Abstract
The thermodynamic properties of beryllium have been evaluated up to 2800 K. A further evaluation is justified by the inclusion of not only new specific heat measurements at low temperature but also new enthalpy measurements at high temperature which lead to a reassessment of the enthalpies and entropies of transition and fusion. Selected values include an enthalpy of sublimation of 324 ± 5 kJ/mol at 298.15 K and a boiling point at one atmosphere pressure of 2745 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
This work is one of a series of articles on thermodynamic properties of the elements to appear in the Journal of Phase Equilibria and Diffusion. The articles are expected to be especially useful to those who are updating thermodynamic databases for phase diagram prediction and for other applications as well. Each article in the series will list the properties of a single element, including Ag,[1] Au,[2] Be, Cr, Cu,[3] Hf,[4] Nb, Ta, V, or W. References are given for papers that have already been published. In addition, the series will also include two summary papers on selected values of the melting points and boiling points of the elements and on the enthalpies and entropies of fusion and transition of the elements.
Previous reviews on beryllium were by Hultgren et al.,[5] Spencer,[6] Alcock et al.,[7] Gurvich et al.[8] and by JANAF (Chase[9]). The values given for the condensed phases by CODATA (Cox et al.[10]) are the same as those of Gurvich et al. A further evaluation for this element is justified by new measurements of the specific heat below 110 K by Swenson[11] and further determinations of the enthalpies of transition and fusion by Kleykamp.[12] The close-packed hexagonal alpha phase transforms to the body-centred cubic beta phase at a selected temperature of 1543 ± 5 K whilst the melting point is selected as 1560 ± 5 K. The selection of these values are reviewed in Part 4.
Wherever possible, values have been corrected to the currently accepted atomic weight of 9.0121831 ± 0.0000005[13] and to the ITS-90 temperature scale using correction factors of Douglas,[14] Rusby,[15] Rusby et al.,[16] and Weir and Goldberg.[17]
Alpha Phase
The superconducting temperature at 0.026 K[18] is below the experimentally determined specific heat values and was therefore not included in the evaluation.
Low temperature specific heat is generally given in terms of the Debye equation where below 4.2 K only the first two terms are considered so that C p = γT + AT 3 where γ is the electronic coefficient and A is usually represented in terms of a limited Debye temperature, θD, where θ 3D = (12/5)π 4 R/A = 1943.770/A where R is the gas constant and A is given in units of J/(mol K4). Values determined for γ and θD are given in Table 1.
Although the values of Ahlers[20] and Swenson[11] agree closely, the latter measurements are selected since they are part of the equation given by Swenson to cover the range up to 108 K. In order to represent the thermodynamic properties up to 298.15 K values derived from this equation were combined with specific heat measurements of Hill and Smith[21] (4-300 K) obtained at 150, 200, 250 and 300 K. It is noted that the measurements of Ahlers deviate up to 4% higher than the selected curve at 30 K whilst below 150 K the measurements of Hill and Smith are up to 67% higher at 20 K but rapidly falling to be only 1% higher at 120 K. Low temperature specific heat equations are given in Table 6 and low temperature thermodynamic properties are summarised in Table 12.
Above 298.15 K the sample 1 enthalpy measurements of Ginnings et al.[22] (367-1170 K) were combined with the enthalpy measurements of Kantor et al.[23] (600-1560 K). The latter were given only in the form of an equation and shown graphically. Based on the number of data points shown on the graph, values were evaluated at 80 K intervals from 600 to 1480 K together with the value at the transition temperature 1543 K. The temperature measurements of Ginnings et al. were corrected to ITS-90 but in view of the manner in which the measurements of Kantor et al. were reported a correction was not applied. Equation 1 represents the selected data over the range 298.15 to 1543 K:
The enthalpy sample 2 of Ginnings et al.[22] (367-1170 K) averaged 0.7% lower than the selected values. The high temperature thermodynamic properties are given in Table 13 whilst derivations of other experimental specific heat and enthalpy measurements are compared with the selected values in Tables 10 and 11 respectively.
A Comparison of Selected Values at 298.15 K
The present values of enthalpy and entropy represent the inclusion of the newer low temperature measurements of Swenson[11] which avoids the previous problems of having to try and reconcile the very different specific heat values given by Ahlers[20] and Hill and Smith[21] below 100 K. The comparison is given in Table 2.
