Abstract
Mixing enthalpies of the liquid binary Al-Sm (0 < xSm < 0.15; 0.37 < xSm < 1), Sm-Sn (0 < xSn < 0.13; 0.56 < xSn < 1) alloys and ternary Al-Sm-Sn alloys (along the three concentration sections: (Sm0.87Sn0.13)1−xAlx (0 < x<0.32); (Sm0.44Sn0.56)1−xAlx (0 < x<0.12); (Al0.85Sm0.15)1−xSnx (0 < x<0.032)) were determined by an isoperibolic calorimetry technique at 1410-1670 K. Thermodynamic properties of liquid Al(Sn)-Sm alloys were described in the whole concentration range using the model of ideal associated solution. Thermodynamic activities of components of the Al(Sn)-Sm melts demonstrate large negative deviation from the ideal behavior, and the mixing enthalpies are characterized by significant exothermic effects. The minimum value of the mixing enthalpy of the Al-Sm melts is −47.1 ± 0.5 kJ/mol at xSm = 0.35 (T = 1500 K, undercooled melt), Sm-Sn is −67.7 ± 0.5 kJ/mol at xSn = 0.48 (T = 1450 K, undercooled melt). The obtained results were compared with the data from survey of the binary Al(Sn)-Sm systems and with the values calculated by the different models from the data for the binary boundary subsystems of the ternary Al-Sm-Sn system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decades, the interest of different scientific groups for the alloys containing aluminum, tin and rare-earth metals (Ln) has been increasing. This was caused by the possibility to obtain the materials with specific physico-chemical properties (such as amorphous and nanocrystalline) based on these elements. However, thermodynamic and other physico-chemical properties of their melts in the wide range of temperature have not been studied yet. At high temperatures, the investigation of such alloys is complicated due to their intensive ability to react with refractory materials and oxygen. So, computational works in this field are predominating; a recent one by Jin at al.[1] contains the review and the estimation of the thermodynamic properties and phase diagrams of the Al-La(Ce, Pr, Nd, Sm) systems. But some of the data obtained[1] significantly differ from the ones determined by our scientific group before. Additionally, the results[1] were derived from quite limited experimental information. This made the further investigation of these objects actual, especially determining the physically based dependences of thermodynamic properties of the Al-Ln alloys on the atomic number of lanthanide.
The properties of alloys of the Al-Sm system cannot be forecasted reliably from the data for the analogous Al-Ln systems due to two reasons. Firstly, the neighbor systems Al-Pm (not investigated because of the radioactivity of Pm) and Al-Eu (with europium having a complex of properties uncommon for the lanthanides) cannot be taken as examples. Secondly, samarium also differs in physical (anomalous volatility) and chemical (existence of the compounds of divalent Sm) properties. Thus, the investigations of the systems containing Sm are especially interesting.
The limited data on the thermodynamic properties of the Ln-Sn alloys are controversial, too. Mostly, they relate to the alloys at low temperatures containing less than 25% Ln. The melting temperatures of the most refractory compounds in the central range of the phase diagrams are still unknown, and their estimated values differ in several hundred degrees. The interaction of Al with the binary Ln-Sn alloy is expected to be intensive at high contents of lanthanides and very weak at high contents of tin. The refractory Ln5Sn3 compound seems to be a delimiter for these types of interaction.
Literature Review
Al-Sm
The Al-Sm system was initially investigated by Casteels and Buschow et al.[2-4] in the aluminum-rich range by thermal, micrographic, x-ray, and microprobe analyses. The liquidus curve was not determined at high Sm contents, because of the high vapor pressure of samarium. The congruent melting of the Al11Sm3 compound was proposed, as well as its catatectic decomposition at 1343 K, unlike the other Al11Ln(Pr, Nd)3 compounds with two allotropic forms in the solid state. Kononenko et al.[5] studied the Al-rich part of the phase diagram by the DTA, metallography, and x-ray analyses. Saccone at al.[6] investigated the whole concentration range of the Al-Sm system using the thermal analysis, micrography, microprobes and x-ray diffraction, and supposed the solid-state transition of Al11Sm3 at 1343 K. Then, Zhou and Napolitano[7] investigated the Al11Sm3 phase using microstructural, microchemical analyses and x-ray diffraction, as well as the first-principles calculation involving VASP.[8] They confirmed the stability of this phase from 1655 down to 1343 K. Gschneidner et al. reported the melting and phase transition temperatures for Sm in Ref 9. Wernick[10] found the Laves-C15 structure for Al2Sm, and Buschow[11] established the Ni2Si-type structure for this compound. Buschow[4] found five intermetallics: Al4Sm, Al3Sm, Al2Sm, AlSm, and AlSm2. However, Saccone[6] and Delsante et al.[12] showed the existence of Al11Sm3 instead of Al4Sm, with structure being similar to the Al11La3;[13] Delsante[12] confirmed the catatectic decomposition reaction of the Sm3Al11.
Pasturel et al.[14] determined the limiting partial enthalpy of Sm in Al \( \left( {\Delta \overline{H}_{\text{Sm}}^{\infty } \, = - 1 7 1. 5 {\text{ kJ}}/{\text{mol}}} \right) \) at 966 K, 0 < xSm < 0.002. Colinet et al.[15] investigated the formation enthalpy of the Al2Sm compound \( \left( {\Delta_{f} H = - 5 4. 3 {\text{ kJ}}/{\text{mol}}} \right) \), through heat of its dissolution in liquid aluminum. Borzone et al.[16] determined the enthalpies of formation of some solid alloys at 0.27 < xSm < 0.83 by direct calorimetry.
The properties of the Al-Sm system were assessed in Ref 6,17,18; Jia et al.[17] used the thermodynamic parameters for liquid phase from Ref 6. Zhou and Napolitano[18] introduced a model considering the formation of the Al2Sm associate. The invariant reaction Liq. → Al(FCC) + Al3Sm is at 919.5 K,[18] that is higher than obtained experimentally by Buscho,[4] 903 K.
