Abstract
A chromium boride–chromium carbide–alumina composite coating was prepared in situ on a Ti-6Al-4V titanium alloy substrate by plasma spraying of Cr2O3-Al-B4C composite powder. The results show that, in the process of plasma spraying the Cr2O3-Al-B4C composite powder, a chemical reaction occurred among Cr2O3, Al, and B4C, and CrB2, Cr3C2, and γ-Al2O3 phases with nanosize were formed in situ in the composite coating. Compared with the coating prepared by plasma spraying CrB2-Cr3C2-Al2O3 composite powder, the coating prepared by the Cr2O3-Al-B4C composite powder has a more uniform and refined microstructure, lower porosity, higher microhardness, better toughness, excellent scratch resistance, and sliding wear resistance. This is attributed to the densification effect and to the fine grain strengthening and toughening effect of nanocrystals produced by the participation of the in situ chemical reactions in the plasma spraying process of the Cr2O3-Al-B4C composite powder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the 1950s, titanium alloys have been widely used as structural materials in machinery, aviation, shipbuilding, biomedicine, and other fields because of their high specific strength, toughness, and corrosion resistance (Ref 1). However, the application of titanium alloys is limited due to their low hardness and poor wear resistance. (Ref 2). In order to improve the surface hardness and wear resistance of titanium alloys, surface alloying and coating methods have been widely used, including, e.g., laser alloying (Ref 3, 4), nitriding (Ref 5), electroless plating (Ref 6, 7), laser cladding (Ref 8,9,10), magnetron sputtering (Ref 11), micro-arc oxidation (Ref 12, 13), etc. Although the above processes improve the surface hardness and wear resistance of the titanium alloy matrix to a certain extent, they also have the disadvantages of expensive equipment, long process cycle, and thin films. In addition to the above processes, the thermal spraying process is also the most widely used surface modification process. Among the thermal spraying processes, plasma spraying is often used in the surface modification of titanium alloys due to its advantages of simple operation, wide range of sprayable materials, high coating thickness, and controllability (Ref 14).
Some oxide and carbide ceramic systems have received more attention in the preparation of wear-resistant coating materials by plasma spraying, including Al2O3-TiO2 (Ref 15,16,17) and a hard cermet system, such as WC-Co (Ref 18), TiC-Co (Ref 19), and Cr3C2-NiCr (Ref 20, 21). However, with the change of service environment, the requirements for wear resistance of titanium alloys are getting higher and higher, but the improvement of wear resistance of the above coating material system is limited. Among the transition metal borides and carbides, CrB2 and Cr3C2 have the characteristics of high melting point, high hardness, excellent wear resistance, corrosion resistance, and high-temperature oxidation resistance, so they can be used as candidate materials for surface modification of titanium alloys (Ref 22, 23). For example, when 15 wt% CrB2 was added to a NiAl coating, the wear rate decreased by about 70 % compared with that of the pure NiAl coating (Ref 24). In previous studies, a CrB2-Cr3C2 binary composite ceramic coating has been prepared on a Ti-6Al-4V alloy substrate by plasma spraying, showing high hardness and wear resistance, but the porosity of the coating was high, and the mechanical properties of the coating needed to be further improved (Ref 25).
Thermite reaction is the reaction between thermite and metal oxide. The heat released by the reaction is high, and it has been widely used in the process of material preparation (Ref 26, 27). Some researchers have prepared composites with two main phases of chromium boride, chromium carbide, and alumina by reactive synthesis. For example, Yeh et al. (Ref 28) used Cr2O3, Al, and B powders as raw materials to obtain chromium boride–alumina composites by introducing a thermite reaction in the process of self-propagating combustion, indicating that B4C as the boron source can reduce the loss of B in the reaction process. Yeh et al. (Ref 29) prepared Cr3C2-Al2O3 composites by self-propagating high-temperature synthesis with Cr2O3, Al, and carbon black as raw materials, and found that the Cr7C3 second phase existed in the material due to a small amount of C loss. In addition, studies have shown that the introduction of Al2O3 can improve the strength and toughness of ceramic materials (Ref 30,31,32). In this work, we considered introducing a thermite reaction into the preparation of a CrB2-Cr3C2 composite ceramic coating, and introducing an Al2O3 phase in situ to improve the compactness and mechanical properties of the coating.
In this study, Cr2O3, Al, and B4C were used as raw materials. Cr2O3-Al-B4C composite powder was prepared by the spray drying method. Then, the chromium boride–chromium carbide–alumina composite ceramic coating was prepared on the Ti-6Al-4V alloy substrate by plasma spraying Cr2O3-Al-B4C composite powder. The phase composition, microstructure, microhardness, toughness, scratch resistance, and sliding wear resistance properties of the coating and the reaction mechanism of the Cr2O3-Al-B4C composite powder have been investigated. Meanwhile, a chromium boride–chromium carbide–alumina composite ceramic coating was prepared by plasma spraying CrB2-Cr3C2-Al2O3 composite powder. The microstructure and properties of the two chromium boride composite coatings have been compared and analyzed.
Materials and Methods
Preparation of Composite Coatings
Commercial CrB2 (1–5 μm, 99.5% purity), Cr3C2 (5–8 μm, 99.0% purity), Al2O3 (40 nm, 99.0% purity), Cr2O3 (1–5 μm, 99.9% purity), Al (6–9 μm 99.8% purity), and B4C (1–5 μm, 99.9% purity) powders were used as raw materials (see Fig. 1). The milled raw powders, deionized water, polymeric binder, and dispersant were mixed to form slurries, and then two composite powders of CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C were obtained by spray drying. The inlet and outlet temperatures of the spray drying were 250 and 120 °C, respectively. The mass ratio was 35.5:21.6:42.9 for CrB2/Cr3C2/Al2O3, and 63.9:13.4:22.7 for Cr2O3/Al/B4C. There were two sizes of the substrate material (Ti-6Al-4V titanium alloy, with high specific strength and comprehensive mechanical properties). One was Ф20 mm × 10 mm for the wear samples, the other was 10 mm × 10 mm × 12 mm for the other samples. Before spraying, the surface of the substrate was sandblasted and degreased to increase the surface roughness Ra to around 6–8 μm). Then, a bond coating of Ni–20wt%Al alloy was deposited on the pretreated substrate surface. Finally, the CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C composite powders were deposited on the bond coating as the top coating. The GP-80-type air plasma spraying equipment (China Jiujiang) was employed to produce the bond coating and top coating with thicknesses of 250 μm. The parameters of plasma spraying were: current 500 A; voltage: 70 V; primary gas (Ar) flow 150 dm3/min; secondary gas (H2) flow 30 dm3/min; spraying distance: 100 mm; powder feeding rate 20–30 g/min; and moving speed of spray gun 200 mm/s. After deposition, the as-sprayed samples were polished using 600-, 1000-, and 1500-grit SiC papers.
