Abstract
Surface coating processes are carried out at high temperatures, so high heat input is applied to the material to be coated and may cause internal structure deterioration. In order to overcome this situation, the ability to reduce the coating temperature with a pretreatment such as Mechanical Activation was investigated in this study. In order to minimize the effect of alloying elements, DIN St28 steel is used. Powders containing B + SiC + KBF4 were mechanically alloyed by planetary ball milling devices to 10 and 20 h, respectively. By mixing boron-containing powder and sodium silicate, the samples were boronized at 923-1173 K temperature and 3-12 h. At the end of the mechanical alloying process, it was determined that the powder particle sizes were in the nanometer scale. According to the microstructure analysis, a single-layer Fe2B structure was successfully obtained on the samples surfaces. While no boride layers were formed on the sample surfaces at temperatures below 1023 K without MA pretreatment, boride layers were formed under these temperatures with MA pre-treatment. It has been observed that the depth of the Fe2B boride layer, which has achieved high diffusivity by creating many defects in the form of nanometer-sized crystal particles, increased with repeated fracture and cold welding of the powder particles with increasing mechanical alloying times. By calculating the activation energies of the powders, their relations with the mechanically unalloyed samples were compared and empirical formulas that could be used for similar experimental conditions were produced. The highest microhardness value was measured as 2200 HV and above.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introductıon
The surfaces of the machine parts that work in harmony with each other must be hard so that they do not use up betimes. Since the machine parts must be resistant to impact loads during operation, it has been desired that the inner parts of the steel exposed to wear are normalized (soft) and the outer parts are hardened, rather than hardening the entire part. Surface hardening processes are carried out to obtain machine parts with this feature. There are techniques that can be hardened by changing or not changing the chemical composition of the surface. While it is possible to harden the surface of steels containing sufficient carbon without changing the chemical composition, it is inevitable to change the chemical composition of the surface for the hardening process in low carbon steels. In general, it is preferred that elements with small atomic diameters such as carbon (called carburizing), boron (called boronizing) and nitrogen (called nitriding) have high hardness in their compounds with Iron. These techniques are some of the surface hardening methods applied for more than a century.
Carburizing; it is a surface hardening process by putting in carbon to the surface in low carbon steels. Nitriding is also based on surface hardening by adding nitrogen to the surface, just like carburizing. Boronizing; It is the process of forming a boride layer by diffusion of boron atoms on the surface of the base metal at high temperature (Ref 1). The technique is one of the methods used to improve the surface properties of iron and steel (Ref 2). Boronizing is basically a chemical process and its purpose is to form a metal-boride layer on the main matrix surface by diffusion of boron atoms onto the sample surface (Ref 3,4,5,6,7). Apart from ferrous materials, boronizing can also be applied to non-ferrous materials such as Ni and Co alloys, metal-bonded carbides, refractory alloys and some super alloys (Ref 4, 8,9,10). In addition to different applications such as solid, liquid and gas (Ref 11, 12), boronizing processes can also be performed with new methods such as plasma, electrochemical, physical or chemical vapor deposition and ion implantation (Ref 10, 13, 14). The superiority of the surface hardening process with boron over other surface hardening methods is that the surface layer is very hard, as well as its low coefficient of friction, resistance to some bases, acids, metal melts and high temperature corrosion. But, like other methods, boronizing is being done at high temperatures such as 1073-1373 K.
The chemical content of the material to be coated with boron is one of the important parameters affecting the boride layer thickness. The most effective parameters on the thickness of the boride layer are the processing temperature and time. As the process temperature and time increase, it is inevitable that there will be changes in the internal structure of the material. In order to overcome this situation, a pre-treatment such as Mechanical Alloying (MA) is applicable to the powders to be used as a boron source. With this process, the activation energy required for diffusion can reduce and thus similar layer thicknesses can be obtained at lower times or temperatures (Ref 15).
The increase in the contact surface of the boron source powder to be used in the material to be borided is important for diffusion, in other words, for the boride layer thickness. With MA, based on repeated fracture welding, the contact surface is indirectly increased by reducing the powder size and activation energy. MA minimizes the effect of product barriers on diffusion kinetics and enhances solid-state diffusion at low temperature (Ref 16).
In this study, the kinetics of the boride coating made with MA pre-treated boron source powders on DIN St28 steel material at different diffusion temperatures and times were investigated. The thicknesses of the boride layers obtained between the temperature of 923-1123 K and the time of 3-12 h were measured and determined as a function of temperature. In this work, the difference between the MA pre-treatment and the conventional boronizing process is presented. In other words, the increase in the layer thickness with the decrease in the activation energy was determined.
