Abstract
Chemical Vapor Deposition (CVD) is an effective method for large-scale graphene growth, but the growth of single-layer graphene using this process is relatively difficult. In this study, the morphological and structural properties of graphene grown by CVD on a copper substrate were investigated at four pressures: 5, 50, 100, and 1000 mbar. The formation mechanism is such that methane gas molecules collide with the copper surface after breaking their own bonds and are absorbed onto the copper surface. Based on Field Emission Scanning Electron Microscopy images, the graphene grown at 5 mbar pressure was a single-layer graphene without wrinkles, while wrinkles were observed at higher pressures due to the high gas density. In addition, according to the Raman results, the number of graphene layers grown increased with increasing pressure, leading to a decrease in the I2D/IG ratio from 2.07 to 0.5 cm−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, two-dimensional materials have attracted a lot of attention due to their good physical, electronic, mechanical, and chemical properties. These materials have been candidates for replacing conventional materials and have brought completely new applications in many areas of technology and industry (Ref 1). However, the major challenge in using them is still producing these materials with a wide surface, high purity, and high electrical resistance. Graphene can be mentioned as one of the materials that have these mentioned properties (Ref 2).
Graphene is a two-dimensional structure of a single layer of carbon honeycomb network in which each carbon atom is connected to three other carbon atoms using its three-electron capacity with three hybridized bonds. Graphene is a single layer of graphite with the fourth bonding electron remaining as a free electron (Ref 3). Due to its exceptional properties in electrical and thermal conductivity, high density, the excitability of charge carriers, optical conductivity, mechanical properties, and very strong, thin, and lightweight structure (Ref 4), graphene has become a unique material, and due to these features, graphene is one of the most important and advanced members among the large family of two-dimensional materials (Ref 5). Bilayer and multilayer graphene have found various applications in a wide range of fields, from flexible transparent electrodes with low resistance (Ref 6), and corrosion protection (Ref 7, 8), to the production of electronic components (Ref 9,10,11). However, precise construction of bilayer or multilayer graphene with a controlled number of layers is very difficult. Several chemical and physical methods have been proposed for producing graphene, and relatively good progress has been made on the laboratory scale in each of these methods, in which graphene is produced with different properties, shapes, and sizes. These methods include mechanical exfoliation (Ref 12), electrochemical exfoliation (Ref 3), liquid phase exfoliation (Ref 5), epitaxial growth on SiC (Ref 1), unzipping CNTs (Ref 13), reduced graphene oxide (Ref 5), and chemical vapor deposition (CVD) (Ref 1). However, the method that has attracted the most attention is the CVD method. In this method, the carbon separated by heat from the carbon precursor is placed on the surface of an active metal and forms a honeycomb network at high temperature and under atmospheric and low pressure (Ref 14). The reasons for using the CVD method for graphene growth include easy installation in research laboratories, long-term successful use in industrial environments, and the possibility of producing large areas of graphene with a controlled number of layers. Furthermore, considering environmental factors and cost, the CVD method is one of the best available methods for synthesizing graphene-based materials (Ref 15).
However, this method faces many challenges such as the impact of growth parameters. For example, the nature of the copper substrate's polycrystalline structure leads to an increase in graphene growth density and nucleation. That is why in recent years, many works have been done to solve this problem. Huang et al. (Ref 16) used metallic catalyst alloys to address this issue and reduce the nucleation of carbon atoms. Also, Liv et al. (Ref 17) suggested that by increasing the temperature of the CVD process, the nucleation rate decreases due to the reduction in surface roughness of the substrate, and it is possible to grow a uniform graphene on the substrate surface.
