Abstract
In this work, AZ31 and AZ31-1 wt.% RE (RE = Nd, Dy, Nd+Dy) alloys were prepared by conventional casting. The effect of single and co-addition of rare earth (RE) on the microstructure, mechanical properties and corrosion behavior of as-cast AZ31 magnesium alloys were investigated at ambient temperature, and after heat treatment at 400 °C for 1 h. The addition of 1 wt.% RE (RE = Nd, Dy, Nd+Dy) preferentially formed the Al2RE phase and completely suppressed the formation of the intermetallic β-Mg17Al12 phase. An excellent ultimate tensile strength (UTS)/ductility combination of 209 MPa/21% and 192 MPa/17% with an adequate yield strength (YS) of 90 MPa was observed for AZ31+Nd sample in as-cast and annealed states, respectively. The work-hardening rate of the AZ31 alloy containing Nd and Dy increased significantly after annealing compared to those of the as-cast state. Fracture analysis indicated that the additive RE did not obviously change the fracture mechanism of the Mg alloy. All specimens exhibit a hybrid fracture with cleavages and dimples. The weight loss test showed that the corrosion resistance of the AZ31 Mg alloy was improved with added RE as it interacts with Al to form Al-RE phase, which upgraded the corrosion resistance of the alloys. The co-addition of RE (RE = Nd+Dy) was proven to enhance corrosion resistance, and also stabilized the corrosion rate. In brief, the co-addition of Nd and Dy significantly improved the corrosion resistance of the AZ31 magnesium alloys than the counterpart of the mechanical properties.
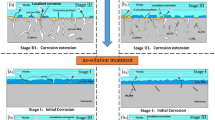
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, magnesium (Mg) alloys as one of the lightest structural material have received significant attention as potential functional parts in automobiles, rail transportation, airplane and biomedical implant materials owing to their specific properties such as lightweight, high damping capacity and good impact energy absorption. However, the poor corrosion resistance and mechanical properties of magnesium alloys limited their widespread applications. Mg alloys have weak corrosion resistance in brine solutions, especially in the presence of aggressive chloride ions, and this has become a significant obstacle to use in corrosion‐sensitive applications (Ref 1, 2). On the other hand, Mg alloys exhibits relatively low formability and poor ductility at room temperature, due to their hexagonal close-packed crystal structure (Ref 3). Furthermore, it has been reported that shear banding plays a key role in recrystallization behavior and texture evolution of Mg alloys. In recent decades, several methods have been proposed to enhance the mechanical properties and corrosion resistance of Mg alloys. Grain refinement through thermomechanical processing and severe plastic deformation methods, including accumulative roll bonding, equal channel angular extrusion, twist extrusion, simple shear extrusion and high-pressure torsion are well-known approaches to improve the mechanical properties of Mg alloys. However, the above-mentioned techniques are not suitable for industrial production, and are still limited to the laboratory scale.
The addition of alloying elements is an effective strategy for improving the mechanical behavior of Mg alloys. The effects of various alloying elements including Al, Li, Mn, Zn, Zr, Ca, Sr on the microstructure, mechanical properties and corrosion behavior of Mg alloys have been studied. However, a solid solution composed of binary or ternary compounds is typically found in Mg alloys because of their low solubility. For high-strength Mg-RE alloys, process control is crucial in addition to alloying design, particularly for Mg alloys with low RE content (Ref 4). Despite the rapid development of RE-free Mg alloys, Mg-RE-based alloys are the most promising high-strength Mg alloys (Ref 5, 6). Rare earth elements (REEs) have been used in Mg-Al alloy for many years. The Mg17Al12 phase formation is diminished by rare earth elements in the Mg-Al-Zn alloys during solidification, as the rare earth (RE) favors the formation of Al-RE intermetallic phases. The addition of rare earth elements into AZ31 alloys leads to the formation of the Al2RE and Al11RE3 phases. However, the phase stability of Al2RE intermetallic compound is higher than that of Al11RE3. At above 150 °C, the Al11RE3 phase decomposes from Al2RE and Al due to the unstable Al11RE3. After phase transformation, the Al retains in the solid solution and forms the β phase which deteriorate the properties at elevated temperature (Ref 7,8,9,10,11,12). Among the rare earth elements, Nd and Dy are effective alternative strengthening elements in Mg alloy for elevated temperature applications. Nd forms a more stable intermetallic with Al and thus restricts the β phase formation (Ref 13, 14). Therefore, the precipitations of REE hardening the Mg alloys are stable and would increase the mechanical strength and corrosion resistance of the magnesium alloys (Ref 15,16,17).
