Abstract
A hydrogen-free surface nitriding process was proposed to enhance the wear properties of TiZrAlV alloy by using double-glow plasma. The structure, morphology, composition, hardness, tribological behavior and corrosion properties of nitrided TiZrAlV alloy have been systematically characterized. A dense and continuous nitrided layer, with a thickness of 110 μm, was formed on the surface of TiZrAlV alloy, mainly containing TiN, and Ti2N phases. The surface hardness of nitrided TiZrAlV alloy was 1437HV, which is 4 times higher than that of as-received alloy. The formation of high-hardness nitrides phases results in remarkable amelioration in specific wear rate and wear resistance of TiZrAlV alloy after nitriding. Moreover, the corrosion resistance of nitrided TiZrAlV alloy, measured in 1 M HCl and 3.5 wt.% NaCl solutions, has been significantly increased. The nitriding treatment reduced the relative proportion of the weight loss after being immersed in 2 M HCl solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Comprehensively, titanium (Ti) alloys are extensively utilized in a wide variety of applications, including aerospace, energy, biomedicine and petrochemical industries due to their excellent properties (Ref 1,2,3). A new series of TiZrAlV alloys, recently, have been developed by adding zirconium (Zr), possessing low density, high strength, excellent high temperature performance, exceptional strength-to-weight ratio and good corrosion resistance (Ref 4,5,6). However, the lower hardness (only about 360 HV) and inferior wear resistance of TiZrAlV alloys seriously restrict their wide utilization as structural materials. Therefore, it is of utmost significance to solve these problems and enhance the potential utilization of TiZrAlV alloys in various practical applications.
Many measures have been taken to further improve the hardness and wear resistance of TiZr alloys. Zhang et al. (Ref 7) showed that the wear resistance of Ti alloys can be improved by adjusting the microstructure. Further, Zhong et al. (Ref 8) studied the effect of different annealing temperature on the wear resistance of TiZr alloys. Compared with the above methods, however, forming nitrides on the surface of titanium alloys is a more effective measure to improve functional properties, such as hardness, wear resistance and corrosion resistance, prolong service life and expand the scope of potential applications (Ref 9,10,11). Various surface treatment techniques are adopted to prepare nitrides films for Ti alloys, such as laser treatment (Ref 6), plasma nitriding (Ref 9, 10) and thermo-diffusion method (Ref 11). Among these methods, the plasma nitriding was widely used as a promising and effective method (Ref 12, 13). Recently, a new plasma nitriding method, named double-glow plasma method, was proposed (Ref 14, 15). Compared with the traditional ion nitriding method, the double-glow plasma method has an auxiliary electrode besides the cathode and anode. The auxiliary electrode with the same composition as the sample played an auxiliary heating role in the experiment (Ref 14), which means that the nitriding efficiency can be further improved and the cost can be reduced. Generally, the nitrogen source of the nitriding process is ammonia (NH3) or the mixture gas of nitrogen and hydrogen (Ref 6, 14,15,16,17,18). Titanium and zirconium alloys, however, are sensitive to hydrogen which can diffuse into these alloys at high working temperature, leading to hydrogen embrittlement and compromising the mechanical properties (Ref 12, 16). Therefore, the hydrogen-free nitriding treatment is required to eliminate the adverse effect of hydrogen embrittlement.
In this work, the double-glow plasma is utilized, as a hydrogen-free nitriding method, to prepare a nitrided layer on the surface of TiZrAlV alloy, and the high-purity nitrogen gas was selected as the nitriding source. Furthermore, the tribological properties and corrosion behavior of the nitrided layer were systematically investigated.
