Abstract
In this study, the impact of multistage solid solution treatment on the microstructure and properties of the Al-6.5Si-5.5Cu-0.2Zr-0.01Sr-0.06Ti-0.2Y alloy was examined using various experimental techniques such as optical microscopy, scanning electron microscopy, x-ray diffraction, transmission electron microscopy, and tensile testing. The findings demonstrated that with an increase in the multistage solid solution temperature and duration, the θ phase (stable Al2Cu) and intermetallic compounds gradually dissolved into the α-Al matrix, leading to a reduction in the residual phase content. The Si phase’s equivalent diameter decreased as the solid solution temperature increased, resulting in the spheroidization of eutectic Si, thereby contributing to dispersion strengthening. Consequently, the gradual rise in dislocation density and sample strength led to an enhancement in tensile strength. The G3 solid solution treatment (470 °C × 2 h + 480 °C × 2 h + 490 °C × 2 h + 500 °C × 2 h + 510 °C × 2 h + 520 °C × 14 h) demonstrated optimal mechanical properties with the ultimate tensile strength reaching 353.58 MPa and a fracture elongation rate of 9.25%. Moreover, this treatment exhibited superior corrosion resistance, as evident from the intergranular corrosion with a maximum depth of 69.36 μm, an electrochemical corrosion potential of − 1.1895 V, and a corrosion current density of 1.9965 × 10−7 A/cm2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the last two decades, the use of casting aluminum alloys has significantly expanded, correlating with an increased demand for improved performance. While numerous new materials have emerged, conventional aluminum alloys remain vital in aerospace and weapon industries due to their stable performance and cost-effectiveness, albeit requiring enhanced performance criteria (Ref 1,2,3,4). Al-Si-Cu casting alloys, renowned for their favorable casting properties, low thermal-expansion coefficient, high specific strength, and superior wear resistance, have found extensive applications in the automotive and aerospace sectors (Ref 5). Notably, in the automotive transmission system, the engine's most intricate component, the cylinder head, predominantly employs Al-Si-Cu casting aluminum alloys. However, these alloys exhibit subpar mechanical properties, notably low tensile strength, and limited elongation post-fracture, owing to coarse α-Al dendrites and flake eutectic Si, consequently restricting their wider applications (Ref 6, 7).
Solid solution treatment stands as a widely employed heat treatment process significantly enhancing alloy properties and microstructure (Ref 8,9,10). For Al-Si-Cu casting alloys, the multistage solid solution treatment, a primary heat treatment process, closely relates the solubility of alloying elements to the solid solution temperature during strengthening (Ref 11). This treatment produces a supersaturated solid solution, significantly boosting the alloy's strength and hardness (Ref 12). Additionally, it effectively dissolves Al2Cu phases and other second-phase metal compounds at the grain boundaries, ensuring a more uniform distribution of elements throughout the alloy (Ref 13). The eutectic Si undergoes spheroidization at angular edges, resulting in a uniformly distributed rounded Si phase along the grain boundaries, crucial for diffusion strengthening (Ref 14).
Multistage solid solution treatment facilitates complete dissolution of alloying elements and yields a finer grain structure. Solid solution temperature and holding time significantly determine the mechanical properties of casting aluminum alloy materials and form pivotal components of heat treatment process parameters (Ref 15).Compared with single-stage solid solution, multistage solid solution treatment has stronger ability to control the second-phase, and can control the diffusion rate of solid solution elements in the process, which is a more excellent and accurate method of solid solution treatment. In addition, the multistage solution treatment is also beneficial to improve the roundness of eutectic silicon and ensure good plasticity (Ref 16). Zhou et al. (Ref 17) investigated effect of various multistage solution treatments on the microstructure and properties of cold-extruded Al alloy. On this basis, the multistage solution treatments are further studied to make up for the effect of multistage (larger than 4) solution treatments on the properties of the Al alloy. In order to further establish the optimum multistage solid solution treatment on the Al-Si-Cu casting aluminum alloy, the maximum solution temperature was gradually increased and each increase was 10 °C to prevent the maximum solution temperature from being too high. To align with the developmental trajectory of cast aluminum alloys and lightweight automotive components, we have proposed three innovative multistage solid solution regimens, adjusting solid solution temperature and holding time. It is imperative to limit the solid solution treatment temperature to below the polyphase eutectic point to avoid the transitional liquid phase. By reasonably elevating the maximum solution temperature to 520 °C and holding it for 14 h while circumventing the transitional liquid phase, residual phases maximally dissolve into the α-Al matrix. This substantially heightens matrix supersaturation, effectively enhancing overall properties and microstructure of the alloy. Through extensive experimentation, we have identified the optimum multistage solid solution treatment for the Al-6.5Si-5.5Cu alloy, achieving tensile strengths exceeding 350 MPa and post-fracture elongation rates exceeding 9%.