The aim of the present work is to investigate the effect of rare earth alloying on the microstructure evolution, mechanical properties and the corrosion behavior of as-cast Mg-Al-REE alloys in 5 wt.% Nacl aqueous solution at ambient temperature.
2 Experimental Section
2.1 Materials Preparation
In this research, AZ31-1 wt.% RE (RE = Nd, Dy, Nd+Dy) alloys were prepared using the conventional casting method at a temperature of about 730 °C (± 20 when added REE) in a protective atmosphere of SF6. For our designed alloy preparation, commercially pure Mg, Al and Zn were melted in a graphite crucible using an induction furnace. REE were added to the above-mentioned alloys by melting commercially pure Nd and Dy in a crucible with an electric resistance furnace at 750 °C. Thereafter, the required amount of REE was added to the melt at 730 °C. Finally, a cast sample was produced with a diameter of about 54.4 mm and a height of 160 mm. The middle portion of the ingots were chosen for metallographic analysis. Then, the effect of the single and combined addition of rare earth Nd and Dy on microstructures, mechanical properties and corrosion weight loss test of as-cast AZ31 magnesium alloys were investigated between ambient temperature and after heat treatment at 400 °C for 1 h. Fig. 1(a) shows the heat treatment cycle used for the AZ31 Mg alloys.
2.2 Mechanical Properties
Tensile strength was measured with a universal tensile tester (SHIMAZU) at room temperature with a strain rate of 1 × 10−3 s−1. Two specimens of each kind of considered alloy were evaluated, and the calculated average value (the average of two measurements value) was used as the final test result. The precise dimensions of the tensile test specimen are shown in Fig. 1(b). All samples tested in this work were cut from the middle portion of the cast ingot to have the similar microstructure throuout the specimen with respect to grain size and precipitate distribution.
2.3 Corrosion Tests
To perform corrosion test, the specimens were cut into φ 12 mm × 5 mm dimensions. Then, the specimens were surface polished with abrasive paper. After polishing, they were immersed into the corrosion solution (5 wt.% NaCl solution). The immersion test was conducted at room temperature and the erosion times were set to 2, 4, 6, 8, 10, 12, and 14 h. At each time point, eight specimens were taken out and the surface corrosion products were removed with a chromic silver nitrate solution (i.e.,, 200 g of CrO3 and 10 g of AgNO3 dissolved in 1 L of deionized water), and then washed with alcohols, to ensure the corrosion products were complete expelled without eliminating any Mg metal. After drying in warm flowing air, the specimens were stored. Finally, the corrosion weight loss was measured.
2.4 Microstructural Analysis
For metallographic examination, specimens were prepared by mounting and polishing in epoxy resin, and were then etched by solutions, including 10 mL acetic acid, 5 g picric acid, and 95 mL ethanol, for 10-20 s. Then, the microstructure and phase distribution were characterized using field emission scanning electron microscopy FESEM-MIRA-LMH II (TESKAN) and the transmission electron microscope (TEM, JEM-F200) equipped with energy-dispersive spectrometer (EDS).
3 Results and Discussion
3.1 Microstructure and Phase Characterizations
The microstructure of both as-cast and heat-treated samples were investigated. Fig. 2 shows the typical SEM morphologies of the various phases in the AZ31 and AZ31-RE (RE = Nd, Dy, Nd+Dy) alloys. There were only small amount of white precipitates in the RE free AZ31 alloy, as shown in Fig. 2. The α-Mg matrix and widely distributed granular β-Mg17Al12 were found in the microstructure of AZ31. Noticeably, AZ31-RE contained dark α-Mg, a bigger grayish white globular-like β-Mg17Al12, white brighter weed-like Al-RE (RE = Nd, Dy, Nd+Dy) and other intermetallic compounds.
When REE is added to the AZ31 alloy, the reaction between the REE and aluminum results in the formation of Al-RE compounds and, the amount of β-precipitates decreases, even diminishes completely, because the preferential reaction of aluminum (Al) and rare earth (RE) leads to the formation of the Al11RE3. The needle-like (or weed-like) Al11RE3 phase was observed at any REE content. It is revealed that, the addition of Nd into AZ31 lead to the formation of Al-Nd, while the addition of Dy resulted in the appearing of Al-Dy phase. The co-addition of Nd and Dy did not induce any new phases, and only the REE-enriched phases appeared after the single addition of Nd or Dy.
