Abstract
Thermal-sprayed aluminum (Al) coatings are widely used for corrosion protection in marine environments. The corrosive nature of seawater and the synergy with marine microbes make for aggressive service environments for Al coatings. In this study, Al coatings were deposited on 316L stainless steel (316L SS) substrates by using wire arc spraying. Chlorella vulgaris, a typical marine fouling algae, was used to investigate the effects of biofilm adhesion on the electrochemical behavior of wire arc-sprayed Al coatings in artificial seawater environment. Electrochemical measurements via dynamic potential polarization curves and electrochemical impedance spectroscopy were used to monitor the corrosion behavior after attachment of microbes on Al coatings and bare, uncoated 316L SS. Analysis of the corrosion products was conducted using scanning electron microscope and energy-dispersive spectrum. The results showed that the Chlorella vulgaris biofilm slowed the corrosion of the Al coatings. This suggested that Chlorella vulgaris biofilm was a critical contributing factor to the corrosion behavior of the Al coatings. Moreover, a mechanism was suggested to illustrate the corrosion behavior of Al coatings in the presence of Chlorella vulgaris.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Corrosion is one of the most critical problems facing marine facilities and is considered to be the most important factor leading to thickness penetration, fatigue cracks, brittle fracture, and instability failure of offshore engineering equipment (Ref 1). Many thermal spray methods have been used to prevent corrosion in the marine environment (Ref 2,3,4,5). For example, Barik et al. showed that HVOF-sprayed nickel-aluminum bronze coating was a suitable material for corrosion resistance under aggressive marine conditions (Ref 3). In recent years, arc spray technology has been considered by many industries as the most suitable anti-corrosion method in the marine environment (Ref 2, 5, 6) due to its simple process, low operating cost and high efficiency. Among them, arc-sprayed Al coatings have high bond strength, low corrosion efficiency and long service life (Ref 6,7,8), which have been proven to possess a service life of up to 20 years in the marine environment (Ref 9).
On the other hand, biofouling is another problem for marine structures and facilities (Ref 10, 11). It should be pointed out that the presence of microorganisms (bacteria, algae, etc.) in the marine environment affects the corrosion process in a variety of ways due to the complexity of the marine environment: cathodic/anodic depolarization, aerobic organisms use oxygen to create a difference in oxygen concentration on metal surfaces, and production of corrosive metabolites such as organic acids and exopolymers (Ref 12). In most cases, the presence of microorganisms accelerates the corrosion process (Ref 13,14,15,16). For example, sulfate-reducing bacteria (SRB), iron-reducing bacteria (IRB), iron-oxidizing bacteria (IOB) and slime-forming bacteria can cause corrosion of steel (Ref 17). However, on the contrary, due to the type of microorganisms and the differences in the attached samples, there are reports in the literature indicating that the presence of microorganisms can impede the corrosion process and reduce the rate of corrosion. For example, Juzeliūnas et al. (Ref 18) found that under identical environmental conditions, Bacillus mycoides accelerated the corrosion of zinc sheets and reduced the corrosion rate of aluminum and showed no effect on the corrosion behavior of mild steel. Garcia et al. (Ref 19) reported that biofilms formed from bacterial strains isolated from the copper surface of potable water pipes and oxidation lagoons reduced the corrosion rate of copper surfaces. It is worth noting that, up to now, only very few studies have been reported on the effects of marine fouling microbial adhesion on the corrosion resistance of thermal spray coatings (Ref 11, 20,21,22). Abdoli et al. (Ref 11) reported the adhesion behavior of Bacillus sp. on thermal-sprayed Al coatings and its effect on their corrosion resistance. Results showed that the formation of Bacillus sp. biofilm accelerated corrosion of stainless steel, but enhanced the corrosion resistance of Al coatings. It is worth noting that due to the diversity of microorganisms in the marine environment, it is not sufficient to focus solely on the effects of bacteria on the corrosion performance of thermal-sprayed coatings. Algae are one of the main marine fouling organisms (Ref 23,24,25,26), unlike bacteria that can actively choose to colonize substrate surfaces, algae mostly passively land on the substrate due to lack of flagella (Ref 27). At the same time, algae will change the dissolved oxygen content of the attached surface through the process of photosynthesis, which will affect the corrosion process of the substrate (Ref 23). Liu et al. (Ref 28) found that Chlorella vulgaris cells in the biofilm could significantly increase the oxygen concentration in the biofilm, thus accelerating the corrosion process of 316L stainless steel (316L SS). Therefore, the microbial corrosion of thermal-sprayed coatings caused by algae cannot be ignored.