Zhou[18] showed the ratio \( \Delta_{mix} H^{liquid} /\Delta_{f} H^{{{\text{Al}}_{2} {\text{Ln}}}} \) (at \( x_{\text{Ln}} = 0. 3 3 3 3 \)) to be relatively constant. Jin[1] determined this ratio to be equal to 0.62 for the Al-Pr(Nd) systems, hence \( \Delta_{mix} H^{liquid} = - 3 4 \), derived from \( \Delta_{f} H^{{{\text{Al}}_{2} {\text{Ln}}}} = - 5 5\;{\text{kJ}}/{\text{mol}} \) of atom.
Kulikova et al.[19] conducted the calculation of mixing enthalpies, excess Gibbs energies, and the activities of components in the Al-Sm melts using the ideal associated solutions model. It is unclear what initial information was taken in that work. Therefore, the obtained minimum value of the integral mixing enthalpy of the Al-Sm melts, \( \Delta H_{\hbox{min} } = - 5 9\;{\text{kJ}}/{\text{mol}} \), strongly differs from the data by other authors, and is difficult to interpret. Moreover, that value was calculated for a very high temperature, 1873 K. As Kulikova et al.[19] stated, “Zhou[18] considered the formation of the only associate (Al2Sm), and in Ref 7 the associate model was not used at all; so the values \( \Delta H_{\hbox{min} } = - 4 4. 5\;{\text{kJs}}/{\text{mol}} \) at 1200 K[18] and −42.5 kJ/mol at 1200 K[7] are underestimated”. This argument seems unacceptable for us. Indeed, by changing the thermodynamic parameters of the associates or the coefficients of the polynomial dependences of the thermodynamic properties, it is possible to obtain any value of \( \Delta H_{\hbox{min} } \). That value depends on the input data used in the model, but not on the number of associates or even whether the association is considered at all.
Actually, the model of associated solutions does not mean the existence of the associates as stable groupings in a real melt. However, the precision of the results obtained with this model is often much better, comparing to the polynomial dependences on concentration.
Zhou and Napolitano did not predict the existence of the temperature dependence of the mixing enthalpies of the Al-Sm melts in Ref 7. On the other hand, their further results[18] are characterized by a significant dependence of \( \Delta H_{\hbox{min} } \) on temperature (−44.5 kJ/mol at 1200 K, −37 kJ/mol at 1873 K). Kulikova[19] gave polynomial coefficients for the integral mixing enthalpies of the Al-Sm melts at three temperatures (1873, 1960 and 2100 K). But it can be seen that all those dependences are erroneous, because they strongly deviate from the concentration dependences presented in the figure and discussed in the text.[19] Such large deviation renders the results obtained by Kulikova et al.[19] very doubtful.
The purpose of our work is calorimetric investigation of the Al-Sm melts in the wide concentration range, and calculation of the thermodynamic properties of the liquid and solid Al-Sm alloys, based on the whole set of the data on thermodynamics and phase equilibria available in literature. At the present time there is quite controversial information on the thermodynamic properties of the Al-Sm melts in literature, and this makes the purpose of our work especially actual.
Sm-Sn
Bulanova et al.[20] made the literature review for the Sm-Sn system in 1994, and there have appeared hardly any new datum on this subject since that time. Percheron[21] investigated the phase diagram of the Sm-Sn system using DTA, x-ray diffraction and MSA. It was determined that the compounds Sm5Sn3 and SmSn3 melt congruently at 1778 and 1363 K, respectively, along with Sm4Sn3, Sm5Sn4, and Sm2Sn3 melting peritectically at 1713, 1693 and 1378 K, respectively. The Sm11Sn10 compound forms and decays peritectically, existing in the narrow temperature interval only (1443-1513 K). The latter data, along with the ones for the formation of Sm4Sn3, are dubious. In accordance with Yeremenko et al.,[22] the melting temperatures of the Sm5Sn3 and Sm5Sn4 compounds are significantly underestimated. So, they were reinvestigated by Bulanova[23] and determined to be 1878 and 1793 K correspondingly. Borzone[24] observed the SmSn2 phase, though before Iandelli[25] this phase was shown not to exist. Weitzer et al.[26] found such phases in the Sm-Sn alloys as SmSn2, Sm3Sn7 and Sm2Sn5. Their compositions are close to each other (xSn = 0.667, 0.7, and 0.714), and their possible equilibria with liquid alloys should be peritectical, situated in the near-horizontal part of liquidus curve at 0.667 < xSn < 0.75. So, the existence of these compounds does not affect the shape of liquidus very much.
No homogeneity ranges for the compounds of samarium with tin were found. In the Sm-Sn system, three eutectic reactions were defined: at 1179 K (11% Sn), 1353 K (67% Sn), and 502 K (99.8% Sn). The catatectic decomposition of β-Sm into α-Sm and liquid is observed at 1189 K. Lebedev et al.[27] determined the solubility of Sn in the solid Sm using e.m.f. method in the temperature range 823-1023 K, and described it by the equation \( \lg x_{\text{Sm}} = 2080 - 3316/T \). Palenzona[28] determined the formation enthalpy of the SmSn3 compound by the direct calorimetry, and Percheron et al.[29] did this (along with the other two compounds) by the dissolution in the liquid Sn. Also, Colinet et al.[30] estimated the formation enthalpies of the compounds in the Sm-Sn system using the Miedema theory (Table 1).
For SmSn3, good agreement between the experimental values of \( \Delta_{f} H \)[28,29] is observed, but the calculated value[30] is much less exothermic. For Sm2Sn3, there is a good agreement between the calculated[30] and experimental[29] values. The formation enthalpy of Sm5Sn3 is doubtful. It is hard to assume that the most refractory compound in this system forms with a lesser heat effect than Sm2Sn3, forming in a peritectical reaction at the temperature in 500 K lower than melting of Sm5Sn3. Percheron et al.[29] considered the formation of these compounds only (Sm5Sn3, Sm2Sn3, SmSn3). The enthalpy and entropy of melting of SmSn3 were determined by Palenzona:[28] \( \Delta {}_{melt}H = 1 6. 7\pm 0. 8\;{\text{kJ}}/{\text{mol}} \) of atom and \( \Delta {}_{melt}S = 1 2. 4\pm 0. 6\,{\text{J}}/\left( {{\text{mol of atom}}\cdot{\text{K}}} \right) \), which corresponds to the melting temperature 1345 K.