To confirm the RE-containing precipitates, EDS measurements of each phase at marked points 1 and 2 are performed for all specimens. The EDS spectrum and elemental compositions are tabulated as in Fig. 3(a) and (b). Fig. 3 represents the SEM images of AZ31 Mg-alloys and the intermetallic phases containing in AZ31-RE (RE = Nd, Dy, Nd+Dy) alloys (a) before HT (as-casted) and (b) after HT (as-annealed) samples, respectively.
3.2 Mechanical Properties
To compare the mechanical properties of AZ31 alloys with and without REE, the tensile test was conducted. In addition to investigate the effects of REE on the mechanical properties and work-hardening rate alloys, their microstructures were examined using TEM. The engineering stress-strain curves of the AZ31 alloys obtained from the uniaxial tensile tests at RT and strain rate of 1 × 10−3 s−1 in as-cast and as-annealed states are shown in Fig. 4. As shown in Fig. 4(a) and (b), the yield strength (YS), ultimate tensile strength (UTS), and elongation of the AZ31 sample in as-cast and/as-annealed states were 95/108 MPa, 167/213 MPa, and 9%/23%, respectively. The results demonstrated that the mechanical properties of AZ31 increased after annealing which could be mainly associated with solid-solution hardening and the presence of MgAl secondary phases in the matrix. On the other hand, it has been reported that the size of precipitations, the amount of distribution and the morphology of the β-Mg17Al12 phase provides the mechanical strength and plasticity to Mg-Al alloys (Ref 18). Although, the formation of coarse secondary phase precipitates in the grain boundary region leads to lower elongation neverthless elongation enhancement of HT-AZ31 alloys can be attributed to the microstructural changes induced by the heat treatment of the alloys. After the addition of rare earth elements, the YS/UTS values significantly changed in both as-cast and annealed states, particualrly the elongation relatively increased for as-cast state samples. The ductility of the AZ31 alloy in as-cast state not only increased with the addition of REEs, but also the tensile strength of the AZ31 alloy increased markedly after adding Nd. It has been reported that the existence of the secondary phase to the metal matrix induces more stress concentration in the matrix, which leads to the nucleation of voids by debonding of the matrix (Ref 19). In addition, the dislocation pile-up at the interface of precipitates-matrix led to initiation and propagation of cracks due to the brittleness of the secondary phases which decrease the ductility of the alloy (Ref 20, 21). An excellent UTS/ductility combination of 209 MPa/21% and 192 MPa/17% with the adequate YS of 90 MPa was observed for AZ31+Nd sample in as-cast and annealed states, respectively. The work-hardening behavior of the alloys under uniaxial tension for as-cast and annealed samples are shown in Fig. 4(c) and (d). The work-hardening rate of the AZ31 in as-cast state was higher than those of the other three REE added samples, suggesting that the accumulation of dislocations is more significant for AZ31 in as-cast state. The work-hardening behavior of alloys after annealing in Fig. 4(d) shown that the work-hardening rate of the AZ31 alloy contain both Nd and Dy elements increased significantly after annealing compared to those of as-cast state. TEM studies were conducted to investigate the work-hardening behaviors and dislocation configuration of alloys during the tensile deformation. Fig. 5 shows TEM images of the AZ31 alloy showing deformed microstructure evolution before and after annealing. The accumulated dislocation density in AZ31 in as-cast state (Fig. 5a, b) appears to be higher than AZ31 alloy after annealing which consistent with the larger hardening rate as shown in Fig. 4(c). All rare earth elements (REEs) form simple eutectic systems with Mg, especially when the alloy contains aluminum. The precipitation hardening can easily occur in magnesium alloys after adding rare earth elements (Ref 22). The hardness of AZ31 alloys correlated to the enhancement of tensile strength with the addition of Nd (RE) because the solubility of Nd (RE) in Mg is low (Ref 22). However, the addition of Dy and Nd+Dy (RE) to the AZ31 alloy fails to enhance the tensile strength of the alloy.