2 Experimental Procedure
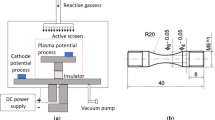
The composition of TiZrAlV alloy used for nitriding experiment is shown in Table 1. The TiZrAlV alloy was cut into a size of Φ40 mm × 4 mm by electric spark wire cutting machine before nitriding treatment. The specimens were mechanically polished, ultrasonically washed and dried in air. The nitriding treatment was carried out using a two-electrode double-glow plasma surface alloying equipment. Herein, one electrode served as the working electrode and the other served as an auxiliary electrode, which possesses the same composition as the sample. The auxiliary electrode played the heating role in the experimental process. The furnace was vacuumed to < 4 Pa and then filled with high-purity nitrogen gas to keep the working pressure at ~ 120 Pa. In order to ensure the nitriding effect and avoid excessive growth of alloy structure at a higher temperature, the working temperature was determined as 850 °C in α + β two-phase region and the working time was 4 h.
The microstructure and composition were analyzed by field emission scanning electron microscope (FESEM, Hitachi S-4800, Tokyo, Japan), equipped with an energy dispersive spectrometer (EDS). A Philips PW1050 x-ray diffractometer (XRD), equipped with CuKα radiations, was used to identify the crystal structure of the nitride layer. The hardness was assessed by using an Axiovert 200 Ma machine under a working load of 50 gf and loading time of 10 s. The surface roughness of specimens before and after nitriding was measured by a profilometer (XP-2™).
Furthermore, a reciprocating ball-on-disk tribological apparatus (Rtec instruments, MFT-5000, USA.) was used to evaluate the dry sliding wear resistance of the as-received and nitrided TiZrAlV alloys in the air. The disk was TiZrAlV specimen with a diameter of 40 mm as well as a thickness of 4 mm, and the counterpart ball with a diameter of 9.5 mm was made of 440C steel. The sliding velocity was 600 rpm with a rotational radius of 15 mm. The applied normal load and loading time were 20 N and 60 min, respectively. During the sliding test, 440C steel ball remained stationary and the specimen kept rotating. A white light interferometer was used to analyze the wear scars profiles.
The polarization curves were measured in 1 M HCl solution and 3.5 wt.% NaCl solution by using a CHI660C electrochemical workstation and a standard three-electrode cell, where saturated calomel electrode (SCE) and platinum plate served as a reference electrode (RE) and counter electrode (CE), respectively. The exposed area of the working electrode (WE) was ~ 1 cm2. The working electrode was scanned in the voltage range of −1.2 to 3 V (versus SCE) at a scan rate of 0.5 mV/s. Prior to the potential dynamic measurements, the specimen was immersed in the electrolyte for 20 min to ensure the stability of working potential. The immersion test was conducted in 2 M HCl solution for 360 h.
3 Results and Discussion
3.1 Nitriding
Figure 1(a) displays the surface morphology of the nitrided TiZrAlV alloy. It can be readily observed that the nitrided surface exhibits a compact hilly feature which may be caused by the accumulation of nitrides, and there is no palpable defects on the surface, such as shedding and holes. The average chemical compositions of nitrided surface were determined by EDS and the results revealed that the nitrogen content is ~ 13.82 wt.%. This result can be used as the evidence of nitrogen enrichment on the sample surface after plasma nitriding (Ref 14). The cross-sectional morphology of the nitrided TiZrAlV alloy is presented in Fig. 1(b), which shows a uniform double-film structure. This special film structure is closely related to a significant nitriding process of TiZrAlV alloy. During the nitriding process, the N2 is decomposed into uncharged N or charged species, and then, N is transferred from the plasma to the TiZrAlV alloy, both by “implantation” of charged species into the surface and the adsorption of uncharged N at the surface. At the same time, the arrival of N from the plasma to surface and the diffusion of N from surface to inside occur simultaneously on the alloy surface. When these two reactions are in equilibrium, only a nitrogen diffusion region will be formed inside the alloy. However, if more nitrogen is provided at the surface than can diffuse inwardly, nitrides can develop at the surface, on top of a diffusion zone. In this work, high-purity nitrogen as a nitriding atmosphere can provide sufficient N for the nitriding process. Therefore, a uniform double-film structure can be observed from Fig. 1(b). Zone-I is an outer nitrides layer with a thickness of ~ 6 μm. Furthermore, it can be seen from the partially enlarged view of Zone-I (shown in the inset image in Fig. 1b) that there is a relatively dense structure of the outer nitrides layer. Zone-II is the diffusion layer with a thickness of ~ 100 μm, and Zone-III refers to the TiZrAlV alloy matrix. It can be seen from Fig. 1(b) that the entire nitrided layer is closely bonded to the TiZrAlV alloy matrix without obvious defects such as holes and cracks indicating a good nitridation quality.