In this study, Al coatings were prepared on the 316L stainless steel substrates by arc spraying. The effect of Chlorella vulgaris on corrosion behavior of Al coating was studied. SEM was used to analyze the behaviors of adhesion and corrosion of Chlorella vulgaris. Polarization curve and EIS were used to study the effect of Chlorella vulgaris adhesion on the corrosion resistance of Al coatings. The equivalent circuit model of aluminum coating immersed in artificial seawater (ASW) with or without Chlorella vulgaris for 7 days and 30 days was fitted. Moreover, the effect of Chlorella vulgaris on the corrosion resistance of the arc-sprayed Al coatings was explained in a schematic way. It provides basic data to research the effect of marine fouling organisms on the corrosion performance of arc-sprayed Al coatings.
Materials and Methods
Sample Preparation
Aluminum wire (∅2 mm, TongBai Metal Tech, China) was used as the feedstock material for the coatings. 316L stainless steel (Ketebong Metal Materials, China) sheets with the size of 20 mm × 20 mm × 2 mm were used as the substrates. The substrates were degreased in acetone and grit blasted with 60 mesh (250 μm) Al2O3 (Jinying Abrasive Technology, China) before the spraying process. Al coatings with a thickness of ~ 150 μm were deposited on the substrate surfaces by arc spraying (AS, Eu Tronic Arc spray 4 HF, Germany). For the spraying, the current and voltage of the arc were set at 100 A and 30 V, respectively, and the spray distance was 140 mm. The compressed air with the pressure of 0.65 MPa was used for arc spraying. To facilitate subsequent electrochemical experiments, the coated and uncoated 316L SS samples were polished to the mirror surface and sealed with epoxy resin, leaving a bare surface of 10 mm × 10 mm.
Algae Cultivation and Inoculation
In this experiment, Chlorella vulgaris (obtained from Ningbo University, China), as typical marine algae, was selected. The ASW was prepared following ASTM D1141-98 (NaCl: 24.53 g/L, MgCl2: 5.20 g/L, Na2SO4: 4.09 g/L, CaCl2: 1.16 g/L, KCl: 0.695 g/L, NaHCO3: 0.201 g/L, KBr: 0.101 g/L, H3BO3: 0.027 g/L, SrCl2: 0.025 g/L, NaF: 0.003 g/L) and sterilized before use. Chlorella vulgaris was cultured in ASW containing Guillard’s (F/2) medium. The algae were incubated at room temperature (~ 22 °C) under a light/dark (12 h:12 h) cycle to simulate the natural conditions. Algae in logarithmic growth stage were selected for adhesion experiment. All reagents were purchased from Aladdin (Aladdin, China).
Algae Adhesion Experiment
Al coating samples (unpolished) and 316L SS (polished) were immersed separately in beakers containing the algae suspension. Each beaker contained 120 mL of suspension. The initial concentration of the algal solution was 5 × 106 mL−1. The beakers were placed in a shaking incubator (to avoid algal deposits). The temperature of the biological incubator was set at 22 °C, the rotation speed was 120 rpm, and the culture was conducted at 12 h:12 h under simulated diurnal conditions. The algae in the beaker were replaced with fresh algae solution every 3 days. At specific test intervals (7 and 30 days), the algae solution was removed by using a pipette gun, and the samples were soaked and washed three times with a sterile ASW to remove the algae that did not adhere to the surface of the material. In addition, the control group was set. Coated and uncoated 316L SS were immersed in aseptic ASW for 7 and 30 days before being taken out and dried.
Surface Analysis
After 7 and 30 days of attachment test, the Al coatings and 316L SS were taken out. To fix the algae, the samples were immersed in a 2.5% glutaraldehyde solution prepared by seawater and stored in a refrigerator at 4 °C for 4 h. Then, the samples were dehydrated through the critical point drying using 25% (5 min), 50% (5 min), 75% (5 min), 90% (5 min) and 100% (2 × 10 min) successively. Then, the samples were dried in a vacuum drying oven at 37 °C for 24 h. After pickling (soaked in 17% HNO3 solution for 1 min), the morphology of the coating was observed after removing the attached biofilm. The samples were finally sputter-coated with gold and observed by scanning electron microscope (FESEM, QUANTA 250 FEG, USA). The chemical composition of Chlorella vulgaris film on the Al coating surface for 30 days was analyzed by EDS. Three samples were taken from each group as parallel samples concurrently. The initial morphology of the Al coatings was observed for comparison. The roughness of coating and substrate was measured by white light confocal interference microscope (UP-Lambda, USA).