TEM images of the AZ31 alloy tensile deformed (a) typical bright-filed TEM images showing the microstructure evolution and dislocation accumulation, (b) higher magnifications illustrating the interaction of dislocation with intermetallics in the AZ31 alloy deformed before heat treatment, (c) TEM image and (d) the corresponding diffraction pattern of AZ31 alloy tensile deformed after heat treatment
3.3 Fracture Morphology
Magnesium alloys tend to crack easily in the form of a cleavage fracture or quasi cleavage fracture, due to their limited slip systems (Ref 23). To investigate the failure mechanisms, the fracture surfaces of the broken tensile specimens were analyzed. Figure 6 shows the SEM images of the room temperature fracture surface and their magnified images of highlighted areas in NHT and HT specimens of the AZ31 and AZ31-RE (RE = Nd, Dy, Nd+Dy) alloys. The fracture morphology of all specimens exhibited hybrid fractures with cleavages and dimples. But the fracture surfaces of the NHT specimens showed a little ductile fracture, which indicates a much higher volume fraction of dimples.
Generally, the failure mechanism of both the AZ31 and AZ31-RE alloys specimens are in fact a combination of trans-granular and inter-granular fractures. In detail, the dimples produced on the fracture surfaces of the NHT and HT specimens of AZ31 and AZ31-Nd appeared to be deep conical dimples, which are inherent in the fracture of plastic materials. Therefore, it can be presumed that an increase in crack resistance is accompanied by an increase in the relative visual depth of dimples, as seen in Fig. 6. But, for AZ31-Dy and AZ31-Nd+Dy, the inter-granular fracture tends to be smaller and shallower than those of AZ31 and AZ31-Nd samples before and after heat treatments. There are some tinier microcracks were noticed in the fracture surfaces of the AZ31-RE (RE = Dy and Nd+Dy) alloys, shown in Fig. 7. These microscopic observations indicates that the change in mechanical properties attributed to the microstructural changes.
3.4 Corrosion Behavior
Immersion tests are commonly conducted using a concentrated chloride solution such as 3.5 wt.% NaCl saturated with Mg (OH)2. Mg corrosion in such a concentrated solution converts metallic Mg to the stable ion Mg2+ via the following reaction: (Ref 24)
In our study, the corrosion rate of the AZ31 magnesium alloy in 5 wt.% NaCl solution accelerated from an initial level and reached a stable corrosion rate after a certain immersion period. The corrosion properties could be altered in the AZ series Mg alloys by varying the amount of additive REE content. The corrosion resistance was improved by adding rare earth metals, since rare earth metals can refine recrystallized grains, which benefits the corrosion resistance (Ref 25). Moreover, AZ31, the wrought alloy, contained a major fraction of solid solution Mg and Al (called the α-phase) and a lower amount of secondary phase (β, Mg17Al12). Despite the fact that the presence of β-phase enhances the performance of Mg-Al-Zn series (AZ series), but it has a negative impact on corrosion properties (Ref 26). Compared with the AZ31 alloy, the AZ31-REE alloys exhibits improved corrosion resistance.
Figure 8 shows the corrosion weight loss curves of HT and NHT samples with respect to immersion time. All samples were soaked in the corrosion solution for a certain time. Initially, there were some bubbles on the surfaces of the samples. One hour later, each sample was corroded to different degrees. The weight loss of AZ31-REE increased steadily with erosion time, which can control the speedy corrosion rate of AZ31 series Mg alloys to some extent. According to the corrosion weight loss of the alloys, the corrosion rates ranked as AZ31 > AZ31Dy > AZ31NdDy > AZ31Nd for samples without heat treatment, and AZ31 > AZ31Dy > AZ31Nd > AZ31NdDy for samples with heat treatment, respectively.
Among the NHT specimens, the corrosion rate following the co-addition of two REE was in between those of the two single-REE alloys, indicating that Nd improved the corrosion resistance of AZ31 than Dy. This was mainly because Nd can purify the melts and interact with Al to form the Al-Nd phase, which upgraded the corrosion resistance of the alloys. But, interestingly among the HT specimens, the corrosion of the co-modified AZ31 Mg alloys was less critical than the single-REE modified AZ31 Mg alloys, indicating that the addition of both Nd and Dy stabilized the corrosion rate and could also enhance the corrosion resistance of the alloys, as the erosion time increased by 2-14 h.
Moreover, the percentage of weight loss of the heat-treated AZ31+Nd, Dy was more stable compared with the first 8 h. The corrosion rate of samples with Dy alone was higher than that with Nd in both the HT and NHT cases. This may be because the Al-Dy phase was more active in Cl- than the Al-Nd phase. This leads to slightly worse corrosion resistance in the AZ31+Dy compared to AZ31+Nd. Hence from these interesting results, it can be understood that the presence of REE in the AZ series Mg alloys has a great influence on corrosion behavior, especially AZ31+Nd, Dy after being heat-treated, and this approach is suggested if the application intended for a corrosive environment.