X-ray diffraction patterns of the as-received and nitrided alloys are shown in Fig. 1(c) and (d). Figure 1(c) shows XRD pattern of the as-received TiZrAlV alloy. The main peaks of the α (Ti) and β phases are observed, indicating the “α + β” two-phase structure of TiZrAlV alloy. Figure 1(d) shows XRD pattern of the nitrided TiZrAlV alloy. It can be seen from Fig. 1(d) that nitrides were formed on the surface of TiZrAlV alloy, which are in agreement with the above analysis. Moreover, the formation of the nitrides can be described by some reactions during the nitriding process (Ref 6, 17):
Surface adsorption:
Nitrogen dissociation:
Nitrides formation:
At the initial stage of nitriding process, the concentration of nitrogen at the gas/metal interface is higher than that of the metal. Based on reaction diffusion rules, N diffuses into the metal and then forms nitrides. For Ti alloys, Ti2N will preferentially form on the metal surface (Ref 17). When the nitrogen concentration at the gas/metal interface is higher than the acceptable concentration in Ti2N, the sample surface will undergo a phase transformation and Ti2N will be transformed into TiN (Ref 17).
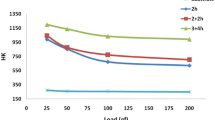
Figure 2 presents the hardness profiles of the as-received and nitrided TiZrAlV alloy. The hardness of nitrided TiZrAlV alloy gradually decreased from 1437 HV near the surface to 365 HV at a depth about 120 μm, which is closely related to the special nitrided layer structure. Firstly, the high hardness of the alloy surface is attributed to the external nitrides layer (Ref 18), and secondly, the gradually decreasing hardness from outside to inside strongly depends on the dissolution of N in the diffusion region. According to the diffusion theory, temperature is one of the important factors affecting the diffusion of atoms, and the higher temperature is conducive to the diffusion of N. Further, the phase diagram for TiN shows that at 860 °C the hcp α-Ti has a large solubility for N (Ref 19). And this temperature region is very close to the nitriding temperature (850 °C) of the TiZrAlV alloy, resulting in a great solubility for N during the nitriding process. Further, the dissolution of N stabilizes the α phases (Ref 19, 20). Thus, a layer of α-Ti develops (zone II) by enhancing the N content in the diffusion zone, while the alloy is α + β underneath (zone III). The nitrogen content in the growing α-Ti layer decreases gradually. Nitrogen is known to be an effective solid solution strengthener in Ti, which explains the occurrence of a hardness gradient, and the similar phenomenon was also observed in other nitride alloys (Ref 6, 14, 21). Further, the surface roughness of TiZrAlV alloy was also measured after nitriding. The results showed that the surface roughness of nitrided specimen is 0.15 μm, while that of the as-received specimen is 0.09 μm.
