Abstract
The microstructures of three atmospheric plasma-sprayed (APS) Al2O3-ZrO2 coatings were investigated using x-ray diffraction, scanning electron microscopy, and transmission electron microscopy. The differences in the microstructures of the three Al2O3-ZrO2 coatings, including their phase compositions, cracks, pores, grain sizes, and solid solutions, were analyzed in detail. A close relationship was observed between the thermal conductivities of the coatings and the microstructures, and the Al2O3-YSZ coatings with more spherical pores, fewer vertical cracks, and finer grains exhibited the lowest thermal conductivity of 0.91 W/m·K. Compared with YSZ coatings, Al2O3-YSZ coatings can exhibit lower thermal conductivity, which may be attributed to the formation of an amorphous phase, smaller grains, and Al2O3-YSZ solid solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thermal barrier coatings (TBCs) have been widely used in advanced gas turbines to protect hot-section metal components from high temperatures, which improve the reliability and durability as well as efficiency of engines (Ref 1-3). TBCs typically consist of 6-8 wt.% yttria-stabilized zirconia (YSZ) deposited by atmospheric plasma spraying (APS) or electron beam physical vapor deposition (EB-PVD) because of its relatively high thermal expansion coefficient and low thermal conductivity (Ref 4-6). However, YSZ TBCs are approaching their durability and temperature limits because of the long-term accelerated sintering and phase transformations occurring above 1200 °C (Ref 7).
Currently, research in this field is increasingly focusing on the development of new TBC systems with higher temperature stability and lower thermal conductivity. Recently, several studies suggested Al2O3-YSZ coatings as potential candidates for TBC applications. In 2011, Tarasi et al. prepared Al2O3-YSZ coatings with good thermal stability at high temperature (as high as 1500 °C) and lower thermal conductivity (0.99 W/m·K at room temperature) (Ref 8). Fabrication, microstructures and mechanical properties of Al2O3-YSZ coatings were also investigated (Ref 9-11). Bo Liang et al. found that the tribological behaviors of the Al2O3-ZrO2 coatings prepared by APS were closely related to their physical and mechanical properties. For instance, a higher thermal conductivity of Al2O3-70 vol.% ZrO2 coatings can contribute to a better wear resistance by reducing the contact temperature and the thermal stress developed underneath the contact (Ref 9). J. Suffner et al. reported that the friction, wear properties, stiffness and bending strength of the as-sprayed materials of near-eutectic composition were superior to those obtained for non-stoichiometric ZrO2-Al2O3 coatings produced by APS plus subsequent quenching with liquid nitrogen-cooled substrates (Ref 10). However, the relationship between the microstructure and low thermal conductivity of Al2O3-YSZ coatings has rarely been reported.
In this study, Al2O3-YSZ coatings with various thermal conductivities were deposited using APS, and the effects of the amorphous phase content, grain size, and crack distribution on the thermal conductivity are discussed.
Experimental Procedure
Preparation of Feedstock and Coatings
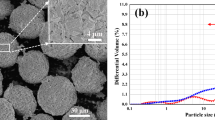
Nanosized Al2O3 (55 wt.%) and YSZ (45 wt.%, 8 mol.% yttria-stabilized zirconia) powders (Jiangsu Lida Hi-Tech Special Material Co. Ltd, China) were mixed by milling as raw materials. Scanning electron microscopy (SEM) images of the nano-Al2O3 powders with sizes <50 nm and nano-YSZ powders with sizes with sizes <50 nm are presented in Fig. 1(a), (b). To ensure a high flowability for the APS system, the nanopowders were agglomerated into microsized, spherical particles ranging from 10 to 70 μm in diameter by spray drying (as shown in Fig. 1c).
The Al2O3-YSZ coatings were fabricated using an A-2000 APS system equipped with a F4-MB plasma gun (Sulzer Metco AG, Switzerland) using three different sets of parameters, as listed in Table 1. The three spraying conditions were labeled A, B, and C.