Electrochemical Measurements
Electrochemical tests were set up for eight group samples, as shown in Table 1. For the corrosion testing of the samples, potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) were acquired using a Solartron Modulab system (2100A, UK). The traditional three-electrode system was used, with a 1-cm2 platinum electrode as the counter electrode, a saturated calomel electrode as the reference electrode and the specimen with an exposed area of 1 cm2 as the working electrode. Potentiodynamic polarization curves were acquired with the potential range of − 500 to 500 mV versus Eocp at a scan rate of 0.5 mV s−1. EIS measurement was taken with a sinusoidal potential excitation signal with an amplitude of 10 mV and the frequency ranging from 100 kHz to 0.01 Hz. After the measurement, the polarization curves were fitted by Tafel method using Nova 2.1 software, and the EIS data were fitted and analyzed using ZSimpWin software based on equivalent circuit models.
Results and Discussion
Initial Coating Morphology
Figure 1 shows the surface deposition morphology of the arc-sprayed Al coating. Aluminum wires were melted into high-speed droplets under the synergistic action of arc and compressed airflow. The flying droplets become lamellas due to high-speed impact on the surface of the substrate, stacked and deposited on each other to form a lamellar structured coating (Ref 29, 30). Figure 1(b) is a magnified image of the selection of Fig. 1(a). Part of the droplets was sputtered around to form irregular lump and granular particles on the surface. Typical Al coating with compact structure and coarse surface is obtained by arc spraying. There are a small number of pores on the coating surface. The reason for the formation of such pores in the thermal spray layer is the peculiarity of the splat layer, which produces rough pores between the imperfect splat layers (Ref 31).
Surface Morphology After Chlorella vulgaris Adhesion Test
After 7 days of the Chlorella vulgaris attachment test, the 316L SS in algae-free (Fig. 2a-1) and algae-containing (Fig. 2a-2) environments was observed under SEM. Due to the short test time, only a few Chlorella vulgaris were observed on the substrate and there is no significant difference in the matrix in these two environments. The topography shows the existence of only a few microetching pits. Figure 2(a-3) shows the corrosion morphology of 316L SS after immersion in sterile ASW for 30 days. It can be seen that there are more corrosion pits on the surface of the sample. For the Al coating in the algae-free sterile ASW (Fig. 2b-1), a large number of corrosion pits appeared on the surface, which destroyed the original morphology of the coating. Direct observation of the Al coating after 30-day immersion in algae-contained ASW showed that the surface of the Al coating had been uniformly covered with a biofilm (Fig. 2b-2). The morphology after removal of the surface Chlorella vulgaris is shown in Fig. 2(b-3). The Al coating structure remained largely unaltered, and only some extremely small pits appeared.
(a-1) Surface morphology of the 316L SS after soaking in sterile ASW for 7 days and morphology of the substrate after removal of Chlorella vulgaris after (a-2) 7 days and (a-3) 30 days of experiment. After 30-day immersion, significant pitting occurred in 316L SS in sterile ASW without Chlorella vulgaris. (b-1) is the surface morphologies of the Al coating in sterile ASW without Chlorella vulgaris after 30 days. After 30-day immersion in ASW with Chlorella vulgaris (b-2), Chlorella vulgaris showed a significant adhesion on the surface of the Al coating to form a biofilm. (b-3) is the topography after removal of surface Chlorella vulgaris
Microbial adhesion experiments have demonstrated that the attachment of Chlorella vulgaris to the surface of the Al coating retards the formation of corrosion pits and reinforced the corrosion resistance. The first step in microbial influence on corrosion is the formation of biofilm, which can remarkably alter the surface physical and chemical characteristics of the material (Ref 32, 33). The elemental composition of the Al coating surface after 30 days of Chlorella vulgaris adhesion was obtained by EDS analysis (Table 2). The coating surface is mainly composed of C, O, Al and a few other elements. Chlorella vulgaris cells secrete extracellular polymeric substances (EPS) that comprised some organic macromolecules. It has been reported that Chlorella vulgaris biofilms have very high protein content and extracellular polysaccharides (Ref 34). The biofilm consists of Chlorella vulgaris, EPS and corrosion products (Ref 23, 35). The extracellular polymer secreted by Chlorella vulgaris acts as a binding material for the adhesion of Chlorella vulgaris to the biofilm matrix.