2 Experimental Method
2.1 Material Preparation
The experimental trials employed an independently formulated casting aluminum alloy, denoted as Al-6.5Si-5.5Cu. The alloy's melting and heat treatment procedures were executed as follows: Initially, pure Al (99.97 wt.%) was combined with intermediate alloys comprising Al-Si (20 wt.%), Al-Cu (50.12 wt.%), and Al-Ti-B (5.11 wt.%), all introduced into a graphite crucible within an SG20 resistance melting furnace, set to 900 °C. Once melted, intermediate alloys of Al-Zr (4.11 wt.%), Al-Sr (9.89 wt.%), and Al-Y (10 wt.%) were added. Subsequently, the furnace temperature was reduced to 750 °C after a 3-h hold, followed by the introduction of a Mg block. The molten alloy was then maintained at a constant temperature of 750 °C for 15 min. Following this, the molten material underwent a resting phase and was degassed with C2Cl6 for two intervals of 15 min each. Finally, the alloy was poured into preheated molds set at 400 °C. The actual composition of the sample, as depicted in Table 1, revealed a composition of Al-6.5Si-5.5Cu-0.2Zr-0.01Sr-0.06Ti-0.2Y.
2.2 Material Characterization
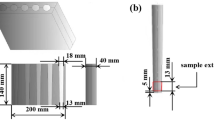
The samples underwent three distinct solid solution treatments, with parameters detailed in Table 2, followed by water cooling. Subsequently, the samples underwent an 18-h aging treatment at 191 °C. Tensile properties were evaluated at room temperature using the WDW-200G tensile tester, employing a tensile speed set at 1 mm/min. Five tensile specimens were taken from each sample, and the tensile samples are shown in Fig. 1. The tensile fracture morphology was analyzed through a JEOL-JSM-IT300-type scanning electron microscope (SEM). Additionally, to test the intergranular corrosion resistance, samples were subjected to the GB/T 7998-2005 standard procedure. The corrosion solution comprised 100 ml H2O + 10 ml H2O2 + 57 g NaCl, and samples were immersed for 6 h at 35 °C. Evaluation of the maximum intergranular corrosion depth was conducted using an optical microscope (OM, 4XC-MS). Qualitative analysis of the microstructure in the solid solution state was performed using the SEM. Quantitative analysis of the phases was executed via a Bruker AXS-D8 ADVANCE-type x-ray diffractometer (XRD), employing basic parameters: Cu-K rays with a wavelength of 0.15406 nm, continuous scanning range of 30-120°, scanning speed of 5°/min, and a data point spacing of 0.02°. After undergoing ion shear thinning, the microstructure of aged samples was observed using a JEM-F200 transmission electron microscope (TEM). Sample preparation involved mechanical grinding to 0.1 mm, followed by electrolytic double spraying and ion thinning.
3 Results and Discussion
3.1 SEM and EDS Analysis
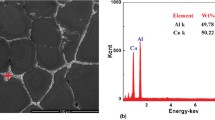
Figure 2 presents distinct morphological traits and Si phase distributions across G1, G2, and G3 samples. Within the figure, the gray areas denote the α-Al matrix, the small black particles represent eutectic Si, and white undissolved phases are evident in all three samples. Detailed observation revealed residual phases predominantly constituted by patchy gray–black bodies and needle-like gray–white structures. EDS qualitative analysis was conducted on these residual phases, specifically on point A with gray–white acicular features and point B with gray–black patches, as shown in Table 3. The primary constituents identified in the residual phases of the gray–white needle-like structures were Al and Cu, pinpointing the insoluble phase at point A as the θ phase (stable Al2Cu). Contrastingly, the gray–black patchy bodies were a composite of Al3Zr, Al3Ti, and Al-Si-Cu-Y four-dimensional compounds. The emergence of this Al-Si-Cu-Y compound was attributed to the large atomic radius and electronegativity of the rare-earth element Y, significantly restricting the degree of solid solution. Wang et al. (Ref 18) proposed an interaction strength model (characterizing the stability and solid solubility of compounds in alloys) between solvent element A and solute element B, which was described by Eq 1:
where R and N are atomic radius of atomic and electronegativity of element, respectively.
Table 4 presents the interaction values between the Y atom and the investigated alloying elements. Notably, the interactions of Y with Al, Si, and Cu exhibit higher reactivity, with corresponding values of 6.2236, 10.1408, and 8.3344, respectively. These findings indicate a pronounced propensity for Y to interact with these three elements, leading to the formation of a four-dimensional Al-Si-Cu-Y compound.