Selected Values of the Transition Temperature and Melting Point
A comprehensive review of the published transition temperatures and melting points was carried out be Alcock et al.[7] A transition temperature of 1543 ± 6 K was selected based on the measurements of Martin and Moore[24] and Loasby and Dearden.[25] A more recent determination by Kleykamp[12] at 1542 ± 1 K is considered to be of equal merit but would not alter the selected value, although with more confidence placed on this value the uncertainty is lowered to ±5 K.
For the melting point, Alcock et al. selected 1563 ± 4 K based on the values of Martin and Moore,[24] Loasby and Dearden[25] and Kantor et al.[23] However Kleykamp[12] obtained a notably lower value of 1556 ± 2 K and suggested that the higher melting point values obtained are in reality the liquidus temperature of oxygen contaminated material in the degenerate eutectic Be-BeO system. On these grounds, the melting point is chosen as a lower value of 1560 ± 5 K in agreement with Gurvich et al.[8] and JANAF (Chase[9]).
Beta Phase and the Enthalpies of Transition and Fusion
The specific heat of the beta phase has not been measured but in agreement with previous reviews was assumed to be 30 J/(mol K). The enthalpy of the alpha phase at the transition temperature is H°1543K − H°298.15K = 32,522 J/mol and for the liquid at the melting point H°1560K − H°298.15K = 47,846 J/mol. The difference is 15,324 J/mol, and if a value of 510 J/mol is subtracted to represent the enthalpy of the beta phase over the range of stability, then the combined enthalpies of transition and fusion are 14,814 J/mol. In the literature, vastly different values have been reported for both the transition enthalpy and the enthalpy of fusion. However, it was noted that for any set of measurements the ratio of the enthalpies of transition and fusion are very similar. On these grounds, reported values of the transition enthalpies have been normalised so that their sum is 14,814 J/mol as given in Table 3. This enthalpy ratio technique was also used by both Alcock et al.[7] and JANAF (Chase[9]). The discrepant measurements of Aldinger and Petzow[27] were rejected and the remainder averaged to selected values of 6855 ± 500 and 7959 ± 500 J/mol for the enthalpies of transition and fusion respectively, where the assigned accuracies take into account the diversities of the original measurements. The enthalpy of the beta phase can then be represented by Eq 2:
Derived thermodynamic properties are also given in Table 13.
Liquid Phase
Kantor et al.[23] represented the enthalpy of the liquid, 1560-2200 K, by means of a quadratic equation which is probably more representative of a possible scatter in the data. Rather than try to reduce this to a linear equation, which would tend to diminish the representation of the actual data, in agreement with Alcock et al.[7] the original equation is retained and can be represented by Eq 3:
Derived thermodynamic properties are also given in Table 13.
Monatomic Gas Phase
Values are based on one bar standard state pressure and are calculated from the fourteen energy levels below 61,000 cm−1 selected by Kraminda and Martin[29] Thermodynamic properties were calculated using the method of Kolsky et al.[30] and the 2010 Fundamental Constants[31,32] and are given in Table 14.
Diatomic Gas Phase
Based on very limited data, both Gurvich et al.[8] and JANAF (Chase[9]) estimated the thermodynamic values of the diatomic gas. However, both sets of estimates suggest that the contribution of the diatomic gas to the total vapour pressure at the boiling point was only of the order of 2.7 × 10−5 bar (0.0027%). On these grounds, the diatomic gas was not considered further in the evaluation.
Enthalpy of Sublimation of the Monatomic Gas
Because of a lack of detail as to what temperature scales were being used then no attempt was made to correct values to ITS-90 from what would be contemporary scales. Values for the enthalpy of sublimation are derived in Table 4. The selected value of 324 ± 5 kJ/mol at 298.15 K was based on the last four sets of measurements and agrees with the value selected by CODATA (Cox et al.[10]) which was also based on these measurements.