Roughness (Ra) is considered to be an essential factor affecting biofilm formation and characteristics (Ref 36,37,38,39). In this study, the roughness of the coating and substrate was about 10.42 and 0.18 μm, respectively. The cell size of Chlorella vulgaris was about 2 μm (Fig. 2b-2). Chlorella vulgaris initially adhered to the voids and cracks on the surface of the Al coating. As the experimental time prolonged, the Chlorella vulgaris gradually grew and multiplied, and the binding force between the individuals was enhanced by secreting the extracellular polymer, and finally a uniform biofilm structure was formed on the surface of the Al coating.
Electrochemical Polarization Curve Analysis
The corrosion behavior of the materials was monitored by electrochemical methods at different time periods. Figure 3 shows the potentiodynamic polarization curves of experimental samples placed with/without Chlorella vulgaris solution at different test times. Corrosion current (Icorr), corrosion potential (Ecorr), anodic (ba) and cathodic (bc) Tafel constants were obtained by Tafel analysis with Nova 2.1 software and are listed in Table 3. Chlorella vulgaris reduced the corrosion current of the 316LSS after 30 days of attachment (Table 3). The corrosion resistance of 316L SS after immersing in sterile ASW for different time is not much different (Fig. 3a). But for 316L SS in a Chlorella vulgaris-containing environment, longer immersion showed slightly better corrosion resistance. Chlorella vulgaris biofilm formation slows substrate corrosion process slightly. Figure 3(b) shows the Tafel curves of Al coatings. After 7 days of the test, the curves of the Al coating with/without Chlorella vulgaris are similar. The fitting data prove that the attachment of Chlorella vulgaris only increases the corrosion current slightly. The Al coatings after 30 days of immersion in Chlorella vulgaris-containing ASW exhibit higher corrosion potential and lower corrosion current than those in sterile ASW. In addition, compared with the coatings at 7 days, the Al coatings after 30 days of Chlorella vulgaris adhesion experiment significantly increased the corrosion potential and reduced the corrosion current.
Generally, the corrosion current determines the corrosion resistance and the corrosion potential is related to the corrosion tendency (Ref 11). An increase in the corrosion potential reduces the tendency of corrosion, and a decrease in the corrosion current reduces the corrosion rate. It can be seen that the corrosion resistance of the Al coating is slightly reduced after 7 days of attachment of the Chlorella vulgaris. After 30 days, the corrosion current of the Al coating was reduced by an order of magnitude, which proved that the corrosion resistance of the coating was greatly improved. The presence of Chlorella vulgaris biofilm reduces the corrosion rate of the Al coating and provides protective effects. The surface topography (Fig. 2) also supports this conclusion.
For the corrosion process, the corrosion rate is closely related to the rate of the cathodic reaction (such as oxygen reduction) and the anodic reaction (Ref 40). In the early stages of attachment, Chlorella vulgaris is primarily attached to the pores of the coating. Because of the short incubation time, the biofilm of Chlorella vulgaris forms oxygen concentration cells on the surface of the coating due to the difference in oxygen concentration between the area covered by the Chlorella vulgaris and the area exposed to the ASW, resulting in accelerated corrosion. After 30 days of the experiment, the Al coating surface was covered by a complete biofilm. The existence of Chlorella vulgaris biofilm filled the pores and cracks on the coating surface, forming a protective structure called “biofilm barrier,” which separated the Al coating from the diffusion of oxygen and slowed the corrosion of the Al coating. It is worth noting that the effect of Chlorella vulgaris attached to the 316L SS on corrosion resistance showed a similar trend as that of the Al coating, but the effect was much smaller. This phenomenon may be related to the fact that, with the increase in immersion time, the biofilm on the surface of the materials was gradually complete, and the effect of the initial surface morphology on the attachment of the organism was reduced. Meanwhile, the initial amount of Chlorella vulgaris attached to the Al coating surface was much larger than that of the 316L SS, which increased the corrosion resistance of the Al coating.
Nyquist Diagram Analysis
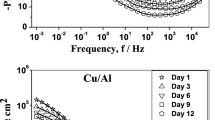
Nyquist plots of the Al coatings and the 316L SS under different experimental conditions were obtained by EIS. The higher the radius of the semi-circular arc, the better the corrosion resistance of the material (Ref 41). For the 316L SS substrate, there is no significant difference in its semi-circular arc in a sterile ASW environment (Fig. 4a). Likewise, the same tendency of Chlorella vulgaris to slow down corrosion is shown on the EIS diagram, indicating that the attachment of Chlorella vulgaris biofilm increases the corrosion resistance of 316L SS. Figure 4(b) shows that the Al coating after 7 days is similar in corrosion resistance in the presence or absence of Chlorella vulgaris. After incubation of the Al coating in ASW containing Chlorella vulgaris for 30 days, the diameter of the capacitive Nyquist semicircle of the Al coating is much higher than the coating in sterile ASW. This situation indicates that due to the formation of corrosion products and bio-adhesion, the charge transfer resistance was increased, and the corrosion resistance was significantly improved.
