be less than 1.0 at.% Zr.
Conclusions
The phase equilibria of the Mo-Ni-Zr system have been systematically investigated via XRD analysis and EPMA measurement, and the 900 °C isothermal section of the Mo-Ni-Zr system was established. The major feature of the three-phase equilibria involves the binary solution phases and two ternary compounds Zr65Mo18−x Ni16.5+x (τ1, 0 ≤ x≤2.5, cF96-Ti2Ni) and Zr65Mo27.3Ni7.7 (τ2, hP28-Hf9Mo4B).
Thirteen three-phase equilibria at 900 °C were well determined. In the present system, a few binary phases dissolve appreciable amounts of the third component. For instance, the maximum solubility of Zr in MoNi3 was determined to be 2.01 at.%. The solubility of Ni in Mo2Zr and Mo in Ni7Zr2 were measured to be 3.98 at.% Ni and 2.48 at.% Mo, respectively.
The four contradictions previously mentioned are clarified and summarized as follows: (1) the existence of the two ternary compounds Zr65Mo18−x Ni16.5+x (τ1, 0 ≤ x≤2.5, cF96-Ti2Ni) and Zr65Mo27.3Ni7.7 (τ2, hP28-Hf9Mo4B) was confirmed; (2) in the Ni-rich corner, the three-phase equilibria of (Ni) + Ni5Zr + Ni7Zr2 were determined; (3) the maximum solubility of the (Mo) phase was identified as 6.94 at.% Zr and 5.22 at.% Ni; and (4) the Ni3Zr binary phase was found at 900 °C in this work.
The presently developed phase equilibria of the Mo-Ni-Zr system at 900 °C can provide new phase diagram data for thermodynamic optimization of the Mo-Ni-Zr system.
References
P. Ettmayer, H. Kolaska, W. Lengauer, and K. Dreyer, Ti(C, N) Cermets—Metallurgy and Properties, Int. J. Refract. Met. Hard Mater., 1995, 13(6), p 343-351
J. Zackrisson and H.-O. Andrén, Effect of Carbon Content on the Microstructure and Mechanical Properties of (Ti, W, Ta, Mo)(C, N)-(Co, Ni) Cermets, Int. J. Refract. Met. Hard Mater., 1999, 17(4), p 265-273
Y. Li, N. Liu, X. Zhang, and C. Rong, Effect of Mo Addition on the Microstructure and Mechanical Properties of Ultra-Fine Grade TiC-TiN-WC-Mo2C-Co Cermets, Int. J. Refract. Met. Hard Mater., 2008, 26(3), p 190-196
X. Zhang, N. Liu, C. Rong, and J. Zhou, Microstructure and Mechanical Properties of TiC-TiN-Zr-WC-Ni-Co Cermets, Ceram. Int., 2009, 35(3), p 1187-1193
X. Zhang and N. Liu, Effects of ZrC on Microstructure, Mechanical Properties and Thermal Shock Resistance of TiC-ZrC-Co-Ni Cermets, Mater. Sci. Eng., A, 2013, 561, p 270-276
M.H. Yang, S.N. Li, Y. Li, J.H. Li, and B.X. Liu, Atomistic Modeling to Optimize Composition and Characterize Structure of Ni-Zr-Mo Metallic Glasses, Phys. Chem. Chem. Phys., 2015, 17(20), p 13355-13365
http://materials.bibliotecabuap.elogim.com/isp/phase-diagram/docs/c_1301041
http://materials.bibliotecabuap.elogim.com/isp/crystallographic/docs/sd_0461606
http://materials.bibliotecabuap.elogim.com/isp/crystallographic/docs/sd_0536140
T. Tokunaga, S. Matsumoto, H. Ohtani, and M. Hasebe, Thermodynamic Analysis of the Phase Equilibria in the Nb-Ni-Zr System, Mater. Trans., 2007, 48(9), p 2263-2271
K. Santhy and K.H. Kumar, Thermodynamic Assessment of Mo-Ni-Ti Ternary System by Coupling First-Principle Calculations with CALPHAD Approach, Intermetallics, 2010, 18(9), p 1713-1721
R. Jerlerud Pérez and B. Sundman, Thermodynamic Assessment of the Mo-Zr Binary Phase Diagram, Calphad, 2003, 27(3), p 253-262
K.P. Gupta, The Mo-Ni-Zr System (Molybdenum-Nickel-Zirconium), J. Phase Equilib., 2000, 21(1), p 95-101
A.V. Virkar and A. Raman, Alloy Chemistry of σ(β-U)-Related Phases. II. Characteristics of δ and Other σ-Related Phases in Some Mo-NiX Systems, Z. MetaIlkd., 1969, 60(7), p 594-600
S.B. Prima, N.V. Dan’ko, and V.M. Petyukh, Phase Equilibria in the Nickel-Zirconium-Molybdenum System at Subsolidus Temperatures and 900 °C, Izv. Vyssh. Uchebn. Zaved. TsvetnMetall., 1991, 3, p 86-94
S.B. Prima and V.M. Petyukh, Phase Relations in Solidification of Nickel-Zirconium-Molybdenum Alloys in the ZrNi-Ni-Mo Region, Metally, 1991, 6, p 161-167
S.B. Prima and V.M. Petyukh, Phase Transformations During Solidification of Nickel-Zirconium-Molybdenum Alloys in the Zr-ZrNi-Mo Region, Metally, 1993, 5, p 205-212
JADE 6.0, Users Guide for XRD Pattern Processing. In: Materials Data I, (Ed.). CA, USA, 2005
L. Bsenko, The Hafnium-Nickel and Zirconium-Nickel Systems in the Region 65-80 at.% Nickel, J. Less-Common Met., 1979, 63(2), p 171-179
Z. Altounian, E. Batalla, J.O. Strom-Olsen, and J.L. Walter, The Influence of Oxygen and Other Impurities on the Crystallization of NiZr2 and Related Metallic Glasse, J. Appl. Phys., 1987, 61(1), p 149
G. Cacciamani, P. Riani, and F. Valenza, Equilibrium Between MB2 (M = Ti, Zr, Hf) UHTC and Ni: A Thermodynamic Database for the B-Hf-Ni-Ti-Zr System, Calphad, 2011, 35(4), p 601-619
F. Valenza, M.L. Muolo, A. Passerone, G. Cacciamani, and C. Artini, Control of Interfacial Reactivity Between ZrB2 and Ni-Based Brazing Alloys, J. Mater. Eng. Perform., 2012, 21(5), p 660-666
Acknowledgments
The financial supports from the National Natural Science Foundation of China (Grant No. 51371199), Ministry of Industry and Information Technology of China (Grant No. 2015ZX04005008) and Project of Innovation-driven Plan in Central South University (Grant No. 2015CX004) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Zhang, C., Lin, C. et al. Measurement of 900 °C Isothermal Section in the Mo-Ni-Zr System. J. Phase Equilib. Diffus. 37, 672–679 (2016). https://doi.org/10.1007/s11669-016-0495-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-016-0495-6