The microstructures of the corroded surface of the AZ31 cast alloys containing various REE (Nd, Dy and Nd+Dy) exposed in 5 wt.% NaCl for 14 h are shown Fig. 9. This microstructure shows that the surface corrosion is distributed to some extent on the surface of the specimens. The corrosion of HT-AZ31 was much more severe than the other specimens. This corrosion is suspected to be sites with the β-Mg17Al12 phase (see in Fig. 2) which exhibit a more passive behavior in NaCl solution than either Al or Mg. Similarly, very small pits were observed in AZ31+Dy and HT-AZ31+Dy. However, the shapes of the pitting corrosion were dissimilar; elliptical in AZ31+Dy and horizontal in HT-AZ31+Dy, before and after heat treatment. Nevertheless, AZ31+Nd, Dy and HT-AZ31+Nd, Dy still suffered much less corrosive attack compared to other AZ31 specimens. Several small shallow corroded pits were found on the surface of the AZ31+Nd and HT-AZ31+Nd specimens. We analyzed the compositions of the corroded surfaces to demonstrate the corrosion inhibition effects of alloying with REE. High-magnification SEM pictures of corroded surfaces are shown in Fig. 10(a) and (b) along with the associated EDS spectra of identified locations. The EDS results of AZ31 and AZ31-RE (RE = Nd, Dy, Nd+Dy) alloys with different RE contents, at.% are tabulated. It is found that the corrosion inhibition effects of AZ31 and AZ31+Dy specimens in the as-casted state (before HT) and in the annealed condition (after HT) seem to have no apparent impact on the distribution of the secondary phase. However, the morphology, size, and composition of precipitate (Al-NdDy) on the corroded surface of HT-AZ31+Nd, Dy specimen, is relatively changed, especially at.% of Dy (RE) contents dramatically decreased. It can turn the inhibition of corrosion effects.
Figure 11 shows the morphology of the cross-sectional corroded surfaces of the AZ31 and AZ31-RE alloys (REE = Nd, Dy and Nd+Dy). It is evident that erosion of the AZ31 magnesium alloys before heat treatment was more severe than samples after heat treatment. Cracking may occur along the grain boundaries, and precipitation can produce regions of minimal corrosion resistance in the immediate vicinity. The corrosion attack was restricted to along the grain boundaries, or immediately adjacent to grain boundaries, since the Al-RE phases are precipitated along the grain boundaries. This type of attack may result from the different compositions of the internal casting alloy, such as coring encountered in it. As we discussed in the former section, the reaction between RE and Al, which are soluble in the α-Mg matrix, originally results in the formation of Al-RE compounds and decreases Al content in the α-Mg matrix. The regions where Al is consumed become the preferential path for corrosion attack or, crack propagation, as illustrated in Fig. 12. The increase in corrosion resistance after heat treatment can be attributed to relieving of stress, and thus, heat treatment has an increasing effect, reducing the amount and distribution of the β-phase, as a result generating supersaturated α-grain.
In the present investigation, the AZ31-RE (RE = Nd, Dy, Nd+Dy) alloys exhibited more corrosion resistance than pure AZ31 alloy. The stability and dissolution kinetics of the surface protection altered by adding alloying elements, particularly rare earth (RE) elements, which increases the corrosion resistance of the Mg alloys (Ref 27). Based on the following factors, microstructural influences on corrosion mechanisms: Under heat treatment conditions, the formation of precipitates are minimized in the morphology of the specimens because this condition has a growing impact on the diminishing amount and distribution of β-phase, as result producing supersaturated α-grains. Cracking occurs at the grain boundaries, and nearby areas that are more susceptible to corrosion due to precipitation develops. As a result of the Al-RE phases precipitating along the grain boundaries, localized corrosion assaults may develop there. The potential mechanism underlying the corrosion attack on AZ31-REE Mg alloys in NaCl solution at various time intervals was illustrated in Fig. 12.