2 Materials and Methods
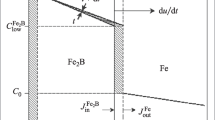
DIN St28 steel, containing 0.13% C, 0.5% (Max) S and 0.5% (Max) P by weight, was chosen because it does not contain different elements (minimally affecting boron). Commercially purchased DIN St28 steel in the form of a cylindrical bar was cut into disks with a diameter of Ø25 mm and a thickness of 7 mm. During the cutting process, coolant was used to prevent thermal input to the sample surface. Subsequently, the surfaces of each sample were sanded with 600-800-1000 and 1200 mesh alumina sandpaper and cleaned with acetone, freed from impurities. Thus, it has been made ready to be boronizing. Elemental boron powder -B- (MERCK- CAS No. 7440-42-8) as boronizing powder, Potassium Tetrafluoroborate -KBF4- (SIGMA-CAS No. 14075-53-7) as activator and 99% pure silicon carbide -SiC- (AKTAŞ Tic, D50 = 9.2 µm) as diluent powders were used. REFSAN, R5127 sodium silicate -Na2O3.SiO2- was used to provide the solid path between the steel and boronizing powder mixture. In addition, the sodium silicate boron source was combined with the powder and brought it to a gel consistency. RETSCH brand PM 200 model planetary type mill was preferred for MA processes were carried out at 400 RPM and 7:1 ball-to-powder ratio with predetermined effective alloying parameters for the equipment and its containers used. ASTM 52100 normed stainless steel balls with Ø10mm diameter were used for MA. Each MA process includes a total of 10 and 20 h of operations at 400 RPM, with a 15-minute break every 30 min. Heat treatment parameters were determined as 923-973-1023-1073-1123 K temperatures and 3-6-12 h. The microstructure of the samples was analyzed using XRD (GI-XRD PANalytical Empyrean) and SEM (Zeiss Sigma 300). Microhardness values were performed using the Tronic DHV-1000 Vicker's hardness tester. The layer thicknesses were calculated by taking the average of the deepest and shallowest points of the boride layer with the help of optical microscope (Nikon ECLIPSE MA200) and are briefly schematized in Fig. 1.
3 Discussion
3.1 Powder Characterization
In Fig. 2, XRD graph of the powders that have been MA treated for 10 to 20 h is given. It is seen that with increasing MA time, the peak intensities decrease, even some peaks disappear and the peak widths increase. It is seen that the intensity of the B4C peaks numbered *1, *2, *5 and *7 decreased with 20 h of alloying. The presence of peaks that decrease, disappear or expand in intensity is accepted as an indication of the mechanical alloying process according to the literature (Ref 17, 18). The intensities of B4C, formed with 10 h of MA pre-treatment and subsequently formed with 20 h of MA treatment, decreased like SiC peaks.
SEM images of mechanically alloyed powders are given in Fig. 3. As can be seen from the images, the average powder particle sizes are below 100 nm. This can be explained by the reduction in size of the grains as a result of their breaking and re-welding as a result of collisions with each other or with the balls or the wall. Increasing MA time resulted in a reduction in the particle size of the powders. It can also be said that after 20 h of alloying, the powder particles agglomerate more than the powder particles that have been MA treated for 10 h. Powder particle size, dislocation density and micro-stress values were calculated using the Scherrer equation as follows (Ref 16).
“D” is particle size, “k” is 0.9 (Scherrer Constant), “λ” is 0.15406 nm (x-ray Wavelength), “β” is Full Weight Half Middle (FWHM) and “θ” represents peak angle (rad).
Similarly, micro-strain is the same (Ref 19)
as above.
β (FWHM) and θ values were calculated using the “Origin 2017” program via by XRD analysis of MA pre-treated powders. For the determination of the average powder particle size, when the average of the particle size is calculated at each peak angle value taken; It is calculated as 22.00798 nm. The average of the δ values were found for the dislocation density. It is calculated as 0.00212 (nm−2). The value found for MicroStress “ε”; it was calculated as 0.00460, which is a dimensionless number.
3.2 Borided Layer Formation Performance
The conditions and boride layer thicknesses of the samples produced in the study are given in Table 1. The presence of a boride layer could not be proven in the samples without MA pre-treatment below 1023 K. In other words, no boride layer was formed on the sample surfaces without MA pretreatment under 1023 K. For this reason, the data of the samples boronized at 923 and 973 K were not included in the preparation of Table 1.