Furthermore, as depicted in Fig. 2, the eutectic Si phase in the G1 sample exhibits primarily angular edges with a substantial presence of undissolved phases between the grains, leading to segregation aggregation. Conversely, in the G2 sample, a moderate degree of spheroidization is observed at the edges of the eutectic Si phase, resulting in finer Si phases after dissolution and spheroidization. While the θ phase (stable Al2Cu) gradually dissolved into the α-Al matrix, there remained a considerable presence of θ phase (stable Al2Cu) and intermetallic compounds at the sample's grain boundaries due to insufficient solution temperature. In terms of the G3 sample, the eutectic Si phase was predominantly spheroidized into rounded dots, showing a sparse and dispersed distribution along the grain boundaries, with a minor portion migrating into the inner grain. Moreover, the θ phase (stable Al2Cu) and intermetallic compounds at the grain boundaries were effectively dissolved into the α-Al matrix. Figure 2(a) and (c) depicts the fragmentation and necking of Si particles, showcasing changes in Si particle morphology. This altered morphology contributed to improved sample properties. Despite coarse acicular silicon platelets acting as internal stress raiser in the microstructure and providing potential fracture paths, the modified Si fibers exhibited greater flexibility during solidification. This structural adaptation resulted in somewhat higher ultimate tensile strength and significantly increased ductility values for the alloy (Ref 19, 20).
Figure 3(a)-(c) depicts statistical diagrams representing the equivalent diameter of the Si phase. Observations revealed a gradual decrease in the Si phase's equivalent diameter with increase in solution temperature, indicating a trend of smaller Si phases post-dissolution and spheroidization. Figure 3(d) presents a line graph illustrating the Si equivalent diameter size alongside the area fraction of residual phases. The measured area fractions of residual phases for the three samples were 9.28%, 7.84%, and 6.62%, respectively. This indicated that with the rise in solution temperature, the θ phase (stable Al2Cu) and intermetallic compounds in the sample progressively dissolved into the α-Al matrix, consequently leading to a reduction in the number of residual phases. Furthermore, the decreasing equivalent diameter and the dispersing distribution of the Si phase also exhibited a correlation with the solution temperature increase.
3.2 XRD Analysis and Dislocation Strength
The XRD patterns and Full Width at Half Maximum (FWHM) of the samples post-solid solution treatment are presented in Fig. 4. The diffraction peak intensity of the θ phase (stable Al2Cu) decreased with the progressive increase in the solution order, consistent with the findings from SEM microstructure analysis. Figure 4(b)-(d) illustrates the half-peak widths of samples G1, G2, and G3, respectively. The FWHM of the G1 sample was lower compared to that of G2 and G3 samples, indicating a larger sub-crystal size in G1. Although the half-peak width of the G3 sample did not notably differ from that of G2, there was a trend of decreasing values, signifying a subsequent rise in solution temperature and an increase in the sub-crystal size of the G3 sample.
According to XRD analysis spectrum, the lattice strain (<e2>1/2) and dislocation density (ρ) can be calculated by Williamson–Hall model. The half-peak width (δ2θ), the angular position of the main highest diffraction peak (θ0), the coherent diffraction region size (d), and the XRD detection ray wavelength (λ) could be described by the following equation (Ref 21, 22):
The Origin software was used to process the data to obtain a linear relationship, as exhibited in Fig. 5. The d and < e2>1/2 values can be attained from the intercept (25 < e2>1/2) and slope (λ/d) of the fitted line, the dislocation density (ρ) can be calculated by this equation (Ref 23):
where the magnitude of Burgers vector b was 0.286 mm, and the relationship between the dislocation strength (σp) could be described according to the following equation (Ref 24):
where M, α and G represent the Taylor factor, numerical factor and shear modulus, and their values were 3.06, 0.24, and 26.9 GPa, respectively (Ref 25,26,27). The relevant dislocation parameters calculated in Eqs 2, 3 and 4 are listed in Table 5.
Table 5 illustrates that the dislocation density and dislocation strength values of the three samples progressively increase with the elevation of the solution temperature. Notably, the G1 sample exhibited the lowest dislocation density and dislocation strength owing to the limited solid solution of the θ phase (stable Al2Cu) in the α-Al matrix, resulting in a weaker pinning effect on dislocation movement. Additionally, the spheroidization degree of eutectic Si and the effect of dispersion strengthening at grain boundaries remained insufficient. Conversely, the G3 sample demonstrated the highest dislocation density and dislocation strength, increasing by 9.8% and 4.8%, respectively, compared to the G1 sample. This increase might be attributed to the comprehensive dissolution of θ phase (stable Al2Cu) into the α-Al matrix, enhancing dislocation resistance. Furthermore, the complete spheroidization of eutectic Si at the grain boundaries significantly contributed to dispersion strengthening. Consequently, the rise in solid solution temperature gradually augmented the dislocation strength of the sample due to the progressive dissolution of θ phase (stable Al2Cu) and intermetallic compounds into the α-Al matrix, accompanied by the gradual specialization and dispersion of eutectic Si, aligning with SEM analysis findings.