4 Conclusions
We investigated the effect of single and co-addition of rare earth elements (REEs) on the mechanical and corrosion properties of the Mg alloys. The findings showed that annealing improved the mechanical characteristics of AZ31, which may primarily be attributed to solid-solution hardening and the existence of MgAl secondary phases in the matrix. The YS/UTS values dramatically changed in both the as-cast and annealed states after the presence of rare earth elements. Interstingly, not only the inclusion of REEs boost the elasticity of the AZ31 alloy in its as-cast form, but also the addition of Nd significantly increased the tensile strength of the alloy. The AZ31 in as-cast form had a higher work-hardening rate than the other three REE-added samples, indicating that the buildup of dislocations in the AZ31 in the as-cast condition. On the other hand, the corrosion resistance was also enhanced by introducing rare earth elements into AZ31 Mg alloys. According to as-cast specimens, the corrosion rate following the co-addition of Nd+Dy elements was in between the values seen for the inclusion of single Nd and Dy elements, indicating that Nd improved the AZ31's corrosion resistance more than Dy because Nd could purify the melts and interact with Al to form the Al-Nd phase, which improved the alloys' corrosion resistance. We can categorically state that the co-REE modified AZ31 Mg alloys in as-annealed specimens stabilized the corrosion rate and suggesting that it would be more promising in the corrosive environment conditions.
References
D. Tie, F. Feyerabend, N. Hort, D. Hoeche, K.U. Kainer, R. Willumeit, and W.D. Mueller, In Vitro Mechanical and Corrosion Properties of Biodegradable Mg-Ag Alloys, Mater. Corros., 2014, 65, p 569-576.
Z.H. Wen, C.J. Wu, C.S. Dai, andF.X. Yang, Corrosion Behaviors of Mg and its Alloys with Different Al Contents in a Modified Simulated Body Fluid, J. Alloy. Compd., 2009, 488, p 392-399.
J. Du, J. Yang, M. Kuwabara, W.F. Li, and J.H. Peng, Effects of Carbon and/or Alkaline Earth Elements on Grain Refinement and Tensile Strength of AZ31 Alloy, Mater. Trans., 2008, 49, p 2303-2309.
J. Zhang, S. Liu, R. Wu, L. Hou, and M. Zhang, Recent Developments in High-Strength Mg-RE-Based Alloys: Focusing on Mg-Gd and Mg-Y Systems, J. Magnes. Alloy., 2018, 6, p 277-291.
S. You, Y. Huang, K.U. Kainer, and N. Hort, Recent Research and Developments on Wrought Magnesium Alloys, J. Magnes. Alloy., 2017, 5, p 239-253.
D.K. Xu, L. Liu, Y.B. Xu, and E.H. Han, The Crack Initiation Mechanism of the Forged Mg-Zn-Y-Zr Alloy in the Super-Long Fatigue Life Regime, Scr. Mater., 2007, 56, p 1-4.
I.P. Moreno, T.K. Nandy, J.W. Jones, J.E. Allison, and T.M. Pollock, Microstructural Stability and Creep of Rare-Earth Containing Magnesium Alloys, Scr. Mater., 2003, 48, p 1029-1034.
M.S. Dargush, G.L. Dunlop, K. Pettersen, Transactions of 19th International Die Casting Congress; Higgins, W., Ed.; North American Die Casting Association: Rosemont, IL, USA, 1997; pp. 131-137.
J.Y. Bai, S. Sun, F. Xue, S. Xue, J. Qiang, and T.B. Zhu, Influence of Annealing on Microstructures, Mechanical and Creep Properties of Mg-4Al-2Sr Alloy, Mater. Sci. Technol., 2006, 22, p 1208-1212.
B. Jing, S. Yangshan, X. Feng, X. Shan, Q. Jing, and T. Weijian, Effect of Extrusion on Microstructures, and Mechanical and Creep Properties of Mg-Al-Sr and Mg-Al-Sr-Ca Alloys, Scr. Mater., 2006, 55, p 1163-1166.
T. Rzychoń, A. Kiełbus, J. Cwajna, and J. Mizera, Microstructure Stability and Creep Properties of Die-Casting Mg-4Al-4RE Alloy, Mater. Charact., 2009, 60, p 1107-1113.
B.R. Powell, V. Rezhets, M.P. Balogh, and R.A. Waldo, Microstructure and Creep Behavior in AE42 Magnesium Die-Casting Alloy, JOM., 2002, 54, p 34-38.
Y.-X. Wang, J.-W. Fu, and Y.-S. Yang, Effect of Nd Addition on Microstructures and Mechanical Properties of AZ80 Magnesium Alloys, Trans. Nonferrous. Met. Soc. China., 2012, 22, p 1322-1328.