Scratch and Wear Test
The scratch resistance of the composite coatings was measured by a WS-2005 automatic scratch tester with an acoustic emission signal detection system (Lanzhou Zhongke Kaihua Technology Development). The scratches were linear and the normal load was progressively increased. The parameters were: indenter was loaded up to 60 N at the rate of 30 N/min, scratch speed was 1 mm/min, and scratch length was 2 mm. Dry sliding wear tests of the composite coatings were carried out by a SFT-2 M ball-on-disc friction and wear tester (Lanzhou Zhongke Kaihua Technology Development). Commercial bearing grade Si3N4 balls with a diameter of 4 mm were used as the counter surface specimen. The parameters were set as: the load was 30 N, the load time was 10 min, track radius was 3 mm, and the speed was 400 rpm. The measurement method was cyclic friction, and wear experiments were conducted on the polished coating surface (roughness Ra was about 1 μm). The wear rate was calculated according to following Eq 1:
where V is the wear volume (mm3), r is the track radius (m), n is the speed (rpm), t is the running time (min), and N is the load (N). The number of repeated wear tests was three.
Sample Characterization
An x-ray diffractometer (DMAX-2500; Rigaku) with Cu Kα radiation was used to identify the phase composition of the raw powders, composite powders, and coatings. Based on the SEM images of the powders, the particle size distribution of the powders was obtained by image analysis. The scanning mode of the diffractometer was continuous, the power was 6 kW, the scanning angle ranged from 10 to 80°, and the scanning rate of the powder and coating was 4°/min. A scanning electron microscope (FE-SEM; S-4800; Hitachi) equipped with the EDAX-AMETEK energy spectrometer (EDS) was used for microstructure analyses of the powders and the coatings. The microstructure of the coating obtained by plasma spraying of the Cr2O3-Al-B4C composite powder was characterized by transmission electron microscopy (TEM; Tec-nai F20; Philips). The thermal property of Cr2O3-Al-B4C composite powder was tested using a thermal analyzer (TG/DTA 6300; Seiko) in Ar gas at a heating rate of 10 °C/min up to 1300 °C. The porosity of the coatings were analyzed by 20 scanning images of polished cross-sections using image analysis. These images were taken at ×500 magnification. The microhardness of the coatings was measured using a microhardness tester (HMV-2; SHIMADZU). The indenter was a Vickers pyramid diamond, the load was 0.1 kgf, and the dwell time was 15 s. To reduce the error, 20 measurements were carried out to give an averaged hardness value. The indentation test was performed with a load of 0.3 kgf and a loading time of 15 s. The toughness of the coatings was analyzed by observing the indentation and fracture morphology under SEM.
Results and Discussion
Microstructure of the CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C Composite Powder
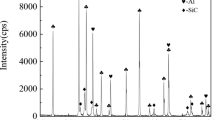
Figure 2(a) and (b) shows the XRD patterns of the CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C composite powders, respectively. It can be seen that the CrB2-Cr3C2-Al2O3 composite powder consists of CrB2, Cr3C2 and α-Al2O3, and that the Cr2O3-Al-B4C composite powder consists of Cr2O3, Al and B4C, which indicates that the phases of the composite powders were consistent with that of the raw powders after spray drying. The SEM micrographs of the CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C composite powders are shown in Fig. 3(a) and (b), respectively, and the spherical or nearly spherical morphology in these two composite powders can be seen. Figure 3(c) and (d) shows the EDS results of region A and region B, respectively, indicating that the two composite powders contain Cr, B, C, O and Al, which is consistent with the XRD results. The particle size distribution of the CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C composite powders are shown in Fig. 3(e) and (f), respectively, and their D50 values are 31 μm and 32 μm, respectively. Table 1 shows the parameters characterizing the fluidity of the two composite powders. The closer the ratio of the tap density to the apparent density is to 1, the better the fluidity. According to the literature (Ref 33), the spray-dried powder (apparent density > 1.7 × 103 kg/m3, D50 > 20 μm) can be directly used to prepare plasma-sprayed coatings without further densification. Therefore, the CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C composite powders used in this experiment meet the requirements of plasma spraying.
Microstructure of the Two Chromium Boride–Chromium Carbide–Alumina Composite Coatings
The coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder consists of CrB2, CrB, Cr3C2, α-Al2O3, γ-Al2O3 and a small amount of Cr2O3, as shown in Fig. 4(a). The XRD result indicated that many CrB2, Cr3C2 and α-Al2O3 were retained in the coating after plasma spraying, and, in addition, new phases CrB, γ-Al2O3 and Cr2O3 were also formed in the coating. A small amount of oxygen in the environment may be drawn into the plasma jet during plasma spraying, resulting in the oxidation of the raw powders. The formation of CrB may be caused by Eq. 2. B2O3 was not detected in Fig. 4(a), the possible reason being that B2O3 volatilized as a gas (Ref 34) or remained in the coating as an amorphous phase (Ref 35). The formation of Cr2O3 may be related to Eq. 2–5:
It has been reported that the phase transformation from α-Al2O3 to γ-Al2O3 can be attributed to the small critical nucleation free energy of γ-Al2O3 (Ref 36), thus molten Al2O3 was more likely to nucleate preferentially γ-Al2O3 instead of α-Al2O3 under plasma spraying conditions (metastable conditions of rapid cooling and solidification). Figure 4(b) shows the XRD pattern of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder. It can be seen from the figure that the coating consists of CrB2, CrB, Cr3C2, Cr7C3, γ-Al2O3 and a small amount of Cr2O3. Compared with Fig. 2(b), it can be seen that a chemical reaction occurred between Cr2O3, B4C and Al in the Cr2O3-Al-B4C composite powder during spraying. The specific reaction process is analyzed in detail in Section 3.3.