3.3 TEM Analysis
To delve deeper into the influence of Y on the aging behavior of Al-Si-Cu system alloys, the G3 sample exhibiting optimal properties was specifically chosen for investigation. Figure 6 presents the TEM image of the G3 sample, revealing various precipitated phases. Conventionally, the precipitation sequence in Al-Si-Cu alloys follows GP (Guinier–Preston) zone \(\to\) θ''\(\to\) θ'\(\to\) θ (Ref 28), where the GP zone represents the Cu Guinier–Preston phase, while θ'' and θ' denote substable phases, and \({\uptheta }\) indicates the equilibrium phase.
Figure 6(a) depicts the STEM morphology of the typical precipitated phase along the [110]Al direction in the G3 sample, illustrating the plate-like morphology of the precipitated phase θ' (substable Al2Cu) with an approximate size of 200 nm, uniformly distributed within grains. Such a distribution pattern facilitated maximum solution strengthening (Ref 29). Figure 6(b) showcases the HRTEM morphology of the GP zone and θ' phase (substable Al2Cu) along the [110]Al direction. These θ' phases (substable Al2Cu) exhibited a coherent orientation relationship with the α-Al matrix, aligning along the interface of \(\left( {110} \right)_{{\theta \prime }} ||\left\{ {110} \right\}_{{\alpha - Al}}\) and in parallel to its wide plane.
Figure 6(c) displays the STEM morphology of other intragranular precipitated phases within the G3 sample, revealing a SADP diffraction pattern characteristic of the precipitated phase, identified as Al8Cu4Y (Ref 30). This rare-earth phase assumed a tetragonal crystal system with a size of approximately 2 μm, representing brittle phases within the material, potentially impacting the alloy's plasticity negatively.
Furthermore, Fig. 6(d) illustrates the STEM morphology at the grain boundaries of the G3 sample, depicting no detectable precipitated phase generation. Consequently, this configuration enhances the corrosion resistance of the alloy. Figure 6(e) presents the STEM morphology at the interface between the Si phase and α-Al within the G3 sample, demonstrating a relatively smooth interface with no evident precipitated phase generation. Additionally, Fig. 6(f) delineates the HRTEM morphology of the Si phase along the [110]Al direction in the G3 sample. The orientation relationship between the Si phase and α-Al matrix lacked coherence with the interface of \(\left( {110} \right)_{{\theta \prime }} ||\left\{ {110} \right\}_{{\alpha - Al}} ,\) effectively enabling dislocation pinning. Post fast Fourier transform (FFT) transformation, the diffraction spots of the Si phase were visible.
Figure 7 primarily displays the Si phase area in the G3 sample for surface scan analysis. The STEM image reveals the presence of a stacking fault phenomenon on the Si phase surface. This occurrence was attributed to the Trace Precipitation and Refinement Effect (TPRE) mechanism activated by Sr and rare-earth (RE) elements within the sample. Consequently, this mechanism hindered the growth of the Si phase, effectively achieving the modification effect. The surface scan analysis results indicated that the precipitated phase identified in the figure is predominantly characterized as Al3M (M = Zr, Ti, Y) at the nanometer scale. These nano-level Al3M entities acted as dislocation inhibitors, contributing to precipitation strengthening, thereby enhancing the overall properties of the sample.
3.4 Electrical Conductivity
Figure 8 depicts the measured electrical conductivity of the sample. The observed changes in conductivity could be attributed to the interaction between mobile electrons and localized strain fields generated by solute atoms, impurities, grain boundaries, and dislocations, which acted as scattering centers for the electrons (Ref 31). Guyot et al. (Ref 32) associated the increase in electrical conductivity with the coarsening and growth of precipitates. Similarly, findings by Liu et al. (Ref 33) suggested that the enhancement in electrical conductivity is facilitated by an increase in the volume fraction of precipitates. It was widely acknowledged that the introduction of lattice distortions due to the dissolution of foreign atoms, crystal dislocations, and thermal vibrations results in elevated electrical resistivity and decreased conductivity (Ref 34).
Within the framework of Matthiessen's rule (Ref 35), the total resistivity can be segmented into several components:
where \(\rho_{0}\) is the lattice resistivity of the Al matrix; \(\rho_{{{\text{gb}}}}\) is the grain boundary resistivity (Ref 36); \(\rho_{{\text{s}}}\) is the resistivity caused by solute atoms dissolving into the matrix; \(\rho_{{\text{P}}}\) is the resistivity due to precipitated phase; \(\rho_{{\text{d}}}\) is the resistivity caused by dislocation; and \(\rho_{{\text{V}}}\) is the resistivity due to vacancy (Ref 37).