Kober, Lebedev et al.[27,31-33] studied the thermodynamic properties of the dilute solutions of Sm in liquid Sn in the two-phase region (melt + SmSn3) by the e.m.f. technique at 700-1030 K (Table 1). In the region of homogeneous solution, the temperature dependence of the activity coefficient of Sm was described by the equation \( \lg \gamma_{\text{Sm}} = 3243{ - }12425/T \).[27] Due to the data for this region, \( \Delta \overline{H}_{\text{Sm}}^{\infty } = - 2 3 7. 9\pm 7. 2\;{\text{kJ}}/{\text{mol}},\;\,\Delta \overline{S}_{Sm}^{ex,\infty } = - 6 2.0 \pm 7.0\;{\text{J}}/\left( {{\text{mol}}\;{\text{K}}} \right). \) From the data of the other works, the \( \Delta \overline{H}_{Sm}^{\infty } = - 20 9. 2\;{\text{kJ}}/{\text{mol at 6}}00{\text{ K}} \),[34] −221.8 ± 8.4 at 750 K,[35] −240.5 ± 10.5 at 828 K,[36] −190.8 at 967 K.[29] As estimated by Colinet,[30] \( \Delta \overline{H}_{Sm}^{\infty } = - 1 7 2\;{\text{kJ}}/{\text{mol,}} \) and \( \Delta H_{\hbox{min} } = - 4 3\;{\text{kJ}}/{\text{mol}} \). The latter value differs strongly from both the data for the solid compounds in the Sm-Sn system and for the melts of some other Ln-Sn systems; so, it is unreliable.
Berrada et al.[37] investigated the mixing enthalpies of alloys of the Sm-Sn system (along with the Pb-Sm system) for 0.69 < xSn < 1 at 1203 K (930°C). They obtained very large negative partial mixing enthalpies of samarium \( \left( {\Delta \overline{H}_{Sm}^{\infty } = - 2 7 7 {\text{ kJ}}/{\text{mol}}} \right) \). However, due to the available phase diagram, the SmSn3 compound melts at 1090°C (quite higher than the temperature by Ref 37). So, these results should represent the properties of heterogeneous alloys at xSn < 0.93, i.e. for the majority of the studied alloys. This also explains why the data[37] are significantly scattered. If the reaction of samarium with the heterogeneous alloy would be complete, the mean value of the observed “\( \Delta \overline{H}_{\text{Sm}} \)“ for 0.75 < xSn < 1 will be equal to
This is rather close to the results.[37] However, such heterogeneous reactions are usually problematical and incomplete, hence the scattered results.
All the data from literature listed above are gathered in the Table 1.
Experimental Part
The whole description of the experimental technique was presented by us in Ref [38]. The experiments were carried out in a high temperature isoperibolic solution calorimeter working up to 1850 K in purified helium atmosphere under a pressure of 150000 Pa. A massive molybdenum block with two crucibles—the melt container and the reference crucible—is placed in the constant-temperature zone of the furnace with a coaxial molybdenum heater. We used aluminum AB00 99.99%, samarium 99.88% and tin 99.99% for experiments. Samples of aluminum had masses 0.005-0.02 g, samarium samples were 0.013-0.035 g, and tin samples 0.01-0.035 g. Being solid at T = 298 K, they were dropped into the melt in the crucible (the calorimetric bath) from a revolving container through a ceramic tube. Molybdenum is inert against samarium and tin, but interacts with aluminum at some point. So, molybdenum crucibles were used for the alloys Al-Sm (0.37 < xSm < 1), Sm-Sn and for the sections (Sm0.87Sn0.13)1−xAlx (0 < x<0.32) and (Sm0.44Sn0.56)1−xAlx (0 < x<0.12) of the ternary system. For the Al-Sm alloys in the range 0 < xSm < 0.15 and the section (Al0.85Sm0.15)1−xSnx (0 < x<0.032), the crucibles made of aluminum oxide were used, because Al2O3 is inert against aluminum, tin and only small concentrations of samarium. However, there should be a threat of the Al2O3 + Sm → Sm2O3 + Al reaction, if the concentration of samarium were greater.
Unfortunately, samarium is quite a volatile metal. Its vapor interacts with some parts of the calorimeter, and that may cause the failure of the device. In addition, the quantity of metal in the crucible decreases with time, and the exact value of this decrease at the given moment is impossible to determine. That is why we attempted to conduct all the experiments at as low temperature as possible, though keeping the alloy liquid. In particular, the Al-Sm alloys in the range 0 < xSm < 0.15 and (Al0.85Sm0.15)1−xSnx (0 < x<0.032) were studied at 1440 K; 0.55 < xSm < 1 at 1410 K, and 0.37 < xSm < 0.55 at 1640-1670 K. In the last case, there was still a possibility of formation of the solid Al2Sm compound in a small amount, so a lesser statistical weight was given to these data in the range 0.37 < xSm < 0.43 while treating the results. The Sm-Sn alloys (0 < x Sn < 0.13) and (Sm0.87Sn0.13)1−xAlx (0 < x<0.32) were studied at 1450 K, Sm-Sn (0.64 < xSn < 1) at 1480 K, Sm-Sn (0.55 < xSn < 0.66) and (Sm0.44Sn0.56)1−xAlx (0 < x<0.12) at 1670 K.
The calibration constant of the calorimeter was determined by adding some samples of the metal, the same as the pure one contained in the crucible (before adding other components)—Al, Sm, or the refractory metal inert to the alloy (Mo). The heat effects observed while the samples dissolved were treated accordingly to the heat balance equation:\( K\int\limits_{0}^{{\tau_{\infty } }} {(T - T_{0} )d} t = \Delta H_{T} + n_{i} \Delta H_{298}^{T} \).