Figure 5(a) and (c) shows the cross-sectional SEM micrographs of the coatings prepared by plasma spraying the CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C composite powders, respectively. As shown in Fig. 5(a) and (c), there are large pores in the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder, compared with the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder, with the microstructure of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder being more compact, showing only a few small spherical pores. The porosity of the coatings prepared by plasma spraying the CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C composite powders are 8.7 ± 1.2 and 5.4 ± 0.7%, respectively, which also indicate that the latter had better compactness. Figure 5(b) is a partial enlarged view of Fig. 5(a), and the EDS results of the gray area A and the black area B are shown in Table 2. The results show that region A contained Cr, B and C elements. Combined with the XRD results, it is speculated that region A may be enriched in CrB2, CrB, and Cr3C2. Region B contains Al and O elements, and, combined with the XRD results, it is speculated that this region may be enriched in the Al2O3 phase. It can be seen from Fig. 5(b) that the microstructure of the coating presents a certain lamellar distribution, but it can also be seen that the gray structure is mostly distributed in blocks, showing a poor melting state. Compared with the borides and carbides of other transition metals (Ti and Zr), the borides and carbides of Cr have relatively low melting points, but higher specific heat capacity. The larger the specific heat capacity, the worse the thermal conductivity, and the powders are difficult to melt. In addition, although the temperature of the plasma jet is high (the temperature at the flame center can be as high as 10,000–15,000 K), the flame velocity is about 200 m/s, so the residence time of powder particles in the flame flow is very short (10−4 to 10−3 s), resulting in the insufficient melting (Ref 37, 38). The black structure was distributed in thin bands and filled in the gaps of the gray block structure, which improved the compactness of the coating to a certain extent, indicating that the melting degree of the nano-Al2O3 in the plasma jet was better. Although the melting point of Al2O3 is also high (2040 °C), compared with CrB2 and Cr3C2, the specific surface area of the nano-Al2O3 is larger, so the heating area of the powders is increased, which results in the improved melting degree. Figure 5(d) is a partial enlarged image of Fig. 5(c), form which it can be seen that the phases in the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder were distributed in lamellae, the microstructure distribution was more uniform and there were fewer microdefects. Figure 5(e) is a high-magnification micrograph of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder. The EDS results of areas C, D, and E in Fig. 5(e) are shown in Table 3. Combined with the XRD results, it is speculated that the dark gray area C is rich in CrB2 and Cr3C2, and that the black area D is rich in γ-Al2O3, and that the spherical particles (region E) are unreacted Cr2O3. It can be seen that region C is composed of particles with a fine and uniform structure. The phase interface of region C and region D is relatively pure, which may be due to the in situ reaction of the composite powder, so as to avoid the pollution of the powder particle surface such as oxidation in the traditional spraying process, reduce defects, and improve the compactness of the coating.
Figure 6 shows TEM images of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder. Figure 6(a) shows that the grain size range of the equiaxed grains is 50–100 nm, which can be speculated as CrB2 nanograins. Figure 6(b) shows that the grain size of the equiaxed grains is about 100 nm, and the diffraction pattern shows the CrB nanograins. The size of short rod-like grains shown in Fig. 6(c) is about 100 nm, and the diffraction pattern is calibrated as nano-γ-Al2O3. Figure 6(d) shows that equiaxed nanograins with size less than 50 nm are also dispersed in the coating. The above results show that the microstructure of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder contains many nanophases, and that the microstructure is obviously refined, which can play a key role in strengthening and toughening.
Reaction Mechanism of the Cr2O3-Al-B4C Composite Powder in the Plasma Spraying Process
By comparing the phase analysis results of the Cr2O3-Al-B4C composite powder and the composite coating, it can be seen that the Cr2O3-Al-B4C system composite powder had an obvious chemical reaction in the process of plasma spraying, and it is speculated that the Cr2O3, Al, and B4C raw powders may react during the plasma spraying process as:
The above reactions were thermodynamically analyzed according to the thermodynamic data manual (Ref 39, 40). The change trend of the Gibbs free energy of Eq. 6–9 with temperature is shown in Fig. 7(a). It can be seen that the Gibbs free energy of the four reactions is less than 0, indicating that the reaction can proceed spontaneously within a given temperature range. The molar enthalpy changes of Eq. 6–9 are shown in Fig. 8, indicating that the four reactions are exothermic. According to the research of Merzhanov et al. (Ref 41), the adiabatic temperature (Tad) of the reaction is not less than 1800 K, indicating that a self-propagating reaction can occur. The heat balance equation of the reaction in adiabatic condition is:
Equation 10 has been rewritten as Eq. 11, where Q represents the heat loss of the reaction system. When Q is equal to 0, it indicates that the heat generated by the reaction is all used to heat the products, the system is in an adiabatic state, and the corresponding temperature is the adiabatic temperature. Figure 7(b) shows the trend of Q with temperature, and Q6–Q9 are the fitting equations of the reaction waste heat, Q, with temperature for Eq. 6–9. If Q = 0, the adiabatic temperature Tad can be estimated. The calculation results are shown in Fig. 8, demonstrating that the adiabatic temperature of the four reactions is greater than 1800 K, indicating that the reactions can occur thermodynamically.
Figure 9 shows the DTA/TG curve of the Cr2O3-Al-B4C composite powder. It can be seen from the DTA curve that there is an endothermic peak near 658.6 °C. Considering that the melting point of Al is 660 °C, it is considered that the endothermic peak represents the melting of Al. When the temperature rose to about 1036.8 °C, an exothermic peak appeared in the DTA curve, indicating that an exothermic reaction occurred between the raw powders near this temperature. In the initial heating stage, due to the volatilization of the adhesive and water in the composite powder, the TG curve decreased slightly. With the continuous rise of temperature, there is a slight oxidation phenomenon in the composite powder. Therefore, the TG curve increased slightly, but the change degree of the curve was very small, indicating that the oxidation degree was very weak.
In order to further determine the phase transformation of the Cr2O3-Al-B4C composite powder during heating, according to DTA/TG curve, three heating temperatures of 700, 900, and 1100 °C were selected for heating, and then the phase analysis of the treated powder was carried out. The results are shown in Fig. 10. It can be seen from Fig. 10(a) that the phase of the composite powder treated at 700 °C is the same as that of the untreated composite powder, indicating that the components in the composite powder did not react below 700 °C. The XRD of the composite powder treated at 900 °C is shown in Fig. 10(b). At this time, new phases of α-Al2O3, Cr, CrC, and AlB10 appeared in the composite powder, indicating that the thermite reaction between Cr2O3 and Al began between 700 and 900 °C (Eq. (12)), and part of B4C was decomposed. The XRD of the composite powder treated at 1100 °C is shown in Fig. 10(c). At this time, the Al phase disappeared, indicating the end of the thermite reaction. In addition, the CrB, Cr3C2 , and Cr7C3 phases were formed, indicating that the Cr generated by the reaction may further react with B4C (see Eq. 13–16). The formation of Al5BO9 may be attributed to the reaction of B2O3 formed by the oxidation of B4C and Al2O3, as shown in Eq. 17:
In order to quantitatively analyze the contribution of the heat released from the thermite reaction to the melting of the compounds, the heat required for the melting of the products in Eq. 12 and the reactant in Eq. 13 was calculated, resulting in:
where nI is the stoichiometric number of the compound, Cp is the average molar heat capacity of the compound from the melting point to room temperature, Tmelt is the melting point of the compound, t0 is room temperature, and H is the phase transition enthalpy of the compound. The results show that the heat required to melt Cr2O3 and Al is 509.255 kJ, the heat required to melt Cr and B4C is 1597.05 kJ, the heat released by Eq. 12 is 545.594 kJ, and the heat released by Eq. 15 is 327.608 kJ. Therefore, the superposition of the heat released by reaction and the heat provided by plasma jet is enough to melt Cr2O3, Al, and B4C.