Given the same alloy composition and grain structure, the lattice resistivity of the Al matrix remained constant, while other types of resistivity were influenced by solid solution. E.V. Bobrun’s study (Ref 38) highlighted that refining the grain to an ultra-fine scale can enhance the alloy's strength without compromising electrical conductivity, suggesting the negligible impact of grain boundary resistivity. As the three samples underwent identical aging treatment, the resistivity attributed to precipitates remained essentially uniform. Relative to \(\rho_{{\text{V}}}\), resistivity from \(\rho_{{\text{s}}}\) and \(\rho_{{\text{d}}}\) more effectively influences electron scattering (Ref 39).
Following Matthiessen's rule analysis, \(\rho_{{\text{s}}}\) gradually diminished due to increased solute atom content within the matrix, while \(\rho_{{\text{d}}}\) decreased gradually owing to heightened dislocation density and strength, thereby increasing resistance to electron transport between grains. Consequently, total resistivity gradually decreased. Figure 8 indicates that the conductivity of the G1 sample is the highest at 39.8%, while that of the G2 and G3 samples decreases to 38.1% IACS and 36.4% IACS, respectively. It was evident that the conductivity of the samples progressively declines with rising solid solution temperature, corroborating the findings derived from Matthiessen's rule analysis.
3.5 Tensile Properties and Fracture Morphology
The tensile properties of the samples are assessed and depicted in Fig. 9 by the stress-strain curves. Table 6 presents the tensile strength and elongation after fracture, while Fig. 10 illustrates the morphological characteristics of the tensile fracture. The findings in Table 6 demonstrated a gradual increase in the tensile strength of the samples with increase in solid solution temperature. Notably, the G3 sample exhibited optimal ultimate tensile strength (UTS) at 353.58 MPa. This enhancement can be primarily attributed to the solubility of the θ phase (stable Al2Cu). In the G3 sample, the complete dissolution of θ phase (stable Al2Cu) into the α-Al matrix and subsequent precipitation of minute Al2Cu during artificial aging facilitated significant precipitation strengthening, contributing substantially to the improved tensile strength of the sample.
Moreover, the considerable rise in tensile strength across samples was also can be attributed to alterations in the size and configuration of eutectic Si particles during the solid solution process. Alongside precipitation hardening offered by Al and Cu, this treatment resulted in the precipitation of sub-microscopic and substable phases containing Si, impeding dislocation motion and thus contributing to precipitation hardening (Ref 40).
Following the attainment of yield strength, the stress in the alloy continued to increase as plastic deformation advances, leading to strain hardening. Two primary hardening mechanisms operate in this process: Δsiso (isotropic strain hardening), caused by changes in dislocation density within the matrix, and Δskin (kinematic strain hardening), induced by dislocation accumulation in the precipitation phases, grain boundaries, and surrounding the eutectic silica. These mechanisms collectively impeded further strain hardening (Ref 41).
The elongation after fracture of the sample exhibited a gradual increase with the rise in solid solution temperature, where the G3 sample demonstrated optimal elongation after fracture, reaching 9.25%. This enhancement was primarily attributed to the morphological variations in the Si phase. At solution temperatures below 520 °C, the profile of the rare-earth-modified fibrous eutectic Si displayed only slight spheroidization due to insufficient solid solution temperatures. However, at 520 °C, the eutectic Si underwent substantial spheroidization, aligning with SEM analysis. Moreover, the alteration of eutectic Si particles' morphology, transitioning from the typical needle-like brittle structure to a more rounded form, contributed to improved ductility. Consequently, as the solid solution temperature increased, the sample's UTS and elongation after fracture reached 353.58 MPa and 9.25%, respectively. These enhancements in mechanical properties were a result of the amplified solid solution strengthening effect and the refinement in the morphology and distribution of the residual phase.
Figure 10 depicts the tensile fracture morphology of the sample observed at room temperature. Figure 10(a) and (b) reveals the fracture morphology of the G1 sample. The presence of thick ligamentous nests, a few tearing prongs, and deconstructed surfaces mixed with small areas was evident. These characteristics were indicative of a brittle fracture, with fractures passing through the crystal structure. Figure 10(c) and (d) displays the fracture morphology of the G2 sample. Equiaxed and densely distributed dimples were observed, accompanied by longer tear edges and cleavage surfaces between dimples. The fracture form also indicated a brittle fracture. Figure 10(e) and (f) illustrates the fracture morphology of the G3 sample. The fracture surface displayed numerous small flocculent dimples, small, dense, and rich tearing edges, and relatively fewer cleavage surfaces. This fracture form was characteristic of ductile fracture, indicating that the G3 sample exhibited the highest plasticity among the samples.