Figure 4 SEM images and XRD graphs of the samples which were boronized at 750 °C for 12 h are given. In SEM images, fringe-like boride layers are seen as one moves from the matrix to the surface. A thin layer of residue is observed in the parts closest to the surface. The appearance of SiC peaks in the XRD analysis is thought to be a source of boronizing powder remaining as a residue on the sample surface.
According to the % atomic result obtained from the EDX analysis, it proves that the leafy-structures under the residual layer (dried and stuck gel containing sodium silicate, KBF4 and Boron) are Fe2B. Fe2B structures have a columnar growth characteristic from the surface to the inside. Borided structures growing random directions can be viewed leafy-shaped during sectional examinations as Fig. 5. In this figure, the leafy-structures are seen under the residual layer.
It is an undisputed fact that Fe2B grows inward from the surface in a 3-dimensional way, it does not grow by the same amount in the same directions. Lateral sections were taken from the samples for microstructure analysis. Meanwhile, Fe2B structures cut by the blade surface are clearly visible. Since the examinations are made in 2 dimensions, the growths in 3 axes can be seen in 2 dimensions at different lengths. Figure 6 has tried to explain this situation (leafy-structures) in more details.
Figure 7 shows the thickness of the boride layer of the borided samples for 3, 6 and 12 h and 0, 10 and 20 h MA times at different temperatures. The thickness of the boride layer obtained under the same heat treatment conditions increased due to the MA pre-treatment of the powders. Boride layer could not be obtained at temperatures below 1023 K without MA pre-treatment. Under the powder composition and conditions used in the experiments, 1023 K can be accepted as the threshold value for the boronizing process in terms of temperature. Diffusion parameters (temperature, time and difference in concentration) can be changed as the process is based on diffusion. Since the FeB phase containing 8.83% B by weight is more brittle than the Fe2B phase (Ref 20), the formation of a single-phase Fe2B layer with a sawtooth morphology is generally preferred over the formation of a biphasic layer containing FeB + Fe2B (Ref 21). The fact that FeB is more brittle than Fe2B, as well as having different heat transfer coefficients and compressive-tensile strengths, that is, Fe2B is more stable, reinforces this preference (Ref 22). Under the same conditions, it is clearly seen in Fig. 5 that the boride layer thicknesses increase significantly with the variable parameter being the MA pretreatment time. It can be concluded that the desired thickness can be reached at a lower temperature and time compared to the traditional boronizing method. In other words, boron atoms were more easily diffused into the α-ferrite, which means diffusion depth was increased. With the decrease in boronizing time or temperature, the material will be exposed to less heat input, so internal structure changes can be minimized. Another conclusion that can be drawn from this situation is that boronizing can be done in steels that are sensitive to internal structure changes.
3.3 Borid Layer Kinetics
This increase in diffusion depth can be explained by the MA pre-treatment time, the decrease in powder size and a parallel increase in surface area. With the kinetic analysis of the boride layers, it is easier to understand the increase in diffusion depth. The following equation was used to calculate the activation values of active boron atoms moving inward from the surface. In this equation; “d” is the layer thickness, “t” is the boronizing heat treatment time, and “K” is the rate constant of the growth of the boride layer.
With the help of the growth rate data obtained, the activation energies of the samples produced on the DIN St28 steel surface under the above-mentioned conditions (heat treatments at the same temperatures) were calculated with the help of the Arrhenious equation.
To find the Activation Energy (Q), ln from both sides of the equation is taken and the following Eq. 5. is obtained.
All calculations made are given in Table 2. In Fig. 7, the LnD − 1/T graph of the boronized samples is given. The activation energy was calculated as 113,555 kJ/mol in the sample (a) boronized using the gel without MA pre-treatment. The activation energy was calculated as 108,814 kJ/mol in the sample (b) boronized using the gel that was MA pre-treated for 10 h. Compared to the experiments without MA, a decrease of 4.74 kJ/mol or 4.33% occurred in the activation energy by performing MA for 10 h, as expected. The activation energy (c) was calculated as 106,746 kJ/mol in the samples boronized using the gel treated with MA for 20 h. Comparing the experiments with non-pretreated powders, it was determined that 20 h of MA pre-treatment reduced the activation energy by about 8 kJ/mol or 6% (Fig. 8).