Recent research has reported that the synthesis of single-layer or multilayer graphene is possible by changing the ratio of carbon to hydrogen or increasing the partial pressure of hydrogen during CVD growth. In the same vein, Lee et al. (Ref 18) investigated the effects of a set of growth parameters on the quality of growth at different pressures. They found that to achieve single-layer and continuous graphene, a short growth time, high temperature, and slow cooling time are necessary. Sharma et al. (Ref 19) reported the successful synthesis of multilayer graphene by controlling the partial pressure of hydrogen. It was also reported that multilayer graphene can be synthesized by dynamic pressure control during the graphene growth process. However, precise control of the growth of single-layer or multilayer graphene with a specific number of layers has not been reported to date. Therefore, the aim of this study was to reduce the influence of partial pressure based on changes in methane gas flow on the growth of single-layer and multilayer graphene, in order to investigate the effect of pressure changes on the growth of graphene with different layer numbers and quality, and to be able to precisely grow single-layer or multilayer graphene.
2 Materials and Methods
In the present study, copper sheets with a purity of 99.999% and a thickness of 30 µm were used as the substrate. Initially, the copper sheets were cut into dimensions of 50 × 20 mm. After cutting, the sheets were placed in a 100% acetic acid solution for 10 min to remove any oxides present on the substrate surface. After completing this step, the samples were washed with distilled water and then placed in a methanol solution in an ultrasonic bath for 5 min to remove any impurities from the surface of the copper sheets. The samples were then washed again with distilled water. After completing the washing step, the sheets were dried using a vacuum oven at a temperature of 100 °C for 10 min.
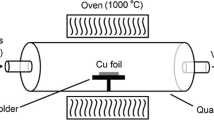
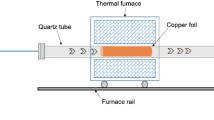
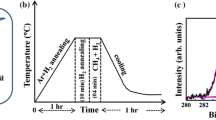
Initially, the samples were placed in a furnace and the air inside the furnace was evacuated using a vacuum pump (to a pressure of 3 × 10–1 mbar). After reaching a suitable vacuum, the furnace was heated to a temperature of 900 °C. Hydrogen gas was used as an auxiliary gas in the growth process and to prevent oxidation of the sample in the chamber. As shown in Fig. 1, hydrogen was introduced into the furnace with a flow rate of 20 sccm at a temperature lower than the oxidation temperature of copper (approximately 200 °C) during the heating process. After reaching a temperature of 900 °C, the pressure in the chamber was adjusted using methane gas at 5, 50, 100, and 1000 mbar. After the end of the coating time (20 min), the methane gas was turned off and the furnace was cooled using a flow of cold hydrogen gas.
To identify and characterize the structural properties of the graphene coatings, FESEM model MIRA3 from TESCAN, Raman spectroscopy in the wavelength range of 530-700 nm, and AFM were used for surface morphology analysis.
3 Result and Discussion
Figure 2 shows SEM images of graphene grown at different pressures. According to the figure, graphene grown at 5 mbar is a single-layer graphene without wrinkles or folds, whereas wrinkles and folds are visible at higher pressures. Additionally, at higher pressures, darker graphene planes are observed in the images, indicating an increase in the density and number of graphene layers with increasing pressure. This may be due to sufficient time for breaking the hydrocarbon bond and accumulating more carbon atoms on the copper foil surface (Ref 20, 21).
In Fig. 3, Raman spectra and the ratios of ID/IG and I2D/IG intensities are shown for different growth pressures. At a pressure of 5 mbar, the intensity of the 2D peak is higher than the G peak, and the I2D/IG ratio is greater than 2, indicating a single-layer graphene coating on the copper surface (Ref 22). At a pressure of 50 mbar (Fig. 3a), the I2D/IG ratio is greater than 1, indicating a small number of layers. As the pressure increases, the single-layer nature decreases, and the increase in the number of layers is visible with a decrease in the I2D/IG ratio. In addition, at pressures of 100 and 1000 mbar, this ratio is less than 1 (< 1), indicating a greater number of layers. In Fig. 3(b), the ratios of I2D/IG and ID/IG are shown. According to the figure, with increasing pressure, I2D/IG decreases and ID/IG increases, indicating an increase in the number of layers and an increase in defects, wrinkles, and folds due to an increase in the number of layers.