3.2 Wear Resistance
Figure 3 shows the typical coefficient of friction (COF) plots of the as-received and nitrided TiZrAlV alloy under the normal load of 20 N. Similarly, the COF plots of the as-received and nitrided TiZrAlV alloy started at a higher value, then decrease with the increase in sliding time and finally tend to be stable. The initial high COF values are due to the initial high roughness of the sample surface. The wear processes can be divided into running-in and steady stages. During the running-in stage, the COF significantly fluctuated and exhibited higher value than the steady stage. At the steady sage, the COF values of the as-received TiZrAlV alloy were found to be 0.59, which is much larger than the nitrided TiZrAlV alloy of 0.22. Further, the COF of the substrate possesses larger fluctuations than the nitrided TiZrAlV alloy. The TiZrAlV alloy has a lower hardness, which is easy to lead to higher and fluctuation COF. However, the nitrided TiZrAlV alloy exhibits excellent friction reducing property and more stable friction process due to the much higher surface hardness than 440C steel ball. The wear rate (ω) can be calculated by using the classical wear equation:
where Δw refers to the weight loss, which can be obtained by measuring the specimens before and after wear tests with an electronic balance. F represents the normal applied load and L denotes the sliding distance. The wear rate of the as-received and nitrided TiZrAlV alloy was 5.89e−8 and 1.88e−8 g N−1 m−1, respectively. Hence, the nitriding process significantly reduced the friction coefficient and wear rate of TiZrAlV alloy.
Ex situ SEM and EDS analysis were employed to investigate the wear mechanism of as-received and nitrided TiZrAlV alloy. Figure 4(a) to (d) displays the wear morphologies of the worn surface of the as-received and nitrided TiZrAlV alloy against 440C steel ball. As presented in Fig. 4(a) and (b), the surface of as-received TiZrAlV alloy exhibits obvious grooves and extensive wear debris, indicating the occurrence of serious abrasive wear. During the dry sliding process, the as-received TiZrAlV alloy with lower hardness is easily scratched by the hard micro-convex on the surface of 440C steel ball and produce wear debris. These hard debris will also cause secondary wear on the as-received TiZrAlV alloy with the relative sliding process. And these effects collectively caused severe abrasive wear on the surface of as-received TiZrAlV alloy. Additionally, some micro-cracks also appeared on the worn surface of as-received TiZrAlV alloy, which represents the fatigue failure appeared (Fig. 4b). However, due to much higher surface hardness of nitrided TiZrAlV alloy than that of the 440C steel ball, the worn surface of the nitrided TiZrAlV alloy exhibits extremely shallow wear scar and almost no wear debris are observed, indicating a very slight abrasive wear (Fig. 4c and d). In addition, no micro-cracks are found on the worn surface of nitrided TiZrAlV alloy.
In order to further clarify the wear mechanism, EDS was applied to analyze the worn surfaces of as-received and nitrided TiZrAlV alloy, and the results are shown in Fig. 4(e) and (f). It can be clearly seen that large quantities of oxygen (O) were observed on the worn surfaces of both kinds of samples, which is mainly ascribed to the accumulation of frictional heat (Ref 22,23,24). During the process of dry sliding, as the time goes on, a large amount of heat gathered on the sliding contact surface, causing the local temperature increase sharply. Since dry sliding tests are carried out in atmosphere, oxides are easily formed on the worn surfaces, resulting in a high oxygen content. Unfortunately, the friction heat is also a vital reason to reduce the shear strength of sliding contact surface leading to fatigue failure (Ref 23, 24). Thus, it caused the micro-cracks on the worn surface of as-received TiZrAlV alloy (Fig. 4b). But, in the case of nitrided TiZrAlV alloy, the nitrided layer composed of high-hardness ceramic phases possessing excellent load-bearing capacity and renders high strength, which can inhibit the initiation and propagation of micro-cracks (Ref 14, 25). Therefore, although friction heat is generated on the worn surface of nitrided TiZrAlV alloy, no micro-cracks formed. Moreover, compared with as-received TiZrAlV alloy, a lot of iron (Fe) and chromium (Cr) elements (15.43 and 3.31 wt.% respectively) deriving from 440C steel ball are observed on the worn surface of the nitrided TiZrAlV alloy, implying the occurrence of materials transfer between the sliding contact surface. The results also corroborate the adhesive wear between the nitrided TiZrAlV alloy and 440C steel ball. In fact, during the relative sliding process between nitrided TiZrAlV alloy with higher hardness and 440C steel ball, some particles or fragments are peeled off from the 440C steel ball and attached to the surface of nitrided TiZrAlV alloy, thus realizing the material transfer. For as-received TiZrAlV alloy, however, the lower hardness means it is difficult to torn off chips from 440C steel ball. Even minute quantity of chips is torn off from 440C steel ball, these chips are difficult to attach to the as-received TiZrAlV alloy due to its serious worn surface. Therefore, no transfer materials were observed on the worn surface of as-received TiZrAlV alloy. Interestingly, the transfer layer formed by transfer materials can partially inhibit the wear degree and reduce the wear rate of substrate (Ref 25). Based on this point of view, the wear rate of nitrided TiZrAlV alloy is lower than as-received TiZrAlV alloy, which is in good agreement with the results of wear rate calculations. In summary, the high-hardness nitride layer plays an important role in improving the wear resistance of TiZrAlV alloy. Figure 5 shows the 2D cross-sectional wear profiles of the as-received and nitrided TiZrAlV alloy measured by white light interferometer. The wear scars of as-received TiZrAlV alloy are much deeper than that of the nitrided TiZrAlV alloy. And some grooves with different depths can be also observed from the 2D wear profiles of as-received TiZrAlV alloy, further proving the severe abrasive wear.