Pieces of stainless steel (128 × 85 × 2 mm3) were used as the substrates. Before the APS process, the surfaces of the substrates were grit blasted to allow for the deposition of well-adhered coatings. The deposited coatings were approximately 1.5 mm in thickness.
Characterization of Feedstock and Corresponding Coatings
The phase compositions of the coatings were identified using x-ray diffraction (XRD) (D8 ADVANCE, Bruker, Germany) using Cu Kα radiation. The XRD measurements were performed in the 2θ range of 10°-80° at a scanning speed of 4°/min. The microstructure was also characterized using SEM (Magellan 400, FEI, USA) equipped with energy-dispersive x-ray spectroscopy (EDS) and electron backscattered diffraction (EBSD) (INCA SERIES, Oxford Instrument, UK) attachments and transmission electron microscopy (TEM) (JEM-2100F, JEOL, Japan).
Thermal-Conductivity Measurements
The deposited coatings were removed from the substrates to produce freestanding parts that could be used for thermal-conductivity tests. The freestanding coatings were then grinded and polished to a desired size for thermal diffusivity and specific heat capacity measurements. The thermal diffusivity (α) of the coatings was measured using the laser flash method, and the corresponding sample size was Φ10 mm × 0.9 mm. The specific heat capacity (Cp) of the coatings was measured using differential scanning calorimetry, and the corresponding sample size was Φ5.5 mm × 0.5 mm. Their respective thermal-conductivity (λ) values were then calculated from their density (ρ), as measured by the Archimedes method, using the following equation:
YSZ (8 mol.% yttria-stabilized zirconia) coatings were also prepared by APS using the parameters as shown in Table 1, and their thermal conductivities were measured to compare to those of APS Al2O3-YSZ coatings.
Results and Discussion
Microstructure of the Coatings
The phase compositions of the Al2O3-YSZ coatings deposited under the three different APS conditions were examined using XRD, and the results are presented in Fig. 2. A single broad halo peak corresponding to a fully amorphous phase was observed at 2θ = 20°-40° for coating A, which indicated that it is almost fully amorphous. In contrast, coatings B and C exhibited some diffraction peaks attributable to crystalline phases such as c-ZrO2 and α-Al2O3 in addition to an amorphous phase. However, there were more crystalline phases in coating C than in coating B, as indicated by the higher crystalline peak intensity in coating C. The formation mechanisms of the amorphous phase and crystalline phase in the Al2O3-YSZ coatings were explained in detail in our previous work (Ref 12). The differences in the phase composition among the three coatings were attributed to the different melting and mixing degrees of the in-flight particles and APS cooling rate in the different APS processes. Note that there was an obvious difference in the phase composition between coating A and coating B for the same APS parameters. The cooling methods can greatly affect the amorphous phase content. It is well known that the heat-transfer rate of a gas is faster than that of a liquid. Therefore, the higher cooling rate obtained for the compressed cooling substrate was more conducive to the formation of the amorphous phase in the preparation of coating A (compared to that of coating B).
Figure 3 presents backscattered electron SEM images of the polished cross sections of the as-sprayed Al2O3-YSZ coatings prepared using the three different APS conditions. The microstructure of the Al2O3-YSZ coatings consist of dense regions intermixed with pores, cracks, or unmelted particles. However, the three Al2O3-YSZ coatings exhibited different structural characteristics. Coating A contains many vertical cracks and some spherical pores (Fig. 3a and b). Coating B contains more horizontal cracks, more spherical pores, and fewer vertical cracks than coating A (Fig. 3c and d). In addition, some scattered grains were embedded in a dense matrix of coating B, which should contribute to the formation of crystalline phase peaks in XRD patterns of coating B. Compared with coatings A and B, a large number of partly melted or unmelted particles were observed in coating C (Fig. 3e and f). Formation mechanism of the different structural characteristics in Al2O3-YSZ coatings can be found in our previous works (Ref 12, 13).