3.6 Corrosion Performance Test
Figure 11 illustrates the intergranular corrosion morphology of the samples observed under an optical microscope after standard testing and the polarization curve of the samples in 3.5 wt.% NaCl solution. Table 7 presents the maximum corrosion depth and intergranular corrosion grade of each sample. Upon reviewing the maximum measured depth of intergranular corrosion and corrosion grades, it was observed that the corrosion depth of the G1 sample measures 104.08 μm, categorized within grade 4. In contrast, both G2 and G3 samples filled within grade 3, with corrosion depths of 99.24 μm for G2 and 69.36 μm for G3. The enhanced intergranular corrosion resistance of the G3 sample could be attributed to the reduced presence of residual phases compared to G1 and G2 samples. Consequently, the lower density of microcells in the G3 sample led to improved corrosion resistance (Ref 42). The gradual dissolution of residual phases into the α-Al matrix in G3 contributed to the enhanced dissolution effect, resulting in its superior intergranular corrosion resistance.
Table 8 presents the corrosion potential (Ecorr) and corrosion current density (Icorr) results. Among the three samples, the G3 sample exhibited the smallest values for Icorr and Ecorr. The Icorr was directly proportional to the corrosion rate, implying that higher Icorr values indicate poorer corrosion resistance of the sample. Meanwhile, the Ecorr value signified the tendency and susceptibility of the alloy to corrosion. Analyzing the polarization curve, the G3 sample exhibited the best corrosion resistance at a solution temperature of 520 °C when compared to temperatures of 500 °C and 510 °C. This finding aligned with the experimental outcomes of intergranular corrosion.
4 Conclusions
This study investigated the effects of three multistage solid solution treatments on the Al-Si-Cu casting aluminum alloy (Al-6.5Si-5.5Cu-0.2Zr-0.01 Sr-0.06Ti-0.2Y). The samples which underwent the G3 solid solution treatment (470 °C × 2 h + 480 °C × 2 h + 490 °C × 2 h + 500 °C × 2 h + 510 °C × 2 h + 520 °C × 14 h) demonstrated optimal mechanical properties. The following conclusions were drawn:
-
(1)
With the gradual increase in solid solution temperature, the θ phase (stable Al2Cu) and intermetallic compounds gradually dissolved into the α-Al matrix, resulting in a reduction in the quantity of residual phases. The microstructure of the G3 sample alloy exhibited the most significant improvement.
-
(2)
As the solid solution temperature gradually rises, the average size of the Si phase also decreased. The spheroidization of eutectic Si gradually exerted a dispersion strengthening effect, while the dislocation density and strength of the samples also increased gradually.
-
(3)
Following the improvement of residual phases and Si phase through the G3 solid solution regimen, the sample exhibited optimal mechanical and corrosion properties. The UTS and elongation after fracture reach 353.58 MPa and 9.25%, respectively. The maximum depth of intergranular corrosion is 69.36 μm. Furthermore, the electrochemical corrosion potential is measured at − 1.1895 V, with a corrosion current density of 1.9965 × 10−7 A/cm2.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Reference
A. Wiengmoon, P. Sukchot, N. Tareelap, J.T.H. Pearce, and T. Chairuangsri, Effects of T6 Heat Treatment with Double Solution Treatment on Microstructure, Hardness and Corrosion Resistance of Cast Al-Si-Cu Alloy, Arch. Metall. Mater., 2015, 60, p 881.
H.C. Fang, H. Chao, and K.H. Chen, Effect of Recrystallization on Intergranular Fracture and Corrosion of Al-Zn-Mg-Cu-Zr Alloy, J. Alloys Compd., 2015, 622, p 166.
K.K. Sankaran, Metallurgy and Design of Alloys with Hierarchical Microstructures., 2017, p 385.
M. Okayasu, K. Ota, S. Takeuchi, H. Ohfuji, and T. Shiraishi, Influence of Microstructural Characteristics on Mechanical Properties of ADC12 Aluminum Alloy, Mater. Sci. Eng. A, 2014, 592, p 189.
L. Jin, K. Liu and X.G. Chen, Evolution of Dispersoids and their Effects on Elevated-Temperature Strength and Creep Resistance in Al-Si-Cu 319 Cast Alloys with Mn and Mo Additions, Mater. Sci. Eng. A, 2020, 770, p 138554.
P. Tang, W. Li, K. Wang, J. Du, X. Chen, Y. Zhao, and W. Li, Effect of Al-Ti-C Master Alloy Addition on Microstructures and Mechanical Properties of Cast Eutectic Al-Si-Fe-Cu Alloy, Mater. Des., 2017, 115, p 147.
S.D. Apelian, Die Soldering: Mechanism of the Interface Reaction Between Molten Aluminum Alloy and Tool Steel, Metall. Mater. Trans. B, 2002, 33, p 465–476.
A.M.A. Mohamed, F.H. Samuel, and S.A. Kahtani, Influence of Mg and Solution Heat Treatment on the Occurrence of Incipient Melting in Al-Si-Cu-Mg Cast Alloys, Mater. Sci. Eng. A Struct. Mater. Prop. Misrostruct. Process., 2012, 543, p 22–34.