3.3 Potentiodynamic Polarization Measurements
The room-temperature potentiodynamic polarization curves of as-received and nitrided-TiZrAlV alloy were measured in 3.5 wt.% NaCl and 1 M HCl solutions, and the results are shown in Fig. 6. It can be seen from Fig. 6(a) and (b) that the current density of the as-received TiZrAlV alloy rapidly increases in NaCl and HCl solutions at 1.505 V (versus SCE) and 1.137 V (versus SCE), respectively, which indicates a pitting tendency. Conversely, the nitrided TiZrAlV alloy displays no pitting corrosion in both solutions. The corrosion parameters, such as corrosion current density (icorr), anodic Tafel slope (βa), cathodic Tafel slope (βc), and corrosion potential (Ecorr), were obtained by using the Tafel extrapolation method, and the results are summarized in Tables 2 and 3. Obviously, the corrosion current density has been significantly decreased both in 3.5 wt.% NaCl and 1 M HCl solutions after nitriding treatment. In particular, the corrosion current density of nitrided TiZrAlV alloy is six times lower than the as-received TiZrAlV alloy in HCl solution. Also, the corrosion potential of the nitrided TiZrAlV alloy has been significantly increased in NaCl and HCl solutions. The polarization resistance (Rp) of the as-received and nitrided-TiZrAlV alloy was calculated by using the Stern–Geary formula:
The polarization curves of the as-received and nitrided TiZrAlV alloy in 3.5% NaCl solution (a) and 1 mol/L HCl solution (b); the SEM morphologies of the corroded surfaces: the as-received TiZrAlV alloy in 3.5% NaCl (c) and 1 mol/L HCl (e) solutions; and the nitrided TiZrAlV alloy in 3.5% NaCl (d) and 1 mol/L HCl (f) solutions
The results indicate that the polarization resistance of the nitrided TiZrAlV alloy is much higher than the as-received TiZrAlV alloy both in 3.5 wt.% NaCl and 1 mol/L HCl solutions. In general, lower icorr corresponds to a lower corrosion rate, whereas higher Ecorr and Rp represent the higher chemical stability and lower corrosion tendency (Ref 26,27,28,29,30). These results imply the improvement of corrosion resistance after nitriding treatment both in HCl and NaCl mediums.