The increase in layer thicknesses during the increasing boronizing parameters (MA times and temperatures) is an expected situation from the point of view of diffusion. With the increase in the MA pre-treatment time, the increase in the thickness of the boride layers proceeds parallel to each other. This situation was also observed at all temperatures and times in all experiments. With the MA pre-treatment process, the activation energy of the powders decreases. Although the decrease in the activation energy continues with the increase in the MA pre-treatment time, the rate of increase in the MA time and the decrease in the activation energy do not show similarity. From the point of view of activation energy, optimum conditions were observed in the samples boronized with gel after MA pre-treatment for 20 h. However, it was calculated that the decrease in the activation energy of the powders forming the gel with 20 h of MA pre-treatment (1.67%) was less than that of 10 h (4.33%). It was observed that the activation energy did not decrease twice with doubling the MA time, but the boride layer thickness increased by 153.37%. The reason for this situation can be explained by the decrease in powder size in SEM images and the increase in agglomeration accordingly. The increase in the amount of amorphization, dislocation density and aggregation in boronizing powder with increasing MA time made diffusion easier (Ref 15).
3.4 Microhardness Distribution of Boride Layers
For the temperature values of 1023 K and below, a boride layer could not be obtained on the sample surfaces prepared without MA pre-treatment. For this reason, samples produced at temperatures of 1023 K and above were used in microhardness measurements, just as in kinetic measurements. The effect of varying MA pre-treatment times on the hardness of the boride layer formed on the surface under the same conditions Fig. 9a. The hardness distribution of the samples produced at a pre-treatment time of 10 h and a temperature of 1073 K but under different boronizing time conditions is shown in Fig. 9b. In addition, the microhardness graph of the samples boronized at different times at a temperature of 1173 K and 20 h of MA pre-treatment time is given in Fig. 9c. Maximum hardness was observed in the sample prepared under the conditions of 12 h boronizing time at 1173 K using 20 h MA pre-treated powder, and it was measured as 2234 HV0,1. Although the maximum hardness was observed in a sample, it seems that values close to each other were measured when looking at the distribution. This is compatible with the measured microhardness values of Fe2B in the literature (Ref 15).
Samples (a) 1073 K boronizing temperature and 12 h boronizing process, different MA pre-treatment times, (b) 10 h MA pre-treatment time and 1073 K boronizing temperature, different boronizing times, (c) 20 h MA pre-treatment time and 1173 K boronizing temperature, microhardness distributions of samples produced under different boronizing times, (d) picture of microhardness measurement
4 Conclusion
The size of the powders subjected to mechanical alloying pre-treatment have been reduced to nano levels. Decreases in powder size and agglomeration were observed with increasing MA pre-treatment times. Single-phase (Fe2B) borides were obtained in boronizing processes as a result of the studies, thanks to both the selected powder chemical composition and the MA pre-treatment times and conditions.
Owing to the MA pre-treatment, similar thicknesses could be obtained at lower temperatures than the conventional boronizing process. It was determined that by increasing the MA pre-treatment compared to the heat treatment under similar conditions, the thickness of the boride layers also increased.
The optimum boronizing temperature depends on the MA process. Using Mechanical Alloying times of 0, 10 and 20 h, the activation energy of boron diffusion was found to be 113,555, 108,814 and 106,746 kJ mol−1, respectively.
Experimental equations have been derived in order to predict the results of the experiments to be performed under similar conditions (such as MA pre-treatment and heat treatment time, temperature).
The surface hardness of the steel with DIN St28 increased up to 2200 HV with boronizing.
4.1 Datasets
The datasets generated and/or analyzed during the current study are not publicly available due [Due to the Turkish Council of Higher Education Thesis Center‘s rule] but are available from the corresponding author on reasonable request.
References
T. Balusamy, T.S.N. Sankara Narayanan, K. Ravichandran, I.S. Park, and M.H. Lee, Effect of Surface Mechanical Attrition Treatment (SMAT) on Pack Boronizing of AISI 304 Stainless Steel, Surf. Coatings Technol., 2013, 232, p 60–67. https://doi.org/10.1016/j.surfcoat.2013.04.053
N.S. Meriç, C. Şahin, S. Backir, and B. Köksal, Investigation of the Boronizing Effect on the Abrasive Wear Behaviour in Cast Irons Mater, Des., 2006, 27, p 751–757.
A.H. Çelebi, G. İpek, M. Bindal, and C. Ücisik, Some Mechanical Properties of Borides Formed on AISI 8620 Steel, Mater. Forum, 2005, 29, p 456–460.
G. Jain and V. Sundararajan, Influence of The Pack Thickness of The Boronizing Mixture on the Boriding of Steel, Surf. Coat. Technol., 2002, 149, p 21–26.
A.H. Özbek, İ Şen, S. İpek, M. Bindal, C. Zeytin, and S. Üçişik, A Mechanical Aspect of Borides Formed on the AISI 440C Stainless-Steel, Vacuum, 2004, 73, p 641–648.