In Fig. 4(a), the percentage of layers as a function of increasing pressure is shown. The I2D/IG ratio for each sample at its corresponding pressure is the average of several Raman analysis points, as shown in Fig. 3(b). Since this ratio indicates the number of graphene layers, the percentage of these points for each sample was taken based on the number of layers, and plotted as a bar graph in Fig. 4(a). It can be seen from the figure that at lower pressures, a large area of graphene is formed that is single-layered (with a higher percentage of single-layeredness), and as the pressure increases, the percentage of single-layeredness decreases. In this figure, the percentage of bilayer and multilayer graphene is also shown as a function of pressure, which allows observing the growth behavior of graphene with different numbers of layers as pressure increases. Figure 4(b) shows the full width at half maximum (FWHM) of the 2D peak as a function of pressure. According to the figure, as the pressure increases, the FWHM value also increases, meaning that the 2D peak broadens and the number of graphene layers increases.
Table 1 shows data for the position and FWHM of the D, G, and 2D bands. With respect to the measured FWHM in the G band, it can be observed that this value increases from 25 to 57 cm−1 as pressure increases, indicating an increase in structural defects and a decrease in order in the graphene structure (Ref 23). As pressure increases, the FWHM of the 2D band becomes broader for all four different pressures. Additionally, the FWHM of the D band (related to graphene defect density and edge effects) increases with pressure. Since the changes in the G band position with respect to 1580 cm−1 indicate topological defects such as size, shape, and distribution of sp2 clusters, this value is equal to 6 cm−1 for 5 mbar pressure, which increases with pressure and is equal to 12 cm−1 for 1000 mbar pressure, indicating a decrease in growth quality. Furthermore, for the 2D band, the position of the band at 2690 cm−1 is one of the criteria for single-layer graphene formation, which according to the growth samples, the position of the 2D band is approximately in the range of 2690 cm−1 for all pressures. Also, based on the values recorded in Table 1, the position of the D band is approximately in the range of 1350 cm−1 for all four pressures, and significant changes are not observed.
In Fig. 5, AFM images of graphene grown at different pressures are shown. Based on the images, graphene layers are visible (bright areas correspond to graphene, and dark areas correspond to copper) and the thickness of these areas is low at a pressure of 5 mbar, indicating a low number of graphene layers. Additionally, the continuity of the graphene layers is low and uniformity is not observed in the dark and bright points. Based on Fig. 5(b), it is observed that most of the surface is covered by graphene. By comparing this figure with Fig. 5(a), the thickness of the graphene grown at 50 mbar is greater than 5 mbar. Because with increasing pressure, the density of methane gas increases, and there is more opportunity for carbon atoms to accumulate, which leads to an increase in the growth rate. Based on Fig. 5(c), the uniformity of the grown graphene is reduced, and in some areas, there are very bright points indicating a high number of graphene layers and nucleation in these areas. Based on this figure, the surface roughness is high, and the continuity is low. The increase in growth rate at high pressures has led to the aggregation of carbon atoms and nucleation in some areas, and the absence of carbon atoms in some areas. However, in regions where graphene has accumulated, layer continuity and uniformity can be observed. Based on Fig. 5(d), continuous layers are not observed, and surface roughness is greater than in other samples. At a pressure of 1000 mbar, the sample has many small bright points that have appeared due to nucleation in these points. In this figure, there is no continuity of the graphene layers, and the thickness of the layers has also increased. Due to the significant increase in pressure, the deposition rate has increased significantly and the surface of the sample has become saturated (Ref 24). Based on previous samples, the quality of this sample is not good.
4 Conclusion
-
1.
According to the results obtained from FESEM images and Raman spectroscopy, as the pressure increased from 5 to 50 mbar, the coherence and deposition rate of carbon atoms increased.
-
2.
With an increase in the pressure of the deposition layer to mbar 1000, the deposition of carbon exceeded the limit and the number of graphene layers increased.
-
3.
At 5 mbar, the percentage of single-layer graphene was higher, but at 50 mbar, the sample coherence was higher with fewer layers.
-
4.
From a practical point of view, there is a need for graphene on a wider scale. Therefore, graphene obtained under the growth conditions at 50 mbar is a better option than the sample obtained at pressures of 5, 10, and 100 mbar.