According to the results of thermodynamic analysis and thermal analysis, the reaction mechanism of Cr2O3-Al-B4C composite powder in the process of plasma spraying is speculated to be: firstly, compared with Cr2O3 and B4C, Al with a lower melting point first melted to form a melt, which wrapped Cr2O3 and B4C particles; secondly, Al melt reacted with Cr2O3 particles to form Cr and Al2O3, and Cr, Al2O3 , and B4C were melted by the heat of thermite reaction and plasma jet; then, Cr and B4C reacted to form chromium boride and chromium carbide phases.
Formation Mechanism of the Two Chromium Boride–Chromium Carbide–Alumina Composite Coatings
The observation of the deposition morphology of powder droplets during plasma spraying is beneficial to the understandng of the formation process of the coating. Figure 11(a) shows the SEM morphology of a single droplet after deposition of the CrB2-Cr3C2-Al2O3 composite powder on the substrate. The droplet-sputtering morphology is obviously disc-shaped. There are obvious finger protrusions at the edge of the droplet and a large void inside the droplet, and the droplet surface is uneven. Figure 11(b) shows an enlarged view of a local area in Fig. 11(a), from which it can be seen that there are more unmelted particles in the droplet. Figure 11(c) shows the spreading morphology of a single droplet obtained by plasma spraying the Cr2O3-Al-B4C composite powder. It can be seen that the droplet surface is relatively flat, there are almost no unmelted particles in the droplet, and there are no massive structures or obvious large defects in the droplet. Figure 11(d) is an enlarged view of a local area in Fig. 11(c), which shows that the droplet structure is uniform and dense. These results show that, compared with the CrB2-Cr3C2-Al2O3 composite powder, the Cr2O3-Al-B4C composite powder showed a better melting and flattening degree and a higher cooling rate in the plasma jet, which is conducive to the formation of a more dense nanocrystalline structure during the deposition process ofthe coating.
A schematic of the formation mechanism of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder is shown in Fig. 12(a). The formation process of the composite coating mainly includes two stages: melting and deposition of feedstocks. The specific process is as follows: the raw materials in the composite powders were mechanically combined under the action of the binder. After the composite powders were fed into the plasma jet, the nano-Al2O3 particles first melted to form the form of molten Al2O3 wrapping the CrB2 and Cr3C2 particles. In the subsequent heating process, some Cr3C2 and CrB2 particles began to melt, and the molten droplets wrapped unmelted particles. In the deposition process, under the carrier of the high-speed plasma jet, the melted droplets and unmelted raw powders impacted the substrate surface. Molten and semi-molten CrB2 and Cr3C2 were deposited on the substrate surface, and the irregular block structure in the coating was formed after cooling, while Al2O3 with good melting state impacted and spread around the CrB2 and Cr3C2 phases to form the lamellar structure in the coating. It can be seen that the microstructure of the coating obtained by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder was simple mechanical stacking, resulting in the high porosity of the composite coating.
Based on the analysis of the reaction mechanism and droplet spreading morphology of the Cr2O3-Al-B4C composite powder, the formation mechanism of the coating prepared by plasma spraying the powder is shown in Fig. 12(b). After the composite powders were fed into the plasma jet, the first stage was that the Al particles melted and wrapped outside the Cr2O3 and B4C particles, thus the contact area between raw materials was increased. In the second stage, with the continuous increase of temperature, a thermite reaction occurred between the molten Al and Cr2O3 to generate Cr and Al2O3. The generated Cr and Al2O3 were fully melted by the superposition of heat released from the thermite reaction and the heat of plasma jet, so the liquid environment for subsequent reactions was formed. In the third stage, Cr generated by the thermite reaction reacted with B4C. The large amount of heat released by the Cr2O3-Al-B4C composite powder in the reaction process can fully melt the generated CrB2, CrB, Cr3C2 , and Cr7C3. The fourth stage was the deposition process. The molten products were carried by the high-speed jet and impacted the substrate surface. A relatively dense composite coating was formed after cooling.
Properties of the Two Chromium Boride–Chromium Carbide–Alumina Composite Coatings
Microhardness
The microhardness is an important index to characterize the properties of ceramic coatings. The microhardness of the coatings prepared by plasma spraying the CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C composite powders are 1057 ± 138 HV0.1 and 1230 ± 115 HV0.1, respectively. As an effective method to evaluate the mechanical properties of materials (Ref 42), the Weibull distribution of hardness can objectively and truly reflect the relationship between the mechanical properties and the microstructure of coatings. The Weibull distribution curve of microhardness of two composite coatings is shown in Fig. 13. The expression of the fitting line in the figure is:
where the larger the m value, the lower the dispersion of the microhardness value and the more stable the performance. Compared with the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder, the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder exhibits higher average microhardness and less dispersion. This can be attributed to two reasons. One is that the porosity of the coating obtained by plasma spraying the Cr2O3-Al-B4C composite powder is lower, and the lower the porosity, the higher the microhardness (Ref 43). Due to the phases in the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder being formed by in situ reaction and the thermite reaction resulting in the higher melting degree of the composite powder in the plasma jet, the coating microstructure uniformity is good, and thus the dispersion of the microhardness value is lower. Secondly, there are many in situ-formed nanocrystalline structures in the coating obtained by plasma spraying the Cr2O3-Al-B4C composite powder, which play a key role in the fine grain strengthening.