Vapor Pressure
As given in Table 5, vapor pressure equations for the alpha phase and the gas were evaluated at 25 K intervals from 750 to 1525 K and the transition temperature and for the liquid phase and the gas at the melting point and 50 K intervals from 1600 to 2800 K. Values for the beta phase were calculated from the vapor pressure values of the alpha phase at the transition temperature and for the liquid phase at the melting point. Values were fitted to the equation:
Comments on Previous Reviews of the Thermodynamic Properties
Using similar enthalpy ratio values as used in the present review, Alcock et al.[7] selected enthalpies of transition and fusion of 6700 and 8000 J/mol respectively whilst JANAF (Chase[9]) selected 6849 and 7895 J/mol, with both sets of measurements showing satisfactory agreement with the presently accepted values as given in Part 5. However, extraordinarily Gurvich et al.[8] accepted the very low original enthalpy of transition value of 2100 J/mol determined by Loasby and Dearden[25] but then ignored an enthalpy of fusion of 2400 J/mol determined by the same authors and instead selected a much higher value of 12,600 J/mol in complete contradiction to the experimental values given in Table 3.
Summary of Representative Equations
Low temperature specific heat equations are given in Table 6 and high temperature specific heat equations are given in Table 7. Gibbs energy equations are given in Table 8 and transition values involved with the Gibbs energy equations in Table 9.
Thermodynamic Tables
Low temperature thermodynamic properties of the solid are given in Tables 12 (Fig. 1) and the high temperature thermodynamic properties of the condensed phases in Table 13 (Fig. 2, 3). Thermodynamic properties of the gas phase are given in Table 14 whilst the vapour pressure summary is given in Table 15.
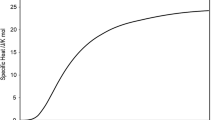
Low temperature specific heat of solid beryllium, taken from Table 12
Specific heat of beryllium for 300 < T < 1700 K, taken from Table 13
High temperature thermodynamic properties of beryllium for 300 < T < 2800 K, taken from Table 13
References
J.W. Arblaster, Thermodynamic Properties of Silver, J. Phase Equilib. Diffus., 2015, 36(6), p 573-591
J.W. Arblaster, Thermodynamic Properties of Gold, J. Phase Equilib. Diffus., 2016, 37(2), p 229-245
J.W. Arblaster, Thermodynamic Properties of Copper, J. Phase Equilib. Diffus., 2015, 36(5), p 422-444
J.W. Arblaster, Thermodynamic Properties of Hafnium, J. Phase Equilib. Diffus., 2014, 35(4), p 490-501
R. Hultgren, P.D. Desai, D.T. Hawkins, M. Gleiser, K.K. Kelley, and D.D. Wagman, Selected Values of the Thermodynamic Properties of the Elements, American Society for Metals, Metals Park, 1973
P.J. Spencer, Thermal Properties, Beryllium: Physico-chemical Properties of Its Compounds and Alloys, Atomic Energy Rev., 1973, 4, p 7-44
C.B. Alcock, M.W. Chase, and V.P. Itkin, Thermodynamic Properties of the Group IIA Elements, J. Phys. Chem. Ref. Data, 1993, 22, p 1-85
L.V. Gurvich, I.V. Veyts, V.A. Medvedev, V.S. Yungman, G.A. Bergman, V.S. Iorish, A.V. Gusarov, V.Y.A. Leonidov, O.V. Dorofeeva, I.I. Nazarenko, E.L. Osina, V.G. Ryabova, M.E. Efimov, E.A. Shenyavskaya, P.I. Tolmach, N.E. Khandamirova, L.N. Gorokhov, and I.V. Sidorova, Thermodynamic Properties of Individual Substances, Vol 3, 4th ed., CRC Press, Boca Raton, 1994
M.W. Chase, Jr., NIST-JANAF Thermochemical Tables. Journal of Physical and Chemical Reference Data Monograph No 9, 4th ed., American Chemical Society, Washington, DC, 1998