J. Zhang, J. Wang, X. Qiu, D. Zhang, Z. Tian, X.D. Niu, D.X. Tang, and M. Jian, Effect of Nd on the Microstructure, Mechanical Properties and Corrosion Behavior of Die-Cast Mg-4Al-Based Alloy, J. Alloy. Compd., 2008, 464, p 556-564.
Y. Lu, Q. Wang, X. Zeng, W. Ding, C. Zhai, and Y. Zhu, Effect of Rare Earths on the Microstructure, Properties and Fracture Behavior of Mg-Al Alloys, Mater. Sci. Eng. A., 2000, 278, p 66-76.
Y. Guangyin, L. Manping, D. Wenjiang, and A. Inoue, Mechanical Properties and Microstructure of Mg-Al-Zn-Si-base Alloy, Mater. Trans., 2003, 44, p 458-462.
C.C. Jain and C.H. Koo, Creep and Corrosion Properties of the Extruded Magnesium Alloy Containing Rare Earth, Mater. Trans., 2007, 48, p 265-272.
F. Guo, D.F. Zhang, X.S. Yang, L.Y. Jiang, and F.S. Pan, Strain-Induced Dynamic Precipitation of Mg17Al12 Phases in Mg-8Al Alloys Sheets Rolled at 748 K, Mater. Sci. Eng. A., 2015, 636, p 516-521.
X.-P. Xu and A. Needleman, Void Nucleation by Inclusion Debonding in a Crystal Matrix, Model. Simul. Mater. Sci. Eng., 1993, 1, p 111-132.
D.K. Xu, L. Liu, Y.B. Xu, and E.H. Han, Effect of Microstructure and Texture on the Mechanical Properties of the as-Extruded Mg-Zn-Y-Zr Alloys, Mater. Sci. Eng. A., 2007, 443, p 248-256.
X.H. Shao, Z.Q. Yang, and X.L. Ma, Strengthening and Toughening Mechanisms in Mg-Zn-Y Alloy with a Long Period Stacking Ordered Structure, Acta Mater., 2010, 58, p 4760-4771.
L.Y. Wei and G.L. Dunlop, The Solidification Behavior of Mg-Al-Rare Earth Alloys, J. Alloy. Compd., 1996, 232, p 264-268.
Z.F. Li, J. Dong, X.Q. Zeng, C. Lu, and W.J. Ding, Influence of Mg17Al12 Intermetallic Compounds on the Hot Extruded Microstructures and Mechanical Properties of Mg-9Al-1Zn Alloy, Mater. Sci. Eng. A., 2007, 466, p 134-139.
G. Song, A. Atrens, and D. St John, An Hydrogen Evolution Method for the Estimation of the Corrosion Rate of Magnesium Alloys, Magnesium Technology. Springer, Cham, 2001, p 255-262
M. Dixit, R.S. Mishra, and K.K. Sankaran, Structure-Property Correlations in Al 7050 and Al 7055 High-Strength Aluminum Alloys, Mater. Sci. Eng. A., 2008, 478, p 163-172.
U.M. Chaudry, A. Farooq, K.-B. Tayyab, A. Malik, M. Kamran, J.-G. Kim, C. Li, K. Hamad, and T.-S. Junis, Corrosion behavior of AZ31 Magnesium Alloy with Calcium Addition, Corrosion Sci., 2022, 199, p 110205.
J. Xie, J. Zhang, Z. Zhang, Q. Yang, K. Guan, Y. He, R. Wang, H. Zhang, X. Qiu, and R. Wu, New Insights on the Different Corrosion Mechanisms of Mg Alloys with Solute-Enriched Stacking Faults or Long Period Stacking Ordered Phase, Corrosion Sci., 2022, 198, p 110163.
Acknowledgments
This research was supported by a grant from a project to develop environment friendly pyrometallurgy processes for high purity HREE and materialization (Project No.: 20000970) by the Korea Evaluation Institute of Industrial Technology (KEIT) in the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lwin, M.L., Shin, Dw., Nam, S.W. et al. Effect of Single and Co-addition of Rare Earth on the Microstructure, Mechanical Properties, and Corrosion Behavior of AZ31 Magnesium Alloys. J. of Materi Eng and Perform (2023). https://doi.org/10.1007/s11665-023-08674-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11665-023-08674-y