References
V.B. Mbayachi, E. Ndayiragije, T. Sammani, S. Taj, E.R. Mbuta, and A.U. Khan, Graphene Synthesis, Characterization and Its Applications: A review, Results Chem., 2021, 3, p 100163. https://doi.org/10.1016/j.rechem.2021.100163
S. Yaragalla, R.K. Mishra, S. Thomas, N. Kalarikkal, and H.J. Maria, Carbon-based nanofillers and their rubber nanocomposites: fundamentals and applications, Elsevier, 2019.
G. Yang, L. Li, W.B. Lee, and M.C. Ng, Structure of Graphene and Its Disorders: A Review, Sci. Technol. Adv. Mater., 2018, 19, p 613–648. https://doi.org/10.1080/14686996.2018.1494493
A. Dideykin, A.E. Aleksenskiy, D. Kirilenko, P. Brunkov, V. Goncharov, M. Baidakova, D. Sakseev, and A. YaVul, Monolayer Graphene from Graphite Oxide, Diam. Relat. Mater., 2011, 20, p 105–108. https://doi.org/10.1016/j.diamond.2010.10.007
Y. Yan, S. Manickam, E. Lester, T. Wu, and C.H. Pang, Synthesis of Graphene Oxide and Graphene Quantum Dots from Miscanthus Via Ultrasound-Assisted Mechano-Chemical Cracking Method, Ultrason. Sonochem., 2021, 73, p 105519.
K.S. Kim, Y. Zhao, H. Jang, S.Y. Lee, J.M. Kim, K.S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi, and B.H. Hong, Large-Scale Pattern Growth of Graphene Films for Stretchable Transparent Electrodes, Nature, 2009, 457, p 706–710. https://doi.org/10.1038/nature07719
S. Chen, L. Brown, M. Levendorf, W. Cai, S.-Y. Ju, J. Edgeworth, X. Li, C.W. Magnuson, A. Velamakanni, R.D. Piner, J. Kang, J. Park, and R.S. Ruoff, Oxidation Resistance of Graphene-Coated Cu and Cu/Ni Alloy, ACS Nano, 2011, 5, p 1321–1327. https://doi.org/10.1021/nn103028d
Y.H. Zhang, B. Wang, H.R. Zhang, Z.Y. Chen, Y.Q. Zhang, B. Wang, Y.P. Sui, X.L. Li, X.M. Xie, G.H. Yu, Z. Jin, and X.Y. Liu, The Distribution of Wrinkles and Their Effects on the Oxidation Resistance Of Chemical Vapor Deposition Graphene, Carbon, 2014, 70, p 81–86. https://doi.org/10.1016/j.carbon.2013.12.075
H.I. Røst, R.K. Chellappan, F.S. Strand, A. Grubišić-Čabo, B.P. Reed, M.J. Prieto, L.C. Tanase, L.S. de Caldas, T. Wongpinij, C. Euaruksakul, T. Schmidt, A. Tadich, B.C.C. Cowie, Z. Li, S.P. Cooil, and J.W. Wells, Low-Temperature Growth of Graphene on a Semiconductor, J. Phys. Chem. C, 2021, 125, p 4243–4252.
L. Gao, G.X. Ni, Y. Liu, B. Liu, A.H.C. Neto, and K.P. Loh, Face-to-Face Transfer of Wafer-Scale Graphene Films, Nature, 2014, 505, p 190–194.