J.D. Cox, D.D. Wagman, and V.A. Medvedev, CODATA Key Values for Thermodynamics, Hemisphere Publishing Corp., New York, 1989
C.A. Swenson, HIP Beryllium: Thermal Expansivity from 4 to 300 K and Heat Capacity from 1 to 108 K, J. Appl. Phys., 1991, 70, p 3046-3051
H. Kleykamp, Thermal Properties of Beryllium, Thermochim. Acta, 2000, 345, p 179-184
Commission on Isotopic Abundances and Atomic Weights (CIAAW), Atomic Weights of the Elements 2015, www.ciaaw.org/atomic-weights.htm, 2015
T.B. Douglas, Conversion of Existing Calorimetrically Determined Thermodynamic Properties to the Basis of the International Practical Temperature Scale of 1968, J. Res. Natl. Bur. Stand., 1969, 73A, p 451-470
R.L. Rusby, The Conversion of Thermal Reference Values to the ITS-90, J. Chem. Thermodyn., 1991, 23, p 1153-1161
R.L. Rusby, R.P. Hudson, and M. Durieux, Revised Values for (t90 − t68) from 630°C to 1064°C, Metrologia, 1994, 31, p 149-153
R.D. Weir and R.N. Goldberg, On the Conversion of Thermodynamic Properties to the Basis of the International Temperature Scale of 1990, J. Chem. Thermodyn., 1996, 28, p 261-276
R.L. Falge, Jr., Superconductivity of Hexagonal Beryllium, Phys. Lett. A, 1967, 24, p 579-580
E. Gmelin, Mesure de la Chaleur Spécifique entre 1.2 et 4.2°K d’un Monocristal de Beryllium, Comptes Rendus, 1964, 259, p 3459-3461
G. Ahlers, Heat Capacity of Beryllium Below 30°K, Phys. Rev., 1966, 145, p 419-423
R.W. Hill and P.L. Smith, The Specific Heat of Beryllium at Low Temperatures, Philos. Mag., 1953, 44, p 636-644
D.C. Ginnings, T.B. Douglas, and A.F. Ball, Specific Heat of Beryllium Between 0° and 900°, J. Am. Chem. Soc., 1951, 73, p 1236-1240
P. Kantor, R. Krasovitskaya, and R. Kisel, Determination of Enthalpy and Thermal Capacity of Beryllium in the Range 600-2200 K, Fiz Met. Metalloved., 1960, 10, p 835-837 (Phys. Met. Metallogr., 1960, 10(6), p 42-44)
A.J. Martin and A. Moore, The Structure of Beryllium with Particular Reference to Temperatures Above 1200°C, J. Less Common Met., 1959, 1, p 85-93
R.G. Loasby and D. Dearden, Transformation Enthalpies in Beryllium, J. Less Common Met., 1977, 52, p 137-144
C. Potard, G. Bienvenu, and B. Schaub, Diagramme de Phases du Systeme Beryllium—Calcium, Thermodynamics of Nuclear Materials 1967, Proceedings of the Symposium, Vienna, Sept 4-8, 1967, International Atomic Energy Agency (IAEA), Vienna, 1968, p 795-807
F. Aldinger and G. Petzow, Constitution of Beryllium and Its Alloys, Beryllium Science and Technology, Vol 1, D. Webster and G.J. London, Ed., Plenum Press, New York, 1979, p 235-305
A. Abey, Pressure Phase Lines and Enthalpies for the Alpha-Beta and Βeta-Liquid Transitions in Beryllium, Rept. UCRL-53567, U.S. Atomic Energy Commission, Washington, DC, 1984
A. Kraminda and W.C. Martin, A Compilation of Energy Levels and Wavelengths for the Spectrum of Neutral Beryllium (Be I), J. Phys. Chem. Ref. Data, 1997, 26, p 1185-1194
H.G. Kolsky, R.M. Gilmer, and P.W. Gilles, The Thermodynamic Properties of 54 Elements Considered as Ideal Monatomic Gases, Rept. LA 2110. U.S. Atomic Energy Commission, Washington, DC, 1957
P.J. Mohr, B.N. Taylor, and D.B. Newell, CODATA Recommendations of the Fundamental Physical Constants: 2010, Rev. Mod. Phys., 2012, 84, p 1527-1605
P.J. Mohr, B.N. Taylor, and D.B. Newell, CODATA Recommendations of the Fundamental Physical Constants: 2010, J. Phys. Chem. Ref. Data, 2012, 41, p 043109-1-043109-84
E. Baur and R. Brunner, Dampfdruckmessungen an Hochsiedenden Metallen, Helv. Chim. Acta, 1934, 17, p 958-969