I.L. Ferreira, D.J. Moutinho, L.G. Gomes, O.L. Rocha, and A. Garcia, Modeling and Experimental Analysis of Macrosegregation During Transient Solidification of a Ternary Al–6 wt% Cu–1 wt% Si Alloy, Philos. Mag. Lett., 2009, 89, p 769.
T.A. Costa, E.S. Freitas, M. Dias, C. Brito, N. Cheung, and A. Garcia, Monotectic Al–Bi–Sn Alloys Directionally Solidified: Effects of Bi Content, Growth Rate and Cooling Rate on the Microstructural Evolution and Hardness, J. Alloys Compd., 2015, 653, p 243.
C. Zhang, Z. Zhang, M. Liu, E. Bao, C. Liang, and G. Zhao, Effects of Single-and Multi-stage Solid Solution Treatments on Microstructure and Properties of As-Extruded AA7055 Helical Profile, Trans. Nonferrous Met. Soc. China, 2021, 31, p 1885.
T. Marlaud, A. Deschamps, F. Bley, W. Lefebvre, and B. Baroux, Influence of Alloy Composition and Heat Treatment on Precipitate Composition in Al–Zn–Mg–Cu Alloys, Acta Mater., 2010, 58, p 248.
P. Ij, Recent Developments in Light Alloys, Mater. Trans. JIM, 1996, 37, p 12.
A.M. Samuel, H.W. Doty, S. Valtierra, and F.H. Samuel, Relationship Between Tensile and Impact Properties in Al–Si–Cu–Mg Cast Alloys and their Fracture Mechanisms, Mater. Des., 2014, 53, p 938.
Y. Huang, J.D. Robson, and P.B. Prangnell, The Formation of Nanograin Structures and Accelerated Room-Temperature Theta Precipitation in a Severely Deformed Al–4 wt.% Cu Alloy, Acta Mater., 2010, 58, p 1643.
S. Tahamtan, A.F. Boostani, and H. Nazemi, Mechanical Properties and Fracture Behavior of Thixoformed, Rheocast and Gravity-Cast A356 Alloy, J. Alloys Compd., 2009, 468, p 107.
Q. Zhou, C. Li, T. Wang, X. Xu, and Y. Luo, Effect of Various Multi-stage Solution Treatments on the Microstructure and Properties of Cold-Extruded Al-9.74Zn-2.59 Mg-0.94Cu-0.2Zr-0.83Ti alloy, J. Mater. Res., 2022, 37, p 3731–3742.
W. Jingtao and C. Jianzhong, Interaction Intensity of Alloying Elements and Its Application in As-Cast Aluminium Alloys With Rare Earth Additions, J. Xi’an Univ. Archit. Technol., 1993, 25, p 445–449.
J. Coutures, R. Verges, and M. Foex, Comparison of Solidification Temperatures of Different Rare Earth Sesquioxides; Effect of Atmosphere, Revue Internationale des Hautes Temperatures et des Refractaires, 1975, 12, p 181.
S.C. Hansen, Antimony modification of aluminum-silicon alloys. The University of Wisconsin-Madison (2000).
K.M. Youssef, R.O. Scattergood, K.L. Murty, and C.C. Koch, Nanocrystalline Al–Mg Alloy with Ultrahigh Strength and Good Ductility, Scr. Mater., 2006, 54, p 251.
X. Xu, H. Shao, J. Gao, K. Chen, and X. Cheng, Effect of SiC Film on Tensile Properties of Nanostructured Ti Produced by Compressive Deformation at Liquid-Nitrogen Temperature, Mater. Sci. Eng. A Struct. Mater. Prop. Misrostruct. Process., 2008, 493, p 195–201.
X. Xu, X. Xu, Z. Jiang, Z. Jiang, V. Tabie, V. Tabie, Q. Mao, Q. Mao, T. Zhang, and T. Zhang, Effect of SiCw Volume Fraction and Cold Pressure on Microstructure and Mechanical Properties of Aluminum Matrix Composites, Mater. Res. Express, 2019, 6, 126597.
X. Chen, D. Xia, J. Zhang, G. Huang, and F. Pan, Ultrafine-Grained Al–Zn–Mg–Cu Alloy Processed Via Cross Accumulative Extrusion Bonding and Subsequent Aging: Microstructure and Mechanical Properties, J. Alloys Compd., 2020, 846, 156306.
C. Zhong-Wei and J. Wan-Qi, Effects of Mg on the Microstructure and Mechanical Property of Al-Si-Mg Casting Alloys, J. Mater. Sci. Eng., 2004, 22, p 647–652.
G. Riontino, S. Abis, and P. Mengucci, DSC Investigation of Natural Ageing in High-Copper AlCuMg Alloys, in International Conference Aluminium Alloys (2000).