Here \( \Delta H_{298}^{T} \) is an enthalpy of heating 1 mol of an additive from 298 K to the temperature of experiment, by Dinsdale;[39] \( K \) is a calibration constant of the calorimeter; \( n_{i} \) is a molar quantity of an additive; \( T - T_{0} = \Delta T \) is a difference between the temperatures of the crucible with the melt and of the isothermal shell of the calorimeter; \( t \) is time.
From the partial mixing enthalpies of one component, the analogous values for the second component were calculated, and then the integral values were obtained (Fig. 1 and 7; Tables 2, 3). These data together with the ones obtained from literature were treated using the model of ideal associated solutions (IAS).[40] Five associates (Al4Sm, Al3Sm, Al2Sm, AlSm, AlSm2) were chosen to model the thermodynamic properties of the Al-Sm system. Three associates (Sm2Sn, SmSn, SmSn3) were taken for the Sm-Sn system; they are close to the compositions of rather stable intermetallics Sm5Sn3, Sm5Sn4, and SmSn3. However, they have simpler compositions, increasing the probability of such atomic combinations in the liquid high-temperature alloys. It appeared that such numbers of the associates are both necessary and sufficient for describing the thermodynamic data within the experimental errors. Given in Tables 2 and 3, these errors were estimated from mean square deviations of experimental points for partial and integral mixing enthalpies from the fitting lines by IAS model. Such estimation gave terminal values of errors, and at other concentrations they were taken proportional to the absolute values of corresponding functions. That is, we suggested the relative errors being constant for a given function \( \Delta \overline{H}_{i} \) or \( \Delta H \). Unfortunately, we could not find more accurate methods of calculating these errors, because of a complex mathematical nature of functions \( \Delta \overline{H}_{i} \) and \( \Delta H \) from concentration of the melt, which is characteristic for IAS model in comparison to polynomial functions.
One of the criticisms of the associated solution model is about the mathematical formalism implying that distinct molecules exist, which is hard to justify.[41] However, actually the model merely implies some underlying structure to the liquid, which is quite reasonable, and it does provide functions which allow for the temperature dependence of the enthalpy of mixing and other thermodynamic functions. In the literature, there is a tendency to use as few associates as possible in the model, based on the assumption that diffraction or other structure-sensitive measurements usually reveal no more than two different kinds of chemical short range order. On the contrary, we think that if one kind of associate is assumed, there are no obstacles to consider as many other kinds as possible. A true reason why this is rarely done in practice is rise in computational difficulties with increase in the number of associates. Nevertheless, some works have used as much as five associates for modeling,[42-45] though most of these imaginable compounds reveal rather low concentrations in the liquid alloys, especially when the composition is far from the stoichiometry of such associates.
Actually, considering a large number of simultaneously possible associates is more general than artificial choosing only one or two among them. So, we cannot agree that an ideal associated model considering five associates is necessarily worse than a regular associated model considering one associate and some interaction energies between the species in the melt. The second variant may seem simpler for someone. But actually we managed to develop an algorithm for quick search of equilibrium point between the species in an ideal associated solution. However, this algorithm fails when the interaction between the species is considered, as in regular associated model. It is similar to the fact that a system of five linear equations is usually simpler to solve than another system of two transcendental equations.
The temperature dependences of the mixing enthalpies of the Al-Sm and Sm-Sn melts are not very large. This may be explained by very intensive interaction between the components, which becomes weaker only at quite high temperatures, >2000 K. So, the differences of experimental temperature within 100-200 K do not cause a violation of the experimental error bounds. Thus, the calculations were made for 1500 K (Al-Sm) and 1450 K (Sm-Sn), which are approximately average of the temperatures of all our experiments conducted in the corresponding systems.
In the end of some series of investigation of mixing enthalpies of binary alloys, several samples of the third component of the ternary Al-Sm-Sn systems were added. This allowed us to obtain partial mixing enthalpies of the third component, and integral enthalpies, along three sections of the Al-Sm-Sn triangle (Tables 2, 3): (Sm0.87Sn0.13)1−xAlx (0 < x<0.32); (Sm0.44Sn0.56)1−xAlx (0 < x<0.12); (Al0.85Sm0.15)1−xSnx (0 < x<0.032).
The obtained set of partial and integral mixing enthalpies and entropies was approximated by the polynomial dependences which give a less exact estimate of the thermodynamic functions, comparing to IAS model, but make the calculation faster in the case of multicomponent system based on the binary Al-Sm and Sm-Sn subsystems. Let us assume
Then the coefficients of the polynomial dependences will have the next values:
Al-Sm \( \left( {x_{2} = x_{Sm} } \right) \):
i | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
\( a_{i} \, \left( {{\text{kJ}}/{\text{mol}}} \right),{ 15}00\;{\text{K}} \) | −183.3 | −222.8 | −306.9 | 4073.9 | −6184.5 | 2730.2 |
\( d_{i} \,\left( {{\text{J}}/\left( {{\text{mol}}\;{\text{K}}} \right)} \right),{ 15}00{\text{ K}} \) | −65.5 | −88.3 | −402.8 | 2569.4 | −3582.0 | 1531.9 |
So, \(\Delta \overline{H}_{\text{Sm}}^{\infty } = - 1 8 3.3 \pm 2.1;\;\,\Delta \overline{H}_{\text{Al}}^{\infty } = - 9 3.4 \pm 1. 9;\,\;\Delta H_{\hbox{min} } \, = - 4 7.1 \pm 0. 5\;{\text{kJ}}/{\text{mol at x}}_{\text{Sm}} = 0. 3 5\)
Sm-Sn \( \left( {x_{2} = x_{\text{Sn}} } \right) \):
i | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
\( a_{i} \,\left( {{\text{kJ}}/{\text{mol}}} \right),{ 145}0\;{\text{K}} \) | −176.6 | −82.1 | −1199.3 | 2822.9 | −1531.8 |
\( d_{i} \left( {{\text{J}}/\left( {{\text{mol}}\;{\text{K}}} \right)} \right),{ 145}0{\text{ K}} \) | −51.1 | 8.5 | −751.1 | 1529.6 | −794.2 |
So, \( \Delta \overline{H}_{\text{Sn}}^{\infty } = - 1 7 6.6 \pm 2. 1;\;\,\Delta \overline{H}_{\text{Sm}}^{\infty } = - 1 6 7.0 \pm 1.9;\,\;\Delta H_{\hbox{min} } \, = - 6 7.7 \pm 0. 5\;{\text{kJ}}/{\text{mol}}\;{\text{at}}\;{\text{x}}_{\text{Sn}} = 0. 4 8. \)
Partial and integral mixing enthalpies of melts of the ternary Al-Sm-Sn system for three sections studied by us, approximated by linear and quadratic functions, are presented in the Fig. 2.