TEM analysis was used to obtain more detailed information about the microstructure of the coatings, and the results are presented in Fig. 4, 5, and 6. In Fig. 4, the diffuse halo rings in the selected area electron diffraction (SAED) pattern indicate that coating A was mainly composed of an amorphous phase. Some amorphous phase was also observed in coating B, as indicated by Fig. 5(a), which was consistent with the broad halo peak spanning a 2θ range of 20°-40° in the XRD patterns of coating B in Fig. 2. Figure 5(f) reveals that some scattered grains with sizes of 500 nm-1 µm were embedded in the amorphous matrix of coating B, which may be the same as the scattered black and white grains observed in the SEM images of the cross section of coating B in Fig. 3(d). The formation of the grains may result from the growth of unmelted nano-Al2O3 and nano-YSZ grains. In addition, significant quantities of nanocrystals with irregular shapes and sizes <100 nm were observed in coating B, as illustrated in Fig. 5(b)-(e). For instance, the diffuse rings in the SAED pattern of Fig. 5(b) and the magnified view of Fig. 5(b) presented in Fig. 5(c) confirm that coating B contained a large amount of a polycrystalline nanostructure. The nanostructure could be the result of the recrystallization during rapid solidification, which could contribute to the formation of the crystalline phase peaks in the XRD patterns of coating B. However, in addition to some structures similar to those in the coating B, the TEM results in Fig. 6 indicated that a large number of partly melted or unmelted grains existed in coating C, which is consistent with the SEM results in Fig. 3(e) and (f). The regular diffraction spots in the SAED patterns in Fig. 6(b) and (e) indicate that the dark grains were cubic zirconia single crystals and the light grains were hexagonal alumina single crystals. Furthermore, the single crystals were not pure ZrO2 or Al2O3 but contained four chemical components of aluminum, zirconium, yttrium, and oxygen, as determined by EDS analysis and shown in Fig. 6(c) and (f), which may be attributed to the formation of Al2O3-YSZ solid solution. This solid solution has often been previously reported in Al2O3-ZrO2 coatings (Ref 14).
(a, d) Brightfield TEM images of a cross section from coating C; (b) SAED patterns corresponding to the c-ZrO2 (cubic YSZ) using the \(\left[ {\bar{1}\bar{1}2} \right]\) zone axis; (c) EDS profile of the dark grain (No. 1); (e) SAED patterns corresponding to the α-Al2O3 (hexagonal alumina) using the \(\left[ {10\bar{1}1} \right]\) zone axis; and (f) EDS profile for the bright grain (No. 2)
Thermal Conductivity of the Coatings
The room-temperature thermal-conductivity measurement results of the three Al2O3-YSZ coatings are presented in Fig. 7. Comparison of the results suggests that coating B exhibits the lowest thermal conductivity of 0.91 W/m·K among the three coatings. For comparison, YSZ coatings were prepared using the same nano-YSZ powders shown in Fig. 1(b) as raw materials using the APS parameters as shown in Table 1. The lowest thermal conductivity reached by the YSZ coatings prepared by the parameter of coating C was 1.17 W/m·K, as shown in Fig. 7, which is higher than that of coating B. Furthermore, the thermal conductivity of coating B is lower than that of Al2O3-YSZ coatings reported in the literature (Ref 8).
Microstructure of the YSZ coating is also characterized by SEM equipped with the EBSD, as shown in Fig. 8. Figure 8(a) shows the SEM image of polished cross section of YSZ coating. It is found that the microstructure of YSZ coating consists of dense regions intermixed with pores, cracks and unmelted particles. The EBSD result in Fig. 8(b) reveals that the dense regions are composed of submicron and micron-sized grains including columnar grains, equiaxed grains and large grains. Obviously, YSZ coating contains more pores and larger grains compared to coating B (Fig. 3c).