B.-S. Lee, S.Y. Kim, and G.S. Kim, Mechanical Properties of Duplex Layer Formed on AISI 403 Stainless Steel By Chromizing And Boronizing Treatment, Surf. Coat. Technol., 2004, 2, p 177–184.
R. Çelikyürek, İ Baksan, B. Torun, and O. Gürler, Boronizing of İron Aluminide Fe72Al28, Intermetallics, 2006, 14, p 136–141.
C. Martini, G. Palombarini, G. Poli, and D. Prandstraller, Sliding and Abrasive Wear Behaviour of Boride Coatings, Wear, 2004, 256(6), p 608–613. https://doi.org/10.1016/j.wear.2003.10.003
C. Özbek and İ Bindal, Mechanical Properties of Boronized AISI W4 Steel, Surf. Coat. Technol., 2002, 154, p 14–20.
E. Bejar and MA, Moreno, Abrasive Wear Resistance Of Boronized Carbon And Low-Alloy Steels, J. Mater. Process. Technol., 2006, 173, p 352–358.
J. Subrahmanyam, Wear Studies On Boronized Mild Steel, Wear, 1984, 95, p 287–292.
S.C. Singhal, A Hard Diffusion Boride Coating for Ferrous Materials, Thin Solid Films, 1977, 45(2), p 321–329.
P.X. Yan et al., Post Boronizing Ion Implantation of C45 Steel, Appl. Surf. Sci., 2002, 195(1–4), p 74–79. https://doi.org/10.1016/S0169-4332(02)00555-X
A. Ersöz, Elektrokimyasal Borlama İle Çeliklerin Yüzey Özelliklerinin Geliştirilmesi. İstanbul Teknik Üniversitesi (2008).
S.O. Yilmaz and S. Karataş, Effect of Mechanical Activation on Jell Boronizing Treatment of the AISI 4140, Appl. Surf. Sci., 2013, 275, p 148–155. https://doi.org/10.1016/j.apsusc.2013.01.059
C. Suryanarayana, Mechanical Alloying and Milling, Prog. Mater. Sci.. Mater. Sci., 2001, 46, p 1–184.
B. Avar, Structural, Thermal and Magnetic Characterization of Nanocrystalline Co65Ti25W5B5 Powders Prepared by Mechanical Alloying, J. Non Cryst. SolidsCryst. Solids, 2016, 432, p 246–253. https://doi.org/10.1016/j.jnoncrysol.2015.10.016
H. Raanaei, S. Abbasi, and S. Behaein, Structural and Magnetic Evolution of Nanostructured Co40Fe10Zr10B40 Prepared by Mechanical Alloying, J. Magn. Magn. Mater.Magn. Magn. Mater., 2015, 384, p 175–180. https://doi.org/10.1016/j.jmmm.2015.02.044
E.A. Badawi, M.A. Abdel-Rahman, A. Mostafa, and M. Abdel-Rahman, Determination of the Crystallite Size & Micro-strain by Novel Method from XRD Profile, Appl. Phys., 2019, 2, p 1–15. https://doi.org/10.31058/j.ap.2019.21001
B. Galibois, A. Boutekno, and O. Voyzele, Mechanisma De Formation Des Couches Borurees Sur Les Aciers A Haut, Carbone-II Tech. des Poudes Acta Met., 1980, 28, p 1765–1771.
O. Ozdemir, M. Usta, C. Bindal, and A.H. Ucisik, Hard Iron Boride (Fe2B) on 99.97 wt% Pure Iron, Vacuum, 2006, 80(11–12), p 1391–1395. https://doi.org/10.1016/j.vacuum.2006.01.022
I. Özbek, Borlama Yöntemiyle (AISI M50, AISI M2) Yüksek Hız Çeliklerinin Ve W1 Çeliğinin Yüzey Performanslarının Geliştirilmesi, Sakarya Üniversitesi (2000).
Acknowledgments
Not applicable.
Author information
Authors and Affiliations
Contributions
MGA: Term, Methodology, Validation, Formal analysis, Investigation, Writing - Review & Editing. EE: Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. The authors whose names are listed immediately below report the following details of affiliation or involvement in an organization or entity with a financial or non-financial interest in the subject matter or materials discussed in this manuscript. Please specify the nature of the conflict on a separate sheet of paper if the space below is inadequate. This statement is signed by all the authors to indicate agreement that the above information is true and correct. The authors named below declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albayrak, M.G., Evin, E. A Different Approach: Effect of Mechanical Alloying on Pack Boronizing. J. of Materi Eng and Perform 33, 9039–9046 (2024). https://doi.org/10.1007/s11665-023-09004-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-023-09004-y