Thermodynamic Modeling
The Properties of the Binary Al(Sn)-Sm Alloys
The activities of pure components and the molar fractions of the associates in the melts of the Al-Sm and Sm-Sn systems are presented in the Fig. 3-4, and the parameters of the IAS model are shown in the Table 4.
Activities of the pure components (ai:  Al,
Al,  Sm) and molar fractions of the associates (xi:
Sm) and molar fractions of the associates (xi:  Al4Sm,
Al4Sm,  Al3Sm,
Al3Sm,  Al2Sm,
Al2Sm,  AlSm,
AlSm,  AlSm2) in the melts of the Al-Sm system at 1500 K, accordingly to the obtained IAS model, compared to estimation by Jin[1] (ai:
AlSm2) in the melts of the Al-Sm system at 1500 K, accordingly to the obtained IAS model, compared to estimation by Jin[1] (ai:  Al, Sm)
Al, Sm)
It is obvious from the Table 4 that the optimized values of the formation enthalpies of the Sm2Sn3 and SmSn3 compounds agree with the experimental data by Percheron et al.[29] For Sm5Sn3, our estimation of the formation enthalpy is more exothermic than obtained in Ref 29. This confirms the speculations by Bulanova et al.[20] that both melting temperatures and enthalpies of formation of the refractory compounds in the Sm-Sn system are underrated. We found the greatest exothermic effect of formation for the Sm5Sn4 compound, not being the most refractory in this system, but closer to the equiatomic composition. This compound stands close to the minimum of the mixing enthalpies of the melts, obtained by extrapolating of our experimental data.
The given parameters of the IAS model correspond to the certain temperature dependences of the thermochemical properties. For the partial mixing enthalpies at infinite dilution \( \left( {\Delta \overline{H}_{i}^{\infty } } \right) \) of the components of melts of the binary Al-Sm and Sm-Sn systems, they are presented in the Fig. 5. For the Sm-Sn system, there were many determinations of \( \Delta \overline{H}_{\text{Sm}}^{\infty } \) at low temperatures,[29,34-36] which did not agree with each other. Our thermodynamic model predicts rather significant temperature dependence of \( \Delta \overline{H}_{\text{Sm}}^{\infty } \) in the Sm-Sn melts. However, this dependence is not as strong as necessary to achieve −230...−250 kJ/mol when the temperature lowers to 700-800 K. Surely, the corresponding experimental data are not reliable enough, probably due to the kinetic effects caused by the low temperature comparing to the melting temperatures of most intermetallics in the Sm-Sn system.
Properties of the Ternary Al-Sm-Sn Melts
For calculating the thermodynamic functions of the ternary alloys from the analogous data for binary boundary subsystems, the latter ones are necessary to transform into the well-known Redlich-Kister polynomial type:\( \Delta H_{A - B} = x_{A} x_{B} \sum\limits_{i} {L_{i}^{AB} (x_{A} - x_{B} )^{i} } . \)
The coefficients of these polynomials are presented in the Table 5.
Then the Redlich-Kister-Muggianu model gives the following description for the mixing enthalpies of the ternary alloys:\( \begin{aligned} \Delta H_{A - B - C} = & \Delta H_{A - B} + \Delta H_{A - C} + \Delta H_{B - C} + x_{A} x_{B} x_{C} (L_{0}^{ABC} + L_{A}^{ABC} x_{A} + L_{B}^{ABC} x_{B} + \cdots ) \\ = & x_{A} x_{B} \sum\limits_{i} {L_{i}^{AB} (x_{A} - x_{B} )^{i} } + x_{A} x_{C} \sum\limits_{i} {L_{i}^{AC} (x_{A} - x_{C} )^{i} } + x_{B} x_{C} \sum\limits_{i} {L_{i}^{BC} (x_{B} - x_{C} )^{i} } \\ & \quad + x_{A} x_{B} x_{C} (L_{0}^{ABC} + L_{A}^{ABC} x_{A} + L_{B}^{ABC} x_{B} + \cdots ) \\ \end{aligned} \).
If the experimental information for the ternary alloys were absent, the \( L_{i}^{ABC} \) coefficients might be assumed as zero. However, in our case, this would give inaccurate values of the partial mixing enthalpies of the components. We found that the coefficients \( L_{0}^{\text{AlSmSn}} = 50,\,\;L_{\text{Al}}^{\text{AlSmSn}} = 60, \) and \( L_{\text{Sm}}^{\text{AlSmSn}} = - 3 60\;\left( {{\text{kJ}}/{\text{mol}}} \right) \) help to achieve agreement for the partial mixing enthalpies at infinite dilution of Al and Sn with our experimental values (Fig. 2). The modeling results are presented in the Fig. 6.
Discussion on the Results
Our results for the mixing enthalpies of melts of the Al-Sm system (Fig. 7) have an interstitial position between insufficiently negative[1,16] and excessively negative[19] data. The data by Saccone[6] are close to our results, though having less asymmetric concentration dependence. Surely, the main source of information for modeling by all these authors was the phase diagram, and this can explain the great divergence between their results. From our experience in modeling the thermodynamic properties of many similar systems (for example, Ref 46), even small inaccuracies in the liquidus temperatures can often lead to significant deviations in the calculated activities of components and mixing enthalpies. Thus, at the present time the only approach to correct those errors is confirming them with the direct experimental results on the mixing enthalpies, and actually this was done in our work. We hope that the accuracy of forecasting the thermodynamic properties will increase for the analogous systems in the future, due to the expansion of the corresponding databases.