Relationship Between the Microstructure and Thermal Conductivity of the Coatings
To explain the differences among the thermal conductivities of the three Al2O3-YSZ coatings and between the Al2O3-YSZ coatings and YSZ coatings, the effect of the microstructure on the thermal conductivity of the Al2O3-YSZ coatings was analyzed in detail. As mentioned in section 3.1, the factors contributing to the different microstructures of the three Al2O3-YSZ coatings include the phase composition, cracks, pores, grain size, and solid solution.
-
(1)
Phase composition The Al2O3-YSZ coatings consisted of an amorphous phase and crystalline phase. However, the amorphous phase content in the three Al2O3-YSZ coatings differed, and the coating A was fully amorphous. The thermal conductivity of a crystalline solid results from changes of lattice vibrations which are usually described in terms of phonons. The expression for the thermal conductivity derived by Debye is (Ref 15)
$$\lambda = \frac{1}{3} l_{\text{p}} C_{\text{v}} v$$(2)where λ is the thermal conductivity, C v is the specific heat, v is the phonon velocity, and l p is the mean free path for scattering of phonons. Equation 2 reveals that the thermal conductivity values of the same material depend sensitively on l p. An amorphous material exhibits a structure with short-range order and long-range disorder. In terms of its heat conduction mechanism, an amorphous structure might be approximately regarded as a “crystal” composed of numerous, extremely fine grains with diameters of a few lattice spacings. The heat conduction mechanism of phonons can be used to describe the heat conduction behavior of an amorphous material. The relevant literature indicates that the maximum of l p corresponds to the diameter of the grain, which happens at low temperature, and the minimum of l p corresponds to a few lattice spacings taking place at high temperature (Ref 15). Intense phonon collision leads to smaller l p values with the increasing temperature. For normal crystals, the value of l p decreases gradually from the diameter of the grain at low temperature to a few lattice spacings at high temperature with the increasing temperature. For an amorphous material, regarded as a “crystal” with extremely fine grains, the value of l p at different temperatures is essentially constant (approximately a few lattice spacings). Therefore, the thermal conductivity of an amorphous phase at any temperature is lower than that of a crystalline phase for the same material. In theory, the thermal conductivity of the coating A should be the lowest of the three Al2O3-YSZ coatings; however, it is not, suggesting that other microstructural features have a significant effect on its thermal conductivity. The actual values of l p depend sensitively on the interface, defects, and impurities, which lead to strong phonon scattering. Stronger phonon scattering results in a smaller value of l p.
In addition, the amorphous phase may be the one reason for the thermal conductivity of coating B being lower than that of the YSZ coatings.
-
(2)
Pores and cracks Spherical pores and horizontal cracks (perpendicular to the direction of heat transfer) increase phonon scattering, leading to a decrease of thermal conductivity. However, vertical cracks (parallel to the direction of heat transfer) promote phonon heat transfer, leading to an increase of thermal conductivity. Hence, more spherical pores and fewer vertical cracks as shown in Fig. 3 result in coating B exhibiting a lower thermal conductivity than coating A with higher amorphous phase content.
However, compared with coating B, more pores in the YSZ coating might not be enough to make it exhibit lower thermal conductivity, suggesting that other microstructural features also have significant influences on thermal conductivity.
-
(3)
Grain size Grain boundaries are one of the important sources of phonon scattering. Smaller grain sizes result in larger grain densities, stronger phonon scattering, and lower thermal conductivity. According to the microstructural analysis in section 3.1, the crystalline phase in coating B is primarily composed of nanocrystals with irregular shapes and sizes <100 nm from the recrystallization, whereas the crystalline phase in coating C mainly consists of 500 nm−1 μm grains resulting from the growth of unmelted nano-Al2O3 and nano-YSZ grains. Therefore, it is apparent that the average grain size of coating C is the largest in the three Al2O3-YSZ coatings when their amorphous phase is regarded as a special “crystal” composed of extremely fine grains with diameters of a few lattice spacings, which explains why coating C exhibits the highest thermal conductivity. To further confirm this hypothesis, an Al2O3-YSZ ceramic was sintered at 1700 °C for 2 h using the same Al2O3-YSZ spray-dried powders shown in Fig. 1(b) as raw materials. Figure 9 presents an SEM image of the surface of the Al2O3-YSZ ceramic. The microstructure of the Al2O3-YSZ ceramic is similar to that of the partly melted or unmelted particles in coating C shown in Fig. 6(d). The average grain size of the Al2O3-YSZ ceramic is 2.6 μm and its thermal conductivity is 7.87 W/m·K, which are much higher than those of coating C.