Interestingly, the capacitive Nyquist semicircle of the Al coating immersed in sterile ASW for 30 days also showed an increase in corrosion resistance relative to the 7-day coating. When the coating samples were immersed in ASW, electrolyte could penetrate through the coating and reach the 316L SS substrate surface which afforded a cathodic protection of the 316L SS substrate (Ref 42). Once the pores of the Al coating were covered with corrosion products, the corrosion rate will slow down (Ref 43). When aluminum dissolved to polarize the 316L SS substrate, alumina/hydroxide formed on the surface of the Al coating which passivated its surface. The formation of the deposition layer led to the decrease in current density over time. When sediment formed, they acted as a barrier protecting the coatings from further corrosion (Ref 44, 45).
Equivalent Circuit Analysis
Based on the EIS results and the electrochemical studies proposed by Szczygiel et al. (Ref 46), equivalent circuit models for the Al coating under different environmental conditions were proposed, as shown in Fig. 5. The equivalent circuit model considers the effects of various phenomena, such as an electric double layer, biofilm formation, layered coating structure and corrosion pits.
Schematic equivalent circuit models simulating the experimental impedance diagrams: (a) the model for the Al coatings soaked in the ASW with/without Chlorella vulgaris after 7 days and without Chlorella vulgaris after 30 days; (b) the model for the Al coatings soaked in the ASW in presence of Chlorella vulgaris after 30 days. Rs: solution resistance, Qb: capacitance of the biofilm, Rb: biofilm resistance, Qcoat: capacitance of the coatings, Rcoat: resistance of the coatings, Qdl: capacitance of the double layer, Rct: charge transfer resistance
For the Al coating after 7 days immersed in ASW with/without Chlorella vulgaris, the two samples had the same two-time constants, in accordance with the two-time constant model, R(Q[R(QR)]), as illustrated in Fig. 5(a). Because the algae incubation time is short, no significant biofilm was formed on the surface of the Al coating, and the surface state is similar to the Al coating in algae-free ASW, so it shows two-time constants. In this equivalent circuit, a constant phase element was used. In EIS experiments, the capacitors presented as a constant phase element (CPE, \(Z_{\text{CPE}} = \frac{1}{{Y_{0} \left( {j\omega } \right)^{n} }}\), where ZCPE is the impedance of the constant phase elements, ω is the angular frequency of alternating current voltage, Y0 and n are the frequency-independent parameters) (Ref 47). The presence of CPE can be explained by the dispersion effect caused by the microroughness of the surface (approximately 13.8 μm). On the surface of these samples there is an Al coating and a double-layer film. The electric double layer exists at the interface between the electrode and the surrounding electrolyte. The double layer is formed as ions from the solution adhere to the electrode surface.
After a further 23 days (i.e., 30th day), the Al coatings in these two environments show different electrochemical mechanisms. For the Al coatings that were not attached by Chlorella vulgaris, the two-time constant model, R(Q[R(QR)]), still applies. However, another time constant appeared on the surface in Chlorella vulgaris-contained environments. For the Al coatings in an algae-contained environment, apart from a passive film and the double layer, Chlorella vulgaris adhered for 30 days formed a continuous biofilm on the Al coating surface (Fig. 2b-2). The three-time constant model of the Al coating, in this case, is as follows: R(Q[R(Q[R(QR)])]). A complete biofilm covers the outermost side of the Al coating, which isolated the Al coating from external corrosive media, thereby improving corrosion resistance.