Partial (a) and integral (b) mixing enthalpies of melts of the Al-Sm system: our experimental data—\( \Delta \overline{H}_{Al} \) at 1410 K ( ), 1640 K (
), 1640 K ( ) and \( \Delta \overline{H}_{Sm} \) at 1440 K (
) and \( \Delta \overline{H}_{Sm} \) at 1440 K ( ); approximated by IAS model at 1500 K (
); approximated by IAS model at 1500 K ( ); data from literature: Saccone,[6] (
); data from literature: Saccone,[6] ( ); Zhou,[18] (
); Zhou,[18] ( ); Jin,[1] 1500 K, MQM (
); Jin,[1] 1500 K, MQM ( ); Kulikova [19] IAS model (dubious) (
); Kulikova [19] IAS model (dubious) ( )
)
It is clear from the Fig. 8 that different modeling methods gave dissimilar results for the excess mixing entropies of melts of the Al-Sm system. They can be divided into two groups: (a) with small deviations from the ideal solutions, −5 to −7 J/(mol K);[1,18]—(b) with large deviations, −18 to −19 J/(mol K)—[6] and our model. Correspondingly, the data (a) have large negative values, and the data (b) have moderate values of the mixing Gibbs energies of the melts (Fig. 9). In our model, large negative mixing entropies correspond to the formation of the stable, strongly ordered associates. Unfortunately, mixing entropies are difficult to evaluate accurately from the experimental data (Fig. 8).
Figure 10 shows that the formation enthalpies of the intermetallics, calculated by us, are slightly less exothermic than obtained by Saccone[6] and Colinet.[15] Nevertheless, they agree with them, as well as with the results of modeling by Jin[1] and Zhou.[18] The greatest deviations are observed for AlSm2 and Al11Sm3. The compound AlSm2 melts at the lowest temperature and has a small area of equilibrium with the melt, so its contribution to the estimation of the liquidus curve of the phase diagram is insignificant. Jin et al.[1] proposed very small exothermic \( \Delta_{f} H \)(Al11Sm3) that corresponds to very steep liquidus curve in the area of equilibrium of this compound with the melt. We calculated the liquidus curve of the Al-Sm phase diagram in accordance to the obtained thermodynamic model based on IAS. It is clear that this curve matches the experimental points[2,5,6] no worse than the modeling,[1] including the area of the equilibrium of Al11Sm3 with the melt. Thus, the value of the formation enthalpy of this compound, equal to −23.9 kJ/mol of atom accordingly to Jin et al.,[1] is hardly possible.
The enthalpies of formation of the associates in the melts ( ) and the intermetallics (
) and the intermetallics ( ) in the solid alloys of the Al-Sm system at 298 K, compared to the data in literature— experimental □ Colinet [15] calorimetry of dissolution in liquid Al;
) in the solid alloys of the Al-Sm system at 298 K, compared to the data in literature— experimental □ Colinet [15] calorimetry of dissolution in liquid Al;  Borzone [16] direct calorimetry; estimations
Borzone [16] direct calorimetry; estimations  Zhou[7] (based on the experiment by Borzone[16]);
Zhou[7] (based on the experiment by Borzone[16]);  Kulikova [19]
Kulikova [19]  Jin [1]
Jin [1]
Moreover, too small negative values of the entropies of formation of the intermetallics in the Al-Sm system, −2···−6 J/(mol of atom K), and even a positive value for Al11Sm3, are proposed by Jin et al.[1] (Fig. 11). This correlates with small negative mixing entropies of the melts. But the equilibrium of the melts with the solid alloys (the liquidus curve) is described quite adequately in Ref 1. For the conclusion, we choose considerably more negative, −13 J/(mol of atom K), entropies of formation of the Al-Sm compounds. They are closer to the values obtained in Ref 6.
The calculated phase diagram (Fig. 12) is an ultimate confirmation of validity of our thermodynamic model. Some small discrepancies with the experimental data are observed for the solubility of aluminum in the high-temperature (BCC) modification of samarium (0.85 < xSm < 1). However, our model is suited firstly for dealing with melts, and our experimental data also refer to melts; so, this model describes solid phases less accurately. Moreover, the experimental data in this area are not determined distinctively.
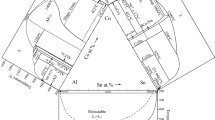
We calculated the liquidus curve of the Sm-Sn phase diagram (Fig. 13) using a model adjusted accordingly to Bulanova et al.[20] The latter data state that the melting temperatures of the most stable intermetallics are higher comparing to the results of the previous works. These higher temperatures correlate with the analogous parameters for other Ln-Sn systems, and they join the regularities in the fundamental lanthanide properties (mainly, the atomic radii). Apparently, a great part of the liquidus curve calculated by us agrees with experiments and predictions by Bulanova et al.[20] There are only two regions of disagreement: 0.45 < xSn < 0.6 (which is uncertain in Ref 20), and 0.9 < xSn < 1. These small inconsistencies do not contradict to the validity of the thermodynamic model obtained by us.
Liquidus curve of the phase diagram of the Sm-Sn system, calculated using our thermodynamic model (the bold curve), compared with the data[20]
As for the ternary Al-Sm-Sn alloys (Fig. 2 and 6), two of their sections—(Sm0.44Sn0.56)1−xAlx (0 < x<0.12) (1670 K) and (Al0.85Sm0.15)1−xSnx (0 < x<0.032) (1440 K)—confirm our expectations that the main part of interaction between the components relates to the binary subsystems. Adding the third component does not give significant energetic effects. The opposite case is observed for the third section—(Sm0.87Sn0.13)1−xAlx (0 < x<0.32) (1450 K). Here we see great negative (−100···−90 kJ/mol) partial mixing enthalpies of aluminum. They are explained by a great fraction of nearly free samarium in the Sm0.87Sn0.13 melt. However, it is difficult to understand the fact that these exothermic effects decrease so slowly with the addition of aluminum—even more slowly than it was observed for pure Sm (Fig. 7). This fact hindered the whole agreement of the mixing enthalpies of the ternary melts with the Redlich-Kister-Muggianu model (Fig. 6). So, this phenomenon is worth being clarified in the future—not surely for Al-Sm-Sn, but at least for some other analogous Al-Ln-Sn systems.