M.J. Deakin, C. Massa, P. Mengucci, and G. Riontino, Investigation on Phase Transformations During Ageing in an AlCuMgAgZn Alloy, Mater. Sci. Forum, 1996, 217–222, p 759.
Y. Wang, Y. Lu, S. Zhang, H. Zhang, and Z. Chen, Characterization and Strengthening Effects of Different Precipitates in Al-7Si-Mg Alloy, J. Alloys Compd., 2021, 885, p 161028.
I.A. Luna, H.M. Molinar, M.C. Román, J.E. Bocardo, and M.H. Trejo, Improvement of the tensile Properties of an Al–Si–Cu–Mg Aluminum Industrial Alloy by Using Multi Stage Solution Heat Treatments, Mater. Sci. Eng. A, 2013, 561, p 1.
J. Li, Y. Zhang, X. Cao, Q. Zeng, Y. Zhuang, X. Qian, and H. Chen, Accelerated Discovery of High-Strength Aluminum Alloys by Machine Learning, Commun. Mater., 2020, 1, p 73.
S.X.W. Jiang, The Effects of Non-isothermal Aging on the Strength and Corrosion Behavior of Al-Zn-Mg-Cu Alloy, J. Alloys Compd. Interdiscip. J. Mater. Sci. Solid-state Chem. Phys., 2016, 681, p 57–65.
P. Guyot and L. Cottignies, Precipitation Kinetics, Mechanical Strength and Electrical Conductivity of AlZnMgCu Alloys, Acta Mater., 1996, 44, p 4161.
D. Liu, B. Xiong, F. Bian, Z. Li, X. Li, Y. Zhang, F. Wang, and H. Liu, Quantitative Study of Precipitates in an Al-Zn-Mg-Cu Alloy Aged with Various Typical Tempers, Mater. Sci. Eng. A, 2013, 588, p 1.
F.A. Chyada, A.R. Jabur, and H.A. Alwan, Effect Addition of Graphene on Electrical Conductivity and Tensile Strength for Recycled Electric Power Transmission Wires, Energy Procedia, 2017, 119, p 121.
A. Matthiessen and M. von Bose, I. On the Influence of Temperature on the Electric Conducting Power of Metals, Philos. Trans. R. Soc. Lond., 1862, 152, p 1–27.
L. Liu, J. Jiang, B. Zhang, W. Shao, and L. Zhen, Enhancement of Strength and Electrical Conductivity for a Dilute Al-Sc-Zr Alloy Via Heat Treatments and Cold Drawing, J. Mater. Sci. Technol., 2019, 35, p 962.
P.K. Rout, M.M. Ghosh, and K.S. Ghosh, Microstructural, Mechanical and Electrochemical Behaviour of a 7017 Al–Zn–Mg Alloy of Different Tempers, Mater Charact, 2015, 104, p 49.
E.V. Bobruk, M.Y. Murashkin, V.U. Kazykhanov, and R.Z. Valiev, Aging Behavior and Properties of Ultrafine-Grained Aluminum Alloys of Al-Mg-Si System, Rev. Adv. Mater. Sci., 2012, 31, p 109.
M.Y. Murashkin, I. Sabirov, V.U. Kazykhanov, E.V. Bobruk, A.A. Dubravina, and R.Z. Valiev, Enhanced Mechanical Properties and Electrical Conductivity in Ultrafine-Grained Al Alloy Processed Via ECAP-PC, Springer, 2013.
A. Mohamed and F.H. Samuel, Influence of Mg and Solution Heat Treatment on the Occurrence of Incipient Melting in Al–Si–Cu–Mg Cast Alloys, Mater. Sci. Eng. A, 2012, 543, p 22.
R. Chen, Q. Xu, H. Guo, Z. Xia, Q. Wu, and B. Liu, Modeling of Strain Hardening Behavior and Mechanical Properties of Al-7Si-Mg Cast Aluminum Alloys During Tensile Process, Acta Metall. Sin., 2017, 53, p 1110–1124.
W. Yingjun, L. Honglei, W. Guojun, D. Kaihui, S. Yingwei, and N.I. Dingrui, Investigation of Anodic Film on a Novel RE-containing Al-Alloy Al-Zn-Mg-Cu-Sc, J. Chin. Soc. Corros. Prot., 2020, 40, p 131.
Acknowledgments
Thanks are due to the financial support from the Key Projects of Equipment Pre-research Foundation of the Ministry of Equipment Development of the Central Military Commission of China (No:6140922010201) and the Key Projects of Research and Development of Zhenjiang (GY2018021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Xu, X., Zhou, Q. et al. Effect of Multistage Solid Solution Treatment on Microstructure and Properties of Al-6.5Si-5.5Cu-0.2Zr-0.01Sr-0.06Ti-0.2Y Alloy. J. of Materi Eng and Perform (2024). https://doi.org/10.1007/s11665-024-09336-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11665-024-09336-3