Mechanism of Chlorella vulgaris Affecting the Corrosion Behaviors of the Al Coating
Figure 6 shows the morphology of the Al coating surface after different application times of Chlorella vulgaris. For the initial aluminum coating, defects such as pores and cracks existed on the surface (Fig. 6a). The existence of these defects provides conditions for the initial attachment and corrosion of Chlorella vulgaris. Therefore, after the adhesion test was performed for 7 days (Fig. 6b), the adhesion of Chlorella vulgaris and corrosion products occurred in the pores and cracks on the Al coating surface. As the incubation time of Chlorella vulgaris increased, the surface of the Al coating was gradually covered with a complete biofilm. Electrochemical data showed that with the extension of the attachment time, the self-corrosion current of the Al coating was reduced and the polarization transfer resistance was increased, which proved that the corrosion resistance had been greatly improved. According to SEM image analysis, compared with the Al coating in an algae-free environment, the Al coating surface after 30-day immersion in Chlorella vulgaris-contained ASW was more complete, indicating that the existence of the biofilm played a protective role (Fig. 6c). The unique microenvironment consists of a Chlorella vulgaris biofilm and an Al coating, which filled holes and cracks on the coating surface, formed a protective structure called a “biofilm barrier,” and insulated the Al coating. This structure isolated the Al coating from external oxygen exchange channels and slowed down the corrosion of the Al coating.
Conclusions
In this study, 30-day adhesion test of Chlorella vulgaris was performed on the wire arc-sprayed Al coating and the 316L SS, respectively. The results of surface morphology and electrochemical tests indicated that Chlorella vulgaris biofilm gradually formed on the sample surface with the extension of culture time. The presence of Chlorella vulgaris biofilm significantly reduced the corrosion rate and increased the charge transfer resistance. The Chlorella vulgaris biofilm plays a barrier role, reducing the contact between the samples and the external environment, thus slowing down the corrosion. The results present here provides a new way of thinking about marine environmental protection.
References
C.G. Soares, Y. Garbatov, A. Zayed, and G. Wang, Influence of Environmental Factors on Corrosion of Ship Structures in Marine Atmosphere, Corros. Sci., 2009, 51(9), p 2014-2026
H.Q. Yang, Z.J. Yao, D.B. Wei, W.B. Zhou, G.X. Yin, and L.X. Feng, Anticorrosion of Thermal Sprayed Al-Zn-Si Coating in Simulated Marine Environments, Surf. Eng., 2014, 30(11), p 801-805
R.C. Barik, J.A. Wharton, R.J.K. Wood, K.S. Tan, and K.R. Stokes, Erosion and Erosion-Corrosion Performance of Cast and Thermally Sprayed Nickel-Aluminium Bronze, Wear, 2005, 259(1–6), p 230-242
A.A.M.M. Singh, P. Arul Franco, J.S. Binoj, S. Sahaya Elsi, and M. Divin Kumar, Evaluation of Corrosion Resistance and Characterization of Ni-Cr Coated Structure Using Plasma Spray Coating for Marine Hulls, Plasmonics, 2018, 14(3), p 595-610
Z. Glogović, V. Alar, Z. Kožuh, I. Stojanović, and S. Kralj, Corrosion Properties of Thermal Sprayed Aluminium (TSA) Coatings Deposited by Powder Flame Spraying, Materialwiss. Werkstofftech., 2011, 42(3), p 224-228
H.S. Lee, M.A. Ismail, and H.B. Choe, Arc Thermal Metal Spray for the Protection of Steel Structures: An Overview, Corros. Rev., 2015, 33(1–2), p 31-61
S. Paul, S. Shrestha, C.M. Lee, and M.D.F. Harvey, Thermally Sprayed Aluminum (TSA) Coatings for Extended Design Life of 22%Cr Duplex Stainless Steel in Marine Environments, J. Therm. Spray Techn., 2013, 22(2–3), p 328-336
W. Zhao, T. Zhang, R. Xin, M. Wang, H. Ai, J. Sun, and Y. Wang, Effects of Thermally Sprayed Aluminum Coating on the Corrosion Fatigue Behavior of X80 Steel in 3.5 wt% NaCl, J. Therm. Spray Techn., 2015, 24(6), p 974-983