In addition, smaller grain size may be another factor resulting in the reduction of thermal conductivity of coating B compared to that of the YSZ coating.
-
(4)
Solid solution According to the above analysis, an Al2O3-YSZ solid solution often appears in Al2O3-YSZ coatings. The formation of a solid solution is often accompanied by vacancies, solutes, and a local strain field, which can reduce l p (Ref 15). For example, a defect chemistry in Al2O3-YSZ coatings can be represented using the following equation:
$$2{\text{ZrO}}_{2} \mathop\rightarrow\limits^{\text{Al}_{2}\text{O}_{3}}2{\text{Al}}_{\text{Zr}}^{ '} + V_{\text{O}}^{ \bullet \bullet } + 3{\text{O}}_{\text{O}}^{ \times }$$(3)\({\text{Al}}_{\text{Zr}}^{ '},\) which represents an Al3+ cation that occupies a Zr4+ cation site, \({\text{V}}_{o}^{ \bullet \bullet }\) is a doubly charged (positive) oxygen vacancy, and \({\text{O}}_{\text{o}}^{ \times }\) is an O2− anion on an oxygen site (neutral charge). The strain field and vacancies can increase the discrete degree of phonons in the lattice when they interact with scattering phonons. The increase of the discrete degree provides more chances of scattering between phonons, which will further reduce the mean free path for the phonons. Therefore, the formation of the Al2O3-YSZ solid solution may be the third factor resulting in the reduction of the thermal conductivity of coating B compared to that of the YSZ coatings.
Conclusions
Three Al2O3-YSZ coatings with different microstructures were prepared using APS. The thermal conductivities of the coatings were closely related to their microstructures. The factors contributing to the different microstructures of the three Al2O3-YSZ coatings included the phase composition, grain size, and the presence of cracks, pores, and a solid solution. The amorphous phase content and presence of horizontal cracks, spherical pores, nanocrystals, and a solid solution caused the Al2O3-YSZ coatings to exhibit low thermal conductivity by reducing the mean free path for phonon scattering. In contrast, the presence of vertical cracks and larger grains increased the thermal conductivity of the coatings by increasing the mean free path for phonon scattering. Thus, the coating containing more spherical pores, fewer vertical cracks, and finer grains exhibited the lowest thermal conductivity of 0.91 W/m·K among the three Al2O3-YSZ coatings. The formation of an amorphous phase, smaller grains, and solid solution in the Al2O3-YSZ coatings may also have contributed to the reduced thermal conductivity of this coating compared to that of the YSZ coating.