Y. Lee, J. Kim, J.H. Koo, T.-H. Kim, and D.-H. Kim, Nanomaterials for Bioelectronics and Integrated Medical Systems, Korean J. Chem. Eng., 2018, 35, p 1–11. https://doi.org/10.1007/s11814-017-0236-5
M. Skoda, I. Dudek, A. Jarosz, and D. Szukiewicz, Graphene: One Material, Many Possibilities–Application Difficulties in Biological Systems, J. Nanomater., 2014 https://doi.org/10.1155/2014/890246
Y. Yao, Z. Li, Z. Lin, K.S. Moon, J. Agar, and C. Wong, Controlled Growth of Multilayer, Few-Layer, and Single-Layer Graphene on Metal Substrates, J. Phys. Chem. C, 2011, 115(13), p 5232–5238. https://doi.org/10.1021/jp109002p
F. Islam, A. Tahmasebi, R. Wang, and J. Yu, Structure of Coal-Derived Metal-Supported Few-Layer Graphene Composite Materials Synthesized Using a Microwave-Assisted Catalytic Graphitization Process, Nanomaterials, 2021, 2021(11), p 1672. https://doi.org/10.3390/NANO11071672
X. Li, W. Cai, L. Colombo, and R.S. Ruoff, Evolution of Graphene Growth on Ni and Cu by Carbon Isotope Labeling, Nano Lett., 2009, 9, p 4268–4272. https://doi.org/10.1021/nl902515k
M. Huang, M. Biswal, H. Park, S. Jin, D. Qu, S. Hong, Z.-L. Zhu, L. Qiu, D. Luo, L. Xiaochi, Z. Yang, Z. Liu, Y. Huang, H. Lim, W. Yoo, F. Ding, Y. Wang, Z. Lee, and R. Ruoff, Highly Oriented Monolayer Graphene Grown on a Cu/Ni(111) Alloy Foil, ACS Nano, 2018 https://doi.org/10.1021/acsnano.8b02444
L. Liu, H. Zhou, R. Cheng, Y. Chen, Y.-C. Lin, Y. Qu, J. Bai, I. Ivanov, G. Liu, Y. Huang, and X. Duan, A Systematic Study of Atmospheric Pressure Chemical Vapor Deposition Growth of Large-Area Monolayer Graphene, J. Mater. Chem., 2011, 22, p 1498–1503. https://doi.org/10.1039/C1JM14272K
B. Lee, W. Chu, and W. Li, Effects of Process Parameters on Graphene Growth Via Low-Pressure Chemical Vapor Deposition, J. Micro Nano Manuf., 2020 https://doi.org/10.1115/1.4048494
I. Sharma, G.S. Papanai, S.J. Paul, and B.K. Gupta, Partial Pressure Assisted Growth of Single-Layer Graphene Grown by Low-Pressure Chemical Vapor Deposition: Implications for High-Performance Graphene FET Devices, ACS Omega, 2020, 5, p 22109–22118. https://doi.org/10.1021/acsomega.0c02132
B. Luo, B. Chen, A. Wang, D. Geng, J. Xu, H. Wang, Z. Zhang, L. Peng, Z. Xu, and G. Yu, Chemical Vapor Deposition of Bilayer Graphene with Layer-Resolved Growth Through Dynamic Pressure Control, J. Mater. Chem. C, 2016, 4, p 7464–7471. https://doi.org/10.1039/C6TC02339H
J. Han, J.Y. Lee, and J.S. Yeo, Large-Area Layer-By-Layer Controlled and Fully Bernal Stacked Synthesis of Graphene, Carbon, 2016, 105, p 205–213. https://doi.org/10.1016/J.CARBON.2016.04.039
S. Trivedi, K. Lobo, and H.S.S.R. Matte, Synthesis Properties and Applications of Graphene, Elsevier, UK, 2019.
X. Li, W. Cai, J. An, S. Kim, J. Nah, D. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, and S.K. Banerjee, Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils, Science, 2009, 324, p 1312–1314.
S. Bhaviripudi, X. Jia, M.S. Dresselhaus, and J. Kong, Role of Kinetic Factors in Chemical Vapor Deposition Synthesis of Uniform Large Area Graphene Using Copper Catalyst, Nano Lett., 2010, 10, p 4128–4133. https://doi.org/10.1021/nl102355e
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noori, A., Eshraghi, M.J., Samiee, M. et al. The Effect of Pressure on the Growth of Single-Layer Graphene on Copper Sheets by Chemical Vapor Deposition Methods. J. of Materi Eng and Perform 33, 1996–2001 (2024). https://doi.org/10.1007/s11665-023-08259-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-023-08259-9