R. Schuman and A.B. Garrett, The Vapor Pressure of Beryllium at 1170-1340°K, J. Am. Chem. Soc., 1944, 66, p 442-444
C.B. Magee, M.J. Pool, W.D. McMullen, R.C. Jerner, and R.H. Cox, Investigation of the Characteristics of Intermetallic Compounds as Thermionic Emitters, University of Denver, Denver, Colorado, Rept. DRI 2137, U.S. Department of Commerce Office of Technology Services, Rept. AD428591, 1963
I. Ansara and E. Bonnier, Tension de Vapeur du Beryllium et de l’Argent Liquide, Conference Internationale Sur la Metallurgie du Beryllium, Grenoble, France, 1965, Presses Universitaires de France, Paris, 1966, p 17-18
R.B. Holden, R. Speiser, and H.L. Johnston, The Vapor Pressures of Inorganic Substances. I. Beryllium, J. Am. Chem. Soc., 1948, 70, p 3897-3899
E.A. Gulbransen and K.F. Andrew, The Kinetics of the Reactions of Beryllium with Oxygen and Nitrogen and the Effect of Oxide and Nitride Films on Its Vapor Pressure, J. Electrochem. Soc., 1950, 97, p 383-395
G.P. Kovtun, A.A. Kruglykh, and V.S. Pavlov, Vapor Pressure of Solid Beryllium, Izv. Akad. Nauk. SSSR Met. i Gornoe Delo, 1964, 2, p 177-179
D.L. Hildenbrand, W.F. Hall, and F. Ju, Unpublished. Quoted by D.L. Hildenbrand and E. Murad, Mass-Spectrometric Determination of the Dissociation Energy of Beryllium Monofluoride, J. Chem. Phys., 1966, 44, p 1524-1529
F. Simon and M. Ruhemann, Untersuchungen über die Spezifischen Wärmen bei Tiefen Temperaturen, Z. Phys. Chem., 1927, 129, p 321-348
E.J. Lewis, Some Thermal and Electrical Properties of Beryllium, Phys. Rev., 1929, 34, p 1575-1587
P. Vernotte and A. Jeufroy, Méthode Simple de Mesure de la Chaleur Spécifique d’un Corps Solide à la Température Ordinaire. Application au Glucinium, Comptes Rendus, 1931, 192, p 612-614
S. Cristescu and F. Simon, Die Spezifischen Wärmen von Beryllium, Germanium und Hafnium bie Tiefen Temperaturen, Z. Phys. Chem. B, 1934, 25, p 273-282
L. Losana, Study of Beryllium. Note 1. Properties of Beryllium of a High Degree of Purity, Alluminio, 1939, 8(2), p 67-75
E.A. Mit’kina, Experimental Determination of the True Specific Heats of Uranium, Thorium and Other Metals, Atomic Energy, 1959, 7, p 163-165 (Sov. J. Atomic Energy, 1961, 7, p 669-670)
B.E. Walker, Jr., C.T. Ewing, and R.R. Miller, Specific Heat of Some High Temperature Materials, J. Chem. Eng. Data, 1962, 7, p 595-597
M.F. Smith, R.D. Watson, J.B. Whitley, and J.M. McDonald, Thermo-mechanical Testing of Beryllium for Limiters on ISX-B and JET, Fusion Technol., 1985, 8, p 1174-1183
R.P. Tye and J.P. Brazel, The Thermophysical Properties of Hot Pressed Beryllium, Rev. Int. Hautes Temp. Refract., 1970, 7, p 326-334
A. Magnus and H. Holzmann, Untersuchungen über die Spezifischen Wärme von Tantal, Wolfram und Beryllium zwischen 100 und 900°C, Ann. Phys., 1929, 5, p 585-613
F.M. Jaeger and E. Rosenbohm, The Exact Measurement of the Specific Heats at High Temperatures. V. Specific Heats and Thermal Hysteresis of Beryllium, Rec. Trav. Chim., 1934, 53, p 451-463
I.B. Fieldhouse, J.C. Hedge, J.I. Lang, and T.E. Waterman, Thermal Properties of High Temperature Materials, Wright Air Development Center, Wright-Patterson Air Force Base, Ohio, WADC Technical Rept. 57-487, 1958
M. Boivineau, L. Arlès, J.M. Vermeulin, and T.H. Thévenin, Thermophysical Properties of Solid and Liquid Beryllium, Int. J. Thermophys., 1993, 14, p 427-439
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arblaster, J.W. Thermodynamic Properties of Beryllium. J. Phase Equilib. Diffus. 37, 581–591 (2016). https://doi.org/10.1007/s11669-016-0488-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-016-0488-5