References
L. Jin, Y.-B. Kang, P. Chartrand, and C.D. Fuerst, Thermodynamic Evaluation and Optimization of Al-La, Al-Ce, Al-Pr, Al-Nd and Al-Sm Systems Using the Modified Quasichemical Model for Liquids, CALPHAD, 2011, 35, p 30-41
F. Casteels, The Aluminium-Rich Parts of the Aluminium-Samarium and Aluminium-Dysprosium Systems, J. Less-Common Met., 1967, 1967(12), p 210-220
K.H.J. Buschow and J.H.N. Van Vucht, The Aluminium-Rich Parts of the Al-Sm and Al-Dy Systems, J. Less-Common Met., 1967, 13, p 369-370
K.H.J. Buschow and J.H.N. Van Vucht, On the Intermediate Phases in the System Samarium-Aluminum, Philips Res. Rep., 1965, 20, p 15-22
V.I. Kononenko and S.V. Golubev, Phase Diagrams of Binary Systems of Aluminum with {La, Ce, Pr, Nd, Sm, Eu, Yb, Sc and Y}, Izvestiya Akademii Nauk SSSR, Metally, 1990, 2, p 197-199, in Russian
A. Saccone, G. Cacciamani, D. Maccio, G. Borzone, and R. Ferro, Contribution to the Study of the Alloys and Intermetallic Compounds of Aluminum with the Rare-Earth-Metals, Intermetallics, 1998, 6, p 201-215
S.H. Zhou and R.E. Napolitano, The Stability of Al11Sm3 (Al4Sm) Phases in the Al-Sm Binary System, Metall. Mater. Trans. A, 2007, 38, p 1145-1151
VASP package. http://cms.mpi.univie.ac.at/vasp/vasp/vasp.html
K.A. Gschneidner and L. Eyring, Eds., Handbook on the Physics and Chemistry of Rare Earths, vol 8, 1986, p 382
J.H. Wernick and S. Geller, Rare Earth Compounds with the MgCu2 Structure, Trans. Metall. Soc. AIME, 1960, 218, p 866-868
K.H.J. Buschow and A.S. Van der Goot, The Crystal Structure of Rare-Earth Aluminium Compounds R2Al, J. Less-Common Met., 1971, 24, p 117-120
S. Delsante, R. Raggio, G. Borzone, and R. Ferro, A Revision of the Al-Rich Region of the Sm-Al Phase Diagram: the Sm3Al11 Phase, J. Phase Equilib. Differ., 2007, 28, p 240-242
A.H. Gomes de Mesquita and K.H.J. Buschow, The Crystal Structure of So-called α-LaAl4 (La3Al11), Acta Cryst., 1967, 22, p 497-501
A. Pasturel, C. Chatillon-Colinet, A. Percheron-Guegan, and J.C. Achard, Thermodynamic Study of the Valence State of Ytterbium in YbAl2 and YbAl3 Compounds, J. Less-Common Met., 1983, 90, p 21-27
C. Colinet, A. Pasturel, and K.H.J. Buschow, Molar Enthalpies of Formation of LnAl2 Compounds, J. Chem. Thermodyn., 1985, 17, p 1133
G. Borzone, A.M. Cardinale, A. Saccone, and R. Ferro, Enthalpies of Formation of Solid Sm-Al Alloys, J. Alloys Compd., 1995, 220, p 122
B.R. Jia, L.B. Liu, D.Q. Yi, Z.P. Jin, and J.F. Nie, Thermodynamic Assessment of the Al-Mg-Sm System, J. Alloys Compd., 2008, 459, p 267-273
S.H. Zhou and R.E. Napolitano, Modeling of Thermodynamic Properties and Phase Equilibria for the Al-Sm Binary System, Metall. Mater. Trans. A, 2008, 39, p 502-512
T.V. Kulikova, A.V. Maiorova, V.A. Bykov, and K.Yu. Shunyaev, Thermodynamic Properties of Melts Based on the Al-Sm System, Russ. J. Phys. Chem. A, 2012, 86(8), p 1185-1188
M.V. Bulanova and V.R. Sidorko, Interaction of Rare-Earth Metals with Tin, Preprint Frantsevich IPMS NAS of Ukraine, Kiev, 1994, p 73, in Russian
A. Percheron, Etude du systeme etain-samarium, Colloq. Int. CNRS, 1970, N180(1), p 165-172
V.N. Yeremenko, M.V. Bulanova, and P.S. Martseniuk, Phase Diagrams of the Binary Systems of Rare-Earth Metals with Tin, Phase Equilibria, Structure and Properties of Alloys, Kiev: Naukova dumka, 1990, 2, p 106-109, in Russian
M.V. Bulanova and P.S. Martseniuk, Forecasting the Temperatures of Some Invariant Equilibria in the Systems of Rare-Earth Metals with p-Elements of the IV Group, Powder Metall. Met. C+, 1991, 5, p 82-85, in Russian
G. Borzone, A. Borsese, and R. Ferro, On the Alloying Behaviour of Cerium with Tin, J. Less-Common Met., 1982, 85(2), p 195-203
A. Iandelli and A. Palenzona, Sulla struttura dei composti intermetallici delle terre rare di formula MSn2, Atti Accad. Naz. Lincei, Rend. Fis., Mat. Nat., 1966, 40(4), p 623-628, in Italian
F. Weitzer, K. Hiebl, and P. Rogl, Crystal Chemistry and Magnetism of Neodymium Stannides Including Compounds of the Structural Series REnSn3n−2, J. Solid State Chem., 1992, 98, p 291-300
V.A. Lebedev, V.V. Yefremov, V.I. Kober et al., Thermodynamic Properties of the Alloys of Samarium with Aluminum, Gallium, Indium, Tin, Antimony, Lead, Bismuth: Alloys of Rare Metals with Particular Physico-Chemical Properties. Nauka, Moscow, 1975, p 96-99, in Russian
A. Palenzona, Dynamic Differential Calorimetry of Intermetallic Compounds. 1. Heats of Formation, Heat and Entropy of Fusion of Rare Earth-Tin Compounds, Thermochim. Acta, 1973, 5(4), p 473-480