Toughness
Vickers indentation is one of the important methods to characterize the toughness of coatings (Ref 44, 45). Figure 14 shows the indentation morphology of the two composite coatings under a load of 300 g. The SEM micrograph of the indentation morphology of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder is shown in Fig. 14(a), from which it can be seen that small cracks appeared in and around the indentation, and that there is no obvious warpage. The SEM micrograph of the indentation morphology of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder is shown in Fig. 14(b), from which it can be seen that the indentation is relatively complete, and that there are no obvious cracks inside and around the indentation. Compared with the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder, the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder shows a better toughness, which may be attributed to fewer defects such as pores and to the nanocrystalline structure in the Cr2O3-Al-B4C composite coating.
The fracture morphology of the composite coating can to a certain extent reflect the microstructure of the coating. At the same time, the fracture toughness can be judged by observing the fracture step and the propagation mode of the crack. The larger the fracture fluctuation, the more complex the crack propagation path. The more energy the material consumes in the fracture process, the higher the strength and toughness of the material. Figure 15 shows the fracture morphology of the composite coatings of the two systems, in which the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder is shown in Fig. 15(a), (c), and (e) and the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder is shown in Fig. 15(b), (d), and (f). Comparing Fig. 15(a) with (b), it can be seen that the fracture morphology of the coatings of the two systems shows obvious brittle fracture characteristics. The lamellar distribution of the microstructure in the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder is not obvious, and the microstructure is mainly in the bulk distribution, while the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder displays obvious a lamellar microstructure and the fracture fluctuates. A long crack is found in area A in Fig. 15(c), which started from the lamellar gap and passed through multiple lamellae during the propagation process, indicating that the coating had poor crack propagation resistance. As can be seen from Fig. 15(d), there are small cracks in area B, but the cracks terminated in a single layer and did not pass between the layers, showing that the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder had a good ability to resist crack propagation. From regions C and D, it can be found that there are many nanoparticles inside the lamellae, and that the microstructure is obviously refined. As can be seen from Fig. 15(e), there is no nanosized structure in the lamellae of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder. As shown in Fig. 15(f), in the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder, the microstructure formed in situ is uniform and dense, and the phase interface is pure, which can result in a higher interface bonding strength, which is conducive to improving the crack propagation energy. It is speculated that the good crack propagation resistance of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder may be attributed to the formation of a nanolamellar structure. Therefore, compared with the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder, the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder shows a better toughness.
Scratch Resistance
The scratch resistance of a coating refers to the ability of the coating surface to resist damage and deformation under a certain normal load. When the load reaches a critical state of coating cracking, cracks occur. At this time, a large strain energy is released inside the coating, and the resulting elastic fluctuation is collected by the acoustic emission equipment, showing a large strength on the signal curve (Ref 46). Figure 16 shows the acoustic emission signals detected by the composite coatings during the scratch process. As can be seen from Fig. 16(a), when the load was 19.9 N, a strong signal fluctuation was detected by acoustic emission, indicating that cracks had begun to appear on the surface of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder. With the increase of the load, the signal fluctuation gradually increased, indicating that the cracks on the coating surface continued to expand and increase. As can be seen from Fig. 16(b), when the load was increased to 40.5 N, a strong signal fluctuation was detected by acoustic emission, indicating that microcracks had begun to appear on the surface of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder at this time. With the increase of the load, the signal fluctuation was less, indicating that the number of cracks on the surface of coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder was less. Compared with the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder, the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder exhibited a greater critical load and better scratch resistance.
Figure 17(a) and (b) shows the morphology SEM micrographs of the scratch end of the two chromium boride–chromium carbide–alumina composite coating after the scratch tests, respectively. Compared with the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder, the scratch depth of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder is relatively shallow and the scratch width is narrow, indicating that the damage of the indenter to the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder was not obvious during the scratch process. Figure 17(c) shows a partial enlarged view of Fig. 17(a). It can be seen that the scratch surface structure is loose, the bonding strength of the phase interface is low, and the cracks are easy to propagate through the phase interface, so that many cracks are generated perpendicular to the scratch direction. Figure 17(d) is a partial enlarged view of Fig. 17(b), from which it can be seen that the scratch surface is still dense. Although cracks are also found, the number and length of the cracks are smaller than in Fig. 17(c). In addition, deflection (region A) and bifurcation (region B) of the cracks can be seen on the scratch surface. Combined with the acoustic emission curve of the composite coatings, the scratch resistance of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder is better than that of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder. The microstructure is the main factor affecting the scratch resistance of the coating. The higher the content of the hard phase in the coating, the denser the structure, the higher the hardness, and the better the scratch resistance of the coating (Ref 47, 48). Combined the cross-sectional morphology with the toughness analysis of the composite coating, the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder has excellent compactness, a more refined microstructure, and high hardness and toughness. Therefore, the scratch resistance of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder is better than that of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder.
Sliding Wear Resistance
The friction coefficient curve of two composite coatings is shown in Fig. 18. It can be seen that the variation trend of the friction coefficient of the two composite coatings first increases and then tends to be stable. The average friction coefficients of the coatings prepared by plasma spraying the CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C composite powders are 0.51 and 0.445, respectively. The coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder has high porosity, more unmelted particles, and more surface microdefects. The coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder has a more uniform and dense structure and relatively low porosity, so the coating surface is smoother and the coating is not easy to peel off during friction with the friction pair. Therefore, the friction coefficient of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder is higher than that of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder.
The wear scar of two composite coatings is shown in Fig. 19, from which it can be seenthat the wear scar of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder is shallower than that of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder. The wear rate of the coatings prepared by plasma spraying the CrB2-Cr3C2-Al2O3 and Cr2O3-Al-B4C composite powders calculated by Eq. 1 are 0.98 × 10−3 and 0.72 × 10−3 mm3/N m, respectively. It can be seen that the wear rate of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder is higher than that of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder because the porosity of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder is relatively high. There are many surface microdefects, and the bonding strength of each layer in the coating is low. In the process of friction, the friction stress can very easily exceed the bonding strength between the layers, resulting in easy peeling of the coating. Therefore, the overall wear of the coating increases. The coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder synthesized by the in situ reaction has a lower porosity, a more refined microstructure, and higher microhardness. Therefore, the wear performance of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder is better than that of the coating prepared by plasma spraying CrB2-Cr3C2-Al2O3 composite powder.