Furthermore, SEM observations were also carried out to analyze the corrosion morphology of as-received and nitrided TiZrAlV alloys and the results are shown in Fig. 6(c) to (f). A large number of corrosion pits with different sizes can be observed on the surface of as-received TiZrAlV alloy (Fig. 6c and e), which illustrates the pitting corrosion during the polarization in NaCl and HCl solutions. This result is consistent with the potentiodynamic polarization curves in Fig. 6(a) and (b). However, as shown in Fig. 6(d) and (f), no corrosion pits are observed on the corroded surfaces of the nitrided TiZrAlV alloy both in NaCl and HCl solutions. These results declare that the nitrided layer can effectively inhibit the pitting corrosion of TiZrAlV alloy both in NaCl and HCl solutions. It is generally believed that chloride-ions attack is one of the important causes of pitting. The point defect model (PDM) proposed by Macdonald et al. (Ref 31,32,33,34) declares that the growth and dissolution of the alloy passivation films a consequence of the balancing fluxes of the migration of oxygen-ion vacancies and metal-ion vacancies. The equilibrium of two kinds of vacancies migration announces that the growth and dissolution of passivation film are balanced. At this moment, the alloy can achieve a stable passivation state and obtain a passivation film with a stable thickness. However, when the solution contains a large amount of Cl−, these Cl− will adsorb on the alloy surface and combine with metal ions on passivation film to form soluble chlorides (Ref 35). Then, the above equilibrium state will be disturbed with the dissolution of the chlorides, thus promoting the dissolution of the passive film and forming the penetrable cracks (Ref 36,37,38,39). Finally, the pitting corrosion occurs. However, a uniform and dense nitrided layer formed on the surface of TiZrAlV alloy after nitriding treatment, which effectively prevented the adsorption of Cl− and reduced the sensitivity of TiZrAlV alloy to chloride-induced attack. Therefore, no corrosion pits are formed on the corroded surface of the nitrided TiZrAlV alloy, indicating a nice corrosion resistance of the nitrided layer.
3.4 Immersion Test
In order to further clarify the corrosion resistance of nitrided TiZrAlV alloy, the immersion test was conducted in 2 M HCl solution for 360 h. The weight loss of the specimens was calculated, and the results are presented in Fig. 7(a). It can be readily observed that the immersion weight loss of as-received TiZrAlV alloy increased with time and reached to 82.8 g/m2 after 360 h. However, the weight loss of nitrided-TiZrAlV alloy was only 4.032 g/m2 after 360 h. Figure 7(b) and (c) presents the SEM images of the as-received and nitrided TiZrAlV alloy after immersion test. As shown in Fig. 7(b), some corrosion pits can be observed on the corroded surface of as-received TiZrAlV alloy, which mainly ascribes to the chloride attack. This indicates the occurrence of pitting corrosion in as-received TiZrAlV alloy, which is consistent with the potentiodynamic polarization measurements. Besides, several α-laths experienced severe corrosion can be also observed, implying the occurrence of homogeneous surface corrosion on the as-received alloy in HCl solution. In the case of nitrided TiZrAlV alloy (Fig. 7c), no corrosion pits and corroded α-lath morphology are observed, indicating that the nitrided layer is not broken down. These results further prove that the nitrided layer has an excellent protective effect on the TiZrAlV alloy matrix in acid solution.
4 Conclusions
In summary, hydrogen-free plasma nitriding treatment was carried out on TiZrAlV alloy, and the structure and tribological properties of nitrided layer have been systematically investigated. The main results of the current study are summarized below:
-
(1)
A homogeneous, dense and continuous nitride layer, with a thickness of 110 μm, was formed on the surface of TiZrAlV alloy. The analysis of cross-sectional hardness revealed a gradient decreasing trend from the surface to the interior, which is caused by the double-film structure of the nitrided layer. The surface hardness of the TiZrAlV alloy increased to 1437 HV after nitriding, which is 4 times higher than the as-received TiZrAlV alloy.
-
(2)
After nitriding, the coefficient of friction of TiZrAlV alloy decreased from 0.59 to 0.22, and the specific wear rate also decreased from 5.89 × 10−7 to 1.89 × 10−7 g N−1 m−1. Further, the wear scars of the nitrided TiZrAlV alloy are much lighter than that of the as-received alloy. These results sufficiently confirm the excellent wear resistance of TiZrAlV alloy after nitriding.
-
(3)
The corrosion potential and polarization resistance of the nitrided-TiZrAlV alloy measured in HCl and NaCl solutions have been significantly increased, whereas the corrosion current density decreased. The corrosion current density of nitrided-TiZrAlV alloy in HCl solution was six times lower than the as-received TiZrAlV alloy.