A. Percheron, J.C. Mathieu, and F. Trombe, Mesure calorimetrique de la chaleur de dissolution du samarium dans l’etain. Determination de l’enthalpie de formation des composes definis du systeme etain-samarium, C.R. Acad. Sci., 1968, C266(12), p 848-851, in French
C. Colinet, A. Pasturel, A. Percheron-Guegan, and J.C. Achard, Experimental and Calculated Enthalpies of Formation of Rare Earth-Tin Alloys, J. Less-Common Met., 1984, 102(2), p 167-177
V.I. Kober, I.F. Nichkov, and S.P. Raspopin, Thermodynamic Properties of Liquid Alloys of Light Rare-Earth Metals with Fusible Metals, Thes. scient. reports V All-Union Conf. on the Structure and Properties of Metallic and Slag Melts. Part 2. Experimental Investigations of Liquid and Amorphous Metals. USC AS USSR, Sverdlovsk, 1983, p 334-336, in Russian
V.I. Kober, I.F. Nichkov, and S.P. Raspopin, Thermodynamic Characteristics of the Compounds of Samarium Rich in Fusible Metal, Izvestiya vuzov. Tsvetnaya Metall., 1987, 5, p 116-118, in Russian
V.I. Kober, I.F. Nichkov, and S.P. Raspopin, Thermodynamic Properties of the Alloys of Samarium with Fusible Metals, Theses of Reports: V Kolsky Seminar on the Electrochemistry of Rare and Non-ferrous Metals. Kolsky Filial of AS USSR, Apatity, 1986, p 51 (in Russian)
R. Boom, Heat of Solution of Metals in Liquid Tin, Scr. Metall., 1974, 8(11), p 1277-1281
R.F. Peluso and M.J. Pool, Proc. of the Fourth Conference on Rare Earth Research. L. Eyring, Ed., Inc. N-Y, 1965, p 269
J.N. Pratt and A.W.H. Morris, Heats of Solution of Some Rare-Earth Elements in Liquid Tin, J. Less-Common Met., 1966, 10(2), p 91-97
A. Berrada, Y. Claire, M.C. Idrissi, and R. Castanet, Calorimetric Investigation on the Pb-Sm and Sn-Sm Alloys, J. Alloys Compd., 1997, 260, p 193-195
M. Ivanov, V. Berezutski, and N. Usenko, Mixing Enthalpies in Liquid Alloys of Manganese with the Lanthanides, J. Mater. Res., 2011, 102(3), p 277-281
A.T. Dinsdale, SGTE Data for Pure Elements, Calphad, 1991, 15(4), p 319-427
M.A. Shevchenko, M.I. Ivanov, V.V. Berezutski, V.G. Kudin, and V.S. Sudavtsova, Thermodynamic Properties of Melts of Mn-Sc(Y, Ln) Systems, Russ. J. Phys. Chem. A, 2012, 86(12), p 1779-1784
N. Saunders and A.P. Miodownik, CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide, Pergamon, Oxford, NY, 1998
V.I. Berdnikov, Yu.A. Gudim, and M.I. Kartelyova, Thermodynamic Models of Regular and Ideal Associated Solutions, Steel Transl., 2009, 39(8), p 615
K. Wasai and K. Mukai, Application of the Ideal Associated Solution Model on Description of Thermodynamic Properties of Several Binary Liquid Alloys, J. Jpn. Inst. Met., 1981, 45(6), p 593, in Japanese
K. Wasai and K. Mukai, A Consideration of Thermodynamic Properties of Binary Liquid Alloys with Negative Deviation of Activities from Raoult’s Law Based on Associated Solution Model, J. Jpn. Inst. Met., 1982, 46(3), p 266, in Japanese
A.I. Zaitsev, M.A. Zemchenko, and B.M. Mogutnov, Thermodynamic Properties of (1 − x) Si + x Fe(I), J. Chem. Thermodyn., 1991, 23, p 831
M.I. Ivanov, V.V. Berezutski, M.O. Shevchenko, V.G. Kudin, and V.S. Sudavtsova, Thermodynamic Properties of Melts of the Al-Y(La, Eu, Yb) Systems, Rep. Acad. Sci. Ukr., 2011, 8, p 85-90, in Ukrainian
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shevchenko, M.O., Berezutski, V.V., Ivanov, M.I. et al. Thermodynamic Properties of Alloys of the Binary Al-Sm, Sm-Sn and Ternary Al-Sm-Sn Systems. J. Phase Equilib. Diffus. 36, 39–52 (2015). https://doi.org/10.1007/s11669-014-0353-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-014-0353-3








 Sm,
Sm,  Sn) and molar fractions of the associates (xi:
Sn) and molar fractions of the associates (xi:  Sm2Sn,
Sm2Sn,  SmSn,
SmSn,  SmSn3) in the melts of the Sm-Sn system at 1450 K, accordingly to the obtained IAS model
SmSn3) in the melts of the Sm-Sn system at 1450 K, accordingly to the obtained IAS model



 ), and the data from literature: Saccone,[
), and the data from literature: Saccone,[ ); Zhou,[
); Zhou,[ ); Jin[
); Jin[ )
)
 ), and the data from literature: Saccone,[
), and the data from literature: Saccone,[ ); Zhou,[
); Zhou,[ ); Jin [
); Jin [ )
)

 ) and the intermetallics (
) and the intermetallics ( ) in the solid alloys of the Al-Sm system at 298 K, compared to the estimations in literature:
) in the solid alloys of the Al-Sm system at 298 K, compared to the estimations in literature:  Zhou;[
Zhou;[ Kulikova;[
Kulikova;[ Jin[
Jin[
 [
[ [
[