The wear scar morphology of two composite coatings is shown in Fig. 20. Comparing Fig. 20(a) and (b), for the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder, the width of the wear scar is relatively large and the wear scar surface is relatively rough, while the width of the wear scar of the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder is more small and the wear scar surface is more smooth. Figure 20(c) is an enlarged view of the middle area of the wear scar in Fig. 20(a), which shows that the wear scar surface has obvious smooth areas and rough areas, the smooth area being the deformation layer generated by the friction between the ceramic ball and the dense structure in the coating, and the rough area being the pores in the coating or the peeling pits caused by the wear (Ref 49). Figure 20(c') is an enlarged view of the edge area of the wear scar in Fig. 20(a). It can be seen that this area is mainly composed of loose particles, indicating that the coating is seriously worn. Figure 20(d) is an enlarged view of the local area in Fig. 20(b), from which it can be seen that the smooth area of the wear scar surface has obvious tissue deformation, but there is still no large lamellar spalling, while the peeling pit area of the rough area is small. Compared with the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder, the coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder does not have large lamellar spalling. The possible explanation is that the coating shows an obvious refined microstructure and high toughness. In the process of friction and wear, the coating has high deformation resistance, difficult crack propagation, and is not easy to fracture. Table 3 shows the data of microhardness, coefficient of friction, and wear rate of the composite coatings containing chromium boride or chromium carbide prepared by plasma spraying. It can be seen that, compared with the composite coatings reported in the literatures, the CrB2-Cr3C2-Al2O3 composite coating synthesized in situ by plasma spraying the Cr2O3-Al-B4C composite powder in this work shows a higher microhardness and a lower coefficient of friction and wear rate, displaying excellent wear resistance.
Conclusions
-
(1)
The coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder consisted of CrB2, CrB, Cr3C2, α-Al2O3 , and γ-Al2O3. The coating prepared by plasma spraying the Cr2O3-Al-B4C composite powder was composed of CrB2, CrB, Cr3C2, Cr7C3 , and γ-Al2O3. The results show that the composite coatings mainly composed of chromium boride and chromium carbide can be prepared in situ by plasma spraying Cr2O3-Al-B4C composite powder.
-
(2)
The coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder had many pores and an uneven microstructure. The microstructure of the composite coating prepared in situ by plasma spraying the Cr2O3-Al-B4C composite powder was obviously lamellar and refined, many nanosized chromium boride and chromium carbide phases were formed, the in situ-formed phases were closely bonded, and the porosity of the coating was low.
-
(3)
The formation mechanism of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder is a melting–deposition mechanism. The formation mechanism of the coating obtained by plasma spraying the Cr2O3-Al-B4C composite powder includes three stages: melting, reaction, and deposition. Al powders with a low melting point melted and wrapped the Cr2O3 and B4C particles. Then, an in situ reaction occurred which was divided into two steps. First, Al and Cr2O3 underwent a thermite reaction to form Cr and Al2O3 phases, and second, Cr and B4C reacted to form chromium boride and chromium carbide phases.
-
(4)
The average microhardness of the coating prepared by plasma spraying the CrB2-Cr3C2-Al2O3 composite powder was 1057 ± 138 HV0.1, the microhardness dispersion was large, the friction coefficient was 0.51, and the wear rate was 0.98 × 10−3 mm3/N m. The microhardness of the coating obtained by plasma spraying the Cr2O3-Al-B4C composite powder was 1230 ± 115 HV0.1, the hardness dispersion was small, the coefficient of friction was 0.445, and the wear rate was 0.72 × 10−3 mm3/N m. The coating obtained by plasma spraying the Cr2O3-Al-B4C composite powder exhibits obvious microstructure refinement, good toughness, scratch resistance, and sliding wear resistance.
References
L.C. Zhang, L.Y. Chen, and L.Q. Wang, Surface Modification of Titanium and Titanium Alloys: Technologies, Developments, and Future Interests, Adv. Eng. Mater., 2020, 22, p 1901258.
V.V. Stolyarov, L.S. Shuster, M.S. Migranov, R.Z. Valiev and Y.T. Zhu, Reduction of Friction Coefficient of Ultrafine-Grained CP Titanium, Mater. Sci. Eng. A., 2004, 371, p 313-317.
L.Y. Zhang, C.T. Peng, X.W. Yao, Q.F. Guan and R.F. Lu, Surface Alloying of Cr on Ti6Al4V Alloy Induced by High-Current Pulse Electron Beam, Surf. Coat. Technol., 2019, 370, p 288-297.
Q. Liu, Y. Liu, X. Li and G.N. Dong, Pulse Laser-Induced Cell-Like Texture on Surface of Titanium Alloy for Tribological Properties Improvement, Wear, 2021, 477, p 203784.
D. Tobola, J. Morgiel and L. Maj, TEM Analysis of Surface Layer of Ti-6Al-4V ELI Alloy After Slide Burnishing and Low-Temperature Gas Nitriding, Appl. Surf. Sci., 2020, 515, p 195942.
X.J. Cai, Y. Gao, F. Cai, L. Zhang and S.H. Zhang, Effects of Multi-Layer Structure on Microstructure, Wear and Erosion Performance of the Cr/CrN Films on Ti Alloy Substrate, Appl. Surf. Sci., 2019, 483, p 661-669.
A.M. Ma, D.X. Liu, C.B. Tang, X.H. Zhang and C.S. Liu, Influence of Glow Plasma Co-Based Alloying Layer on Sliding Wear and Fretting Wear Resistance of Titanium Alloy, Tribol. Int., 2018, 125, p 85-94.
T.G. Zhang, H.G. Zhuang, Q. Zhang, B. Yao and F. Yang, Influence of Y2O3 on the Microstructure and Tribological Properties of Ti Based Wear-Resistant Laser-Clad Layers on TC4 Alloy, Ceram. Int., 2020, 46, p 13711-13723.
F. Wang, H.J. Yu, C.Z. Chen, J.L. Liu, L.J. Zhao, J.J. Dai and Z.H. Zhao, Effect of Process Parameters on the Microstructure Evolution and Wear Property of the Laser Cladding Coatings on Ti-6Al-4V Alloy, J. Alloys Compd., 2017, 692, p 989-996.
Y.N. Liu, L.J. Yang, X.J. Yang, T.G. Zhang and R.L. Sun, Optimization of Microstructure and Properties of Composite Coatings by Laser Cladding on Titanium Alloy, Ceram. Int., 2021, 47, p 2230-2243.
J.W. Qi, Y.M. Yang, M.M. Zhou, Z.B. Chen and K. Chen, Effect of Transition Layer on the Performance of Hydroxyapatite/Titanium Nitride Coating developed on Ti-6Al-4V Alloy by Magnetron Sputtering, Ceram. Int., 2019, 45, p 4863-4869.
X. Yao, X. Zhang, H. Wu, L. Tian, Y. Ma and B. Tang, Microstructure and Antibacterial Properties of Cu-Doped TiO2 Coating on Titanium by Micro-Arc Oxidation, Appl. Surf. Sci., 2014, 292, p 944-947.