F.S. Rogers, Thermal Spray for Commercial Shipbuilding, J. Therm. Spray Techn., 1997, 6(3), p 291-293
R. Piola, A.S.M. Ang, M. Leigh, and S.A. Wade, A Comparison of the Antifouling Performance of Air Plasma Spray (APS) Ceramic and High Velocity Oxygen Fuel (HVOF) Coatings for Use in Marine Hydraulic Applications, Biofouling, 2018, 34(5), p 479-491
L. Abdoli, X. Suo, and H. Li, Distinctive Colonization of Bacillus sp. Bacteria and the Influence of the Bacterial Biofilm on Electrochemical Behaviors of Aluminum Coatings, Colloid. Surface B., 2016, 145, p 688-694
S.E. Coetser and T.E. Cloete, Biofouling and Biocorrosion in Industrial Water Systems, Crit. Rev. Microbiol., 2005, 31(4), p 213-232
A.M. Olszewski, Avoidable MIC-related Failures, J. Fail. Anal. Prev., 2007, 7(4), p 239-246
T. Anzai, K. Nakano, K. Nishio, and K. Matsukawa, Biofilm Adhesion and Microbially Influenced Corrosion of SUS304 Welds Exposed to Dam Water, Weld. Int., 2006, 20(9), p 698-706
B. Cwalina, W. Dec, W. Simka, J. Michalska, and M. Jaworska-Kik, Biofilm Formation on NiTi Surface by Different Strains of Sulphate Reducing Bacteria (Desulfovibrio desulfuricans), Solid State Phenom., 2015, 227, p 302-305
H. Liu, D. Xu, A. Dao, G. Zhang, Y. Lv, and H. Liu, Study of Corrosion Behavior and Mechanism of Carbon Steel in the Presence of Chlorella vulgaris, Corros. Sci., 2015, 101, p 84-93
A. Abdolahi, E. Hamzah, Z. Ibrahim, and S. Hashim, Application of Environmentally-Friendly Coatings Toward Inhibiting the Microbially Influenced Corrosion (MIC) of Steel: A Review, Polym. Rev., 2014, 54(4), p 702-745
E. Juzeliunas, R. Ramanauskas, A. Lugauskas, K. Leinartas, M. Samulevicene, and A. Sudavicius, Influence of Wild Strain Bacillus mycoides on Metals: From Corrosion Acceleration to Environmentally Friendly Protection, Electrochim. Acta, 2006, 51(27), p 6085-6090
F. Garcia, A. Leonor Rivera Lopez, J. Campos Guillén, L. Hernández Sandoval, C. Regalado González, and V. Castaño, Corrosion Inhibition in Copper by Isolated Bacteria, Anti-Corros. Method. M., 2012, 59(1), p 10-17
X. He, Y. Liu, J. Huang, X. Chen, K. Ren, and H. Li, Adsorption of Alginate and Albumin on Aluminum Coatings Inhibits Adhesion of Escherichia coli and Enhances the Anti-Corrosion Performances of the Coatings, Appl. Surf. Sci., 2015, 332, p 89-96
X. He, Y. Liu, Y. Gong, C. Zhou, and H. Li, Autoclaving-Induced In-Situ Grown Alumina on Arc-sprayed Aluminum Coatings: Multiscaled Topography Facilitates Antifouling Performances, Surf. Coat. Techn., 2017, 309, p 295-300
L. Abdoli, J. Huang, and H. Li, Electrochemical Corrosion Behaviors of Aluminum-based Marine Coatings In the Presence of Escherichia coli Bacterial Biofilm, Mater. Chem. Phys., 2016, 173, p 62-69
J. Landoulsi, K.E. Cooksey, and V. Dupres, Review-Interactions between Diatoms and Stainless Steel: Focus on Biofouling and Biocorrosion, Biofouling, 2011, 27(10), p 1109-1124
Y. Mi, Z. Zhou, Z. Wang, G. Wang, and X. Zhang, Research Progress of Antifouling Coatings for Maritime Structures, Mater. Review, 2015, 29(2A), p 35-39
Q. Wang and S. Song, Progress in Marine Biologically Influenced Corrosion Study, J. Chin. Soc. Corros. Prot., 2002, 22(3), p 184-188
M. Wahl, Marine epibiosis. I. Fouling and Antifouling: Some Basic Aspects, Mar. Ecol. Prog. Ser., 1989, 58, p 175-189
S. Cao, J. Wang, H. Chen, and D. Chen, Progress of Marine Biofouling and Antifouling Technologies, Chin. Sci. Bull., 2010, 56(7), p 598-612
H. Liu, M. Sharma, J. Wang, Y.F. Cheng, and H. Liu, Microbiologically Influenced Corrosion of 316L Stainless Steel in the Presence of Chlorella vulgaris, Int. Biodeterior. Biodegrad., 2018, 129, p 209-216
Y. Chen, X. Liang, Y. Liu, and B. Xu, Development of Electric Arc Spray Forming Technology, J. Mater. Eng., 2010, 2, p 91-96