References
R.A. Miller, Current Status of Thermal Barrier Coatings: An Overview, Surf. Coat. Technol., 1987, 30(1), p 1-11
N.P. Padture, M. Gell, and E.H. Jordan, Thermal Barrier Coatings for Gas-Turbine Engine Applications, Science, 2002, 296(5566), p 280-284
S.O. Chwa and A. Ohmori, Microstructures of ZrO2-8wt.%Y2O3 Coatings Prepared by a Plasma Laser Hybrid Spraying Technique, Surf. Coat. Technol., 2002, 153(2-3), p 304-312
W. Beele, G. Marijnissen, and A.V. Lieshout, The Evolution of Thermal Barrier Coatings Status and Upcoming Solutions for Today’s Key Issues, Surf. Coat. Technol., 1999, 121, p 61-67
D. Stöver, G. Pracht, H. Lehmann, M. Dietrich, J.E. Döring, and R. VaBsen, New Material Concepts for the Next Generation of Plasma-Sprayed Thermal Barrier Coatings, J. Therm. Spray Technol., 2004, 13(1), p 76-83
B. Saruhan, P. Francois, K. Fritscher, and U. Schulz, EB-PVD Processing of Pyrochlore-Structured La2Zr2O7-Based TBCs, Surf. Coat. Technol., 2004, 182(2-3), p 175-183
D. Basu, C. Funke, and R.W. Steinbrech, Effect of Heat Treatment on elastic properties of separated thermal barrier coatings, J. Mater. Res., 1999, 14(12), p 4643-4650
F. Tarasi, M. Medraj, A. Dolatabadi, J. Oberste-Berghaus, and C. Moreau, High-Temperature Performance of Alumina-Zirconia Composite Coatings Containing Amorphous Phases, Adv. Funct. Mater., 2011, 21(21), p 4143-4151
B. Liang, G. Zhang, H.L. Liao, C. Coddet, and C.X. Ding, Friction and Wear Behavior of ZrO2-Al2O3 Composite Coatings Deposited by Air Plasma Spraying: Correlation with Physical and Mechanical Properties, Surf. Coat. Technol., 2009, 203(20-21), p 3235-3242
J. Suffner, H. Sieger, H. Hahn, S. Dosta, I.G. Cano, J.M. Guilemany, P. Klimczyk, and L. Jaworska, Microstructure and Mechanical Properties of Near-Eutectic ZrO2-60 wt.% Al2O3 Produced by Quenched Plasma Spraying, Mater. Sci. Eng. A, 2009, 506(1-2), p 180-186
I. Zukerman, V.N. Zhitomirsky, G. Beit-Ya’akov, R.L. Boxman, A. Raveh, and S.K. Kim, Vacuum Arc Deposition of Al2O3-ZrO2 Coatings: Arc Behavior and Coating Characteristics, J. Mater. Sci., 2010, 45(23), p 6379-6388
X.M. Song, T. Suhonen, T. Varis, L.P. Huang, X.B. Zheng, and Y. Zeng, Fabrication and Characterization of Amorphous Alumina-Yttria-Stabilized Zirconia Coatings by Air Plasma Spraying, J. Therm. Spray Technol., 2014, 23(8), p 1302-1311
X.M. Song, T. Suhonen, C. Sun, L.P. Huang, X.B. Zheng, and Y. Zeng, Microstructures, Microhardness, and Crystallization Behaviors of Amorphous Al2O3-YSZ Coatings Prepared by Air Plasma Spraying, Surf. Rev. Lett., 2015, 22(3), p 1550047
A.L. Vasiliev, N.P. Padture, and X.Q. Ma, Coatings of Metastable Ceramics Deposited by Solution-Precursor Plasma Spray: I. Binary ZrO2-Al2O3 System, Acta Mater., 2006, 54(18), p 4913-4920
T.G. Xi, Thermophysical Properties of Inorganic Materials, Shanghai Science and Technology Press, Shanghai, 1981
Acknowledgments
Financial support for this study was provided by the Research Project Fund of the International Science & Technology Cooperation Project of China (2013DFG52290), Science & Technology Innovation Key Project of Shanghai Institute of Ceramics (Y37ZC4141G), Shanghai Technical Platform for Testing and Characterization on Inorganic Materials (14DZ2292900) and the CAS Key Technology Talent Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, X., Liu, Z., Kong, M. et al. Effect of Microstructure on the Thermal Conductivity of Plasma-Sprayed Al2O3-YSZ Coatings. J Therm Spray Tech 25, 770–777 (2016). https://doi.org/10.1007/s11666-016-0389-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-016-0389-5