Q.Q. Guo, D.P. Xu, W. Yang, Y.C. Guo, Z. Yang, J.P. Li and P.H. Gao, Synthesis, Corrosion, and Wear Resistance of a Black Microarc Oxidation Coating on Pure Titanium, Surf. Coat. Technol., 2020, 386, p 125454.
R.B. Heimann, Plasma-Spray Coating, VCH Publishers, New York, NY, 1996.
Y. Wang, C.G. Li, W. Tian, and Y. Yang, Laser Surface Remelting of Plasma Sprayed Nanostructured Al2O3-13wt%TiO2 Coatings on Titanium Alloy, Appl. Surf. Sci., 2009, 225, p 8603-8610.
I.D. Utu, G. Marginean, I. Hulka, V.A. Serban and D. Cristea, Properties of the Thermally Sprayed Al2O3-TiO2 Coatings Deposited on Titanium Substrate, Int. J. Refract. Met. Hard Mater., 2015, 51, p 118-123.
L. Wang, S.Y. Liu, J.F. Gou, Q.W. Zhang, F.F. Zhou, Y. Wang and R.Q. Chu, Study on the Wear Resistance of Graphene Modified Nanostructured Al2O3/TiO2 Coatings, Appl. Surf. Sci., 2019, 492, p 272-279.
F. Ghadami, M. Heydarzadeh Sohi and S. Ghadami, Effect of TIG Surface Melting on Structure and Wear Properties of Air Plasma-Sprayed WC-Co Coatings, Surf. Coat. Technol., 2015, 261, p 108-113.
Y.X. Du, H.F. Chen, G. Yang, B. Liu, Y.F. Gao and H.J. Luo, Effect of Cobalt Content on High-Temperature Tribological Properties of TiC-Co Coatings, Ceram. Int., 2018, 44, p 14186-14194.
S. Matthews, Development of High Carbide Dissolution/Low Carbon Loss Cr3C2-NiCr Coatings by Shrouded Plasma Spraying, Surf. Coat. Technol., 2014, 258, p 886-900.
Q. Liu, Y. Bai, H.D. Wang, G.Z. Ma, M. Liu, C.Y. Chu, Y.W. Sun, W. Fan, F. Ding, B. Zhao and Y.T. Wang, Microstructural Evolution of Carbides and its Effect on Tribological Properties of SAPS or HVOF Sprayed NiCr-Cr3C2 Coatings, J. Alloys Compd., 2019, 803, p 730-741.
V. Reddy, J.K. Sonber, K. Sairam, T.S.R.C. Murthy, S. Kumar, G.V.S.N. Rao, T.S. Rao and J.K. Chakravartty, Densification and Mechanical Properties of CrB2-MoSi2 Based Novel Composites, Ceram. Int., 2015, 41, p 7611-7617.
K. Hirota, K. Mitani, M. Yoshinaka and O. Yamaguchi, Simultaneous Synthesis and Consolidation of Chromium Carbides (Cr3C2, Cr7C3 and Cr23C6) by Pulsed Electric-Current Pressure Sintering, Mater. Sci. Eng. A., 2005, 399, p 154-160.
O. Poliarus, J. Morgiel, P. Bobrowski, M. Szlezynger, O. Umanskyi, M. Ukrainets, L. Maj and O. Kostenko, Effect of Powder Preparation on the Microstructure and Wear of Plasma-Sprayed NiAl/CrB2 Composite Coatings, J. Therm. Spray Technol., 2019, 28, p 1039-1048.
Y.W. Wang, X.W. Sun, L. Wang, Y. Yang, X.X. Ren, Y.D. Ma, Y.H. Cui, W.W. Sun, X.Y. Wang and Y.C. Dong, Microstructure and Properties of CrB2-Cr3C2 Composite Coatings Prepared by Plasma Spraying, Surf. Coat. Technol., 2021, 425, p 127693.
J. Mei, R.D. Halldearn and P. Xiao, Mechanisms of the Aluminum-Iron Oxide Thermite Reaction, Scripta Mater., 1999, 41, p 541-548.
Y. Fang, X.H. Liu, Y.X. Feng, J.F. Zhu and W. Jiang, Microstructure and Mechanical Properties of Ti3(Al, Ga)C2/Al2O3 Composites Prepared by In Situ Reactive Hot Pressing, J. Adv. Ceram., 2020, 9(6), p 782-790.
C.L. Yeh and J.Z. Lin, Effects of Boron Source on Combustion Synthesis of Chromium Boride/Al2O3 Composites, Mater. Manuf. Process., 2013, 28, p 1335-1339.
C.L. Yeh and G.T. Liou, A Novel Route for Synthesis of Alumina-Chromium Carbide Composites from PTFE-Activated Cr2O3/Al/C Combustion, Ceram. Int., 2018, 44, p 19486-19491.
W. Zheng, J.M. Wu, S. Chen, C.S. Wang, C.L. Liu, S.B. Hua, K.B. Yu, J. Zhang, J.X. Zhang and Y.S. Shi, Influence of Al2O3 Content on Mechanical Properties of Silica-Based Ceramic Cores Prepared by Stereolithography, J. Adv. Ceram., 2021, 10(6), p 1381-1388.
X.Y. Wang, W. Yang, Y.X. Shao, Y. Yang, Z.L. Yang, Y.W. Wang, Y.H. Cui, Y.D. Ma, W.W. Sun and W. Li, Effect of Nano-Al2O3 on the Microstructure and Properties of NbB2-NbC Composite Coatings Prepared by Plasma Spraying, J. Am. Ceram. Soc., 2022, 105, p 712-727.
M. Dai, X.M. Song, C.C. Lin, Z.W. Liu, W. Zheng and Y. Zeng, Investigation of Microstructure Changes in Al2O3-YSZ Coatings and YSZ Coatings and Their Effect on Thermal Cycle Life, J. Adv. Ceram., 2022, 11(2), p 345-353.
X.Q. Cao, R. Vassen, S. Schwartz, W. Jungen, F. Tietz and D. Stöever, Spray-Drying of Ceramics for Plasma-Spray Coating, J. Eur. Ceram. Soc., 2000, 20, p 2433-2439.
A. Godavarty, A. Agarwal and N.B. Dahotre, Neural Networks in Studies on Oxidation Behavior of Laser Surface Engineered Composite Boride Coatings, Appl. Surf. Sci., 2000, 161, p 131-138.