M.H.A. Malek, N.H. Saad, S.K. Abas, and N.M. Shah, Thermal Arc Spray Overview, IOP Conf. Series: Mater. Sci. Eng., 2013, 46, p 1-10
I.C. Park and S.J. Kim, Cavitation Damage Behavior in Seawater for Al-Mg Alloy Arc Thermal Spray Coating with Mg Content, Acta Phys. Pol., A, 2016, 129(4), p 572-577
F.M. AlAbbas, C. Williamson, S.M. Bhola, J.R. Spear, D.L. Olson, B. Mishra, and A.E. Kakpovbia, Influence of Sulfate Reducing Bacterial Biofilm on Corrosion Behavior of Low-Alloy, High-Strength Steel (API-5L X80), Int. Biodeterior. Biodegrad., 2013, 78, p 34-42
F.J. Passman, Microbial Contamination and Its Control in Fuels and Fuel Systems Since 1980-A Review, Int. Biodeterior. Biodegrad., 2013, 81, p 88-104
H. Liu, T. Gu, G. Zhang, Y. Cheng, H. Wang, and H. Liu, The Effect of Magneticfield on Biomineralization and Corrosion Behavior of Carbon Steel Induced by Iron-Oxidizing Bacteria, Corros. Sci., 2016, 102, p 93-102
R.P. George, P. Muraleedharan, N. Parvathavarthini, H.S. Khatak, and T.S. Rao, Microbiologically Influenced Corrosion of AISI, Type 304 Stainless Steels under Fresh Water Biofilms, Mater. Corros., 2000, 51(4), p 213-218
K. Pedersen, Biofilm Development on Stainless-Steel and PVC Surfaces in Drinking-Water, Water Res., 1990, 24(2), p 239-243
S.H. Flint, J.D. Brooks, and P.J. Bremer, Properties of the Stainless Steel Substrate, Influencing the Adhesion of Thermo-Resistant Streptococci, J. Food Eng., 2000, 43(4), p 235-242
N. George, M. Mahon, and A. McDonald, Bactericidal Performance of Flame-sprayed Nanostructured Titania-Copper Composite Coatings, J. Therm. Spray Techn, 2010, 19(5), p 1042-1053
Y. Cheng, G. Feng, and C.I. Moraru, Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review, Front. Microbiol., 2019, 10(4), p 1-16
D. Ornek, T.K. Wood, C.H. Hsu, and F. Mansfeld, Corrosion Control Using Regenerative Biofilms (CCURB) on Brass in Different Media, Corros. Sci., 2002, 44(10), p 2291-2302
A. Fattah-alhosseini and S. Vafaeian, Influence of Grain refinement on the Electrochemical Behavior of AISI, 430 Ferritic Stainless Steel in an Alkaline Solution, Appl. Surf. Sci., 2016, 360, p 921-928
B. Syrek-Gerstenkorn, S. Paul, and A.J. Davenport, Use of Thermally Sprayed Aluminium (TSA) Coatings to Protect Offshore Structures in Submerged and Splash Zones, Surf. Coat. Technol., 2019, 374, p 124-133
A. Lopez-Ortega, R. Bayon, and J.L. Arana, Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications, Materials, 2019, 12(8), p 1-23
R.G. Echaniz, S. Paul, and R. Thornton, Effect of Seawater Constituents on the Performance of Thermal Spray Aluminum in Marine Environments, Mater. Corros., 2019, 70(6), p 996-1004
A. López-Ortega, J.L. Arana, E. Rodríguez, and R. Bayón, Corrosion, Wear and Tribocorrosion Performance of a Thermally Sprayed Aluminum Coating Modified by Plasma Electrolytic Oxidation Technique for Offshore Submerged Components Protection, Corros. Sci., 2018, 143, p 258-280
B. Szczygiel and M. Kolodziej, Composite Ni/Al2O3 Coatings and Their Corrosion Resistance, Electrochim. Acta, 2005, 50(20), p 4188-4195
X. Sheng, Y.P. Ting, and S.O. Pehkonen, The Influence of Sulphate-Reducing Bacteria Biofilm on the Corrosion of Stainless Steel AISI, 316, Corros. Sci., 2007, 49(5), p 2159-2176
Acknowledgments
This study was supported by the Chinese Academy of Sciences President’s International Fellowship Initiative (Grant # 2020VEA0005), CAS-Iranian Vice Presidency for Science and Technology Joint Research Project (Grant # 174433KYSB20160085) and a Natural Sciences and Engineering Research Council of Canada Discovery Grant (Grant # NSERC RGPIN-2018-04298).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, H., Zhang, H., Chen, X. et al. Effect of Chlorella vulgaris Biofilm Adhesion on Electrochemical Behaviors of Wire Arc-Sprayed Aluminum Coatings. J Therm Spray Tech 29, 1991–2000 (2020). https://doi.org/10.1007/s11666-020-01113-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-020-01113-7