J.K. Sonber, T.S.R.C. Murthy, C. Subramanian, S. Kumar, R.K. Fotedar and A.K. Suri, Investigation on Synthesis, Pressureless Sintering and Hot Pressing of Chromium Diboride, Int. J. Refract. Met. Hard Mater., 2009, 27, p 912-918.
Y.D. Chen, Y. Yang, Z.H. Chu, X.G. Chen, L. Wang, Z. Liu, Y.C. Dong, D.R. Yan, J.X. Zhang and Z.L. Kang, Microstructure and Properties of Al2O3-ZrO2 Composite Coatings Prepared by Air Plasma Spraying, Appl. Surf. Sci., 2018, 431, p 93-100.
J.R. Davis, Handbook of Thermal Spray Technology, ASM International, Materials Park, 2004.
H.M. Bian, Y. Yang, Y. Wang and W. Tian, Preparation of Nanostructured Alumina-Titania Composite Powders by Spray Drying, Heat Treatment and Plasma Treatment, Powder Technol., 2012, 219, p 257-263.
D.L. Ye and J.H. Hu, Handbook of Thermodynamic Data of Practical Inorganic Substances, 2nd ed. Metallurgical Industry Press, Beijing, 2002. ((in Chinese))
M. Binnewies and E. Milke, Thermochemical Data of Elements and Compounds, Wiley-VCH Verlag GmbH, Weinheim/New York, 2002.
A.G. Merzhanov, D.Y. Kovalev, V.M. Shkiro and V.I. Ponomarev, Equilibrium of Products of Self-Propagating High-Temperature Synthesis, Dokl. Phys. Chem., 2004, 394(4-6), p 34-38.
A. Nevarez-Rascon, A. Aguilar-Elguezabal and E. Orrantia, Compressive Strength, Hardness and Fracture Toughness of Al2O3 Whiskers Reinforced ZTA and ATZ Nanocomposites-Weibull Analysis, Int. J. Refract. Met. Hard Mater., 2011, 29(3), p 333-340.
R. Venkataraman, G. Das, S.R. Singh, L.C. Pathak, R.N. Ghosh, B. Venkataraman and R. Krishnamurthy, Study on Influence of Porosity, Pore Size, Spatial and Topological Distribution of Pores on Microhardness of as Plasma Sprayed Ceramic Coatings, Mater. Sci. Eng. A., 2007, 445-446, p 269-274.
B.R. Lawn, Physics of Fracture, J. Am. Ceram. Soc., 1983, 66, p 83-91.
J. Wan, M. Zhou, X.S. Yang, C.Y. Dai, Y. Zhang, W.G. Mao and C. Lu, Fracture Characteristics of Freestanding 8wt%Y2O3-ZrO2 Coatings by Single Edge Notched Beam and Vickers Indentation Tests, Mater. Sci. Eng. A., 2013, 581, p 140-144.
R. Jaworski, L. Pawlowski, F. Roudet, S. Kozerski and F. Petit, Characterization of Mechanical Properties of Suspension Plasma Sprayed TiO2 Coatings Using Scratch Test, Surf. Coat. Technol., 2008, 202(12), p 2644-2653.
Y. Bautista, J. Gonzalez, J. Gilabert, M.J. Ibanez and V. Sanz, Correlation Between the Wear Resistance, and the Scratch Resistance, for Nanocomposite Coatings, Prog. Org. Coat., 2011, 70(4), p 178-185.
M.S. Kabir, P. Munroe, Z.F. Zhou, and Z.H. Xie, Study of the Structure, Properties, Scratch Resistance and Deformation Behaviour of Graded Cr-CrN-Cr(1-x)AlxN coatings, Ceram. Int., 2018, 44, p 11364-11373.
H.T. Chi, L.T. Jiang, G.Q. Chen, P.C. Kang, X. Lin and G.H. Wu, Dry Sliding Friction and Wear Behavior of (TiB2+h-BN)/2024Al Composites, Mater. Design, 2015, 87, p 960-968.
Q. Liu, Y.B. Hu, N.J. Chong, Y. Bai, G.Z. Ma, Z.D. Li, M.Y. Bao, H. Zhan, R.J. Wang, Y.W. Sun, Y. Wang and Y.S. Ma, Quantitative Characterization of Carbide Loss and Correlation with Microstructure and Performance of Plasma-Sprayed NiCr-Cr3C2 Metal Carbide Coatings, J. Therm. Spray Technol., 2021, 30, p 457-470.
L. Lin, G.L. Li, H.D. Wang, J.J. Kang, Z.L. Xu and H.J. Wang, Structure and Wear Behavior of NiCr-Cr3C2 Coatings Sprayed by Supersonic Plasma Spraying and High Velocity Oxy-Fuel Technologies, Appl. Surf. Sci., 2015, 356, p 383-390.
D. Davis, M. Srivastava, M. Malathi, B.B. Panigrahi and S. Singh, Effect of Cr2AlC MAX Phase Addition on Strengthening of Ni-Mo-Al Alloy Coating on Piston Ring: Tribological and Twist-Fatigue Life Assessment, Appl. Surf. Sci., 2018, 49, p 295-303.
S. Natarajan, A.E. Edward, K.S. Akhilesh, A. Rajagopal and P.P. Nambiar, Effect of Graphite Addition on the Microstructure, Hardness and Abrasive Wear Behavior of Plasma Sprayed NiCrBSi Coatings, Mater. Chem. Phys., 2016, 175, p 100-106.
F.Y. Zhang, S. Yan, C. Li, Y. Ding, J.N. He, and F.X. Yin, Synthesis and Characterization of MAX phase Cr2AlC Based Composite Coatings by Plasma Spraying and Post Annealing, J. Eur. Ceram. Soc., 2019, 39, p 5132-5139.
S.M. Huang, D.Q. Sun, W.Q. Wang, and H.Y. Xu, Microstructures and Properties of In-Situ TiC Particles Reinforced Ni-Based Composite Coatings Prepared by Plasma Spray Welding, Ceram. Int., 2015, 41, p 12202-12210.
Acknowledgment
The authors gratefully acknowledge the financial supports of the National Natural Science Foundation of China (52072110), the Natural Science Foundation of Hebei Province (E2018202034).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Yw., Yang, Y., Li, H. et al. Microstructure and Properties of Chromium Boride–Chromium Carbide–Alumina Composite Coatings Prepared by Reactive Plasma Spraying. J Therm Spray Tech 32, 2262–2282 (2023). https://doi.org/10.1007/s11666-023-01636-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-023-01636-9