-
(4)
The results of the immersion test in 2 M HCl solution indicate the lower weight loss of nitrided TiZrAlV alloy than the as-received TiZrAlV alloy. The nitrided layer can effectively prevent the alloy matrix from chloride-induced attack.
References
A.V. Tyunkov, D.A. Golosov et al., Nitriding of Titanium in Electron Beam Excited Plasma in Medium Vacuum, Surf. Coat. Technol., 2020, 383, p 125241.
P. Kwasniak and H. Garbacz, Screw Dislocation Mediated Solution Strengthening of Substitutional α-Ti Alloys–First Principles Investigation, Acta Mater., 2017, 141, p 405–418.
Y. Yang, R.-R. Chen et al., Improving Microstructure and Mechanical Properties of Alloy Ti43Al5Nb0.1B by Addition of Fe, Rare Met., 2019, 38, p 1024.
R. Jing, S.X. Liang et al., Effect of the Annealing Temperature on the Microstructural Evolution and Mechanical Properties of TiZrAlV Alloy, Mater. Des., 2013, 52, p 981–986.
S.X. Liang, Z.H. Feng et al., The Effects of V Content on the Microstructure and Mechanical Properties of Hot Rolled TiZr Based Alloys, J. Alloys Compd., 2016, 664, p 11–18.
Z.-H. Feng, X.-Y. Sun et al., Microstructure and Microhardness of a Novel TiZrAlV Alloy by Laser Gas Nitriding at Different Laser Powers, Rare Met., 2020, 39, p 270–278.
G.-S. Zhang, D.-F. Guo, M. Li et al., Tailoring Microstructure and Tribological Properties of Cold Deformed TiZrAlV Alloy by Thermal Treatment, Acta Metall. Sin., 2016, 10, p 1007.
H. Zhong, L.Y. Dai, Y. Yue et al., Friction and Wear Behavior of Annealed Ti-20Zr-65Al-4V Alloy Sliding Against 440C Steel in Vacuum, Tribol Int., 2017, 109, p 571–577.
A. Nishimoto, H. Nii et al., Simultaneous Duplex Process of TiN Coating and Nitriding by Active Screen Plasma Nitriding, Surf. Coat. Technol., 2013, 228, p S558–S562.
S. Ahmed, D. Chakrabarty et al., Plasma Nitriding on Titanium Surface for Adhesion Promotion, Surf. Eng., 2015, 31, p 616–622.
D. Rodriguez, F.J. Gil, and J.A. Planell, Wear Resistance of the Nitrogen Diffusion Hardening of the Ti-6Al-4V alloy, J. Biomech., 1998, 1001, p 49.
P. Olsson, K. Kese et al., On the Role of Hydrogen Filled Vacancies on the Embrittlement of Zirconium: An ab Initio Investigation, Nucl. Mater., 2015, 467, p 311–319.
E. Galvanetto, F.P. Galliano et al., Corrosion Resistance Properties of Plasma Nitrided Ti-6Al-4V Alloy in Hydrochloric Acid Solutions, Corros. Sci., 2002, 44, p 1593–1606.
Z. Xu, X. Liu, P. Zhan et al., Double Glow Plasma Surface Alloying and Plasma Nitriding, Surf. Coat. Technol., 2007, 201, p 4822–4825.
X.Y. Ai, H.L. Lin et al., Hydrogen-Free Nitriding of ZrTiAlV by Double Glow Plasma Discharge Improving the Wear Resistance, Mater. Sci. Technol., 2018, 34, p 1303–1305.
N.S. Mashovets, I.M. Pastukh, and S.M. Voloshko, Aspects of the Practical Application of Titanium Alloys after Low Temperature Nitriding Glow Discharge in Hydrogen-Free-Gas Media, Appl. Surf. Sci., 2017, 392, p 356–361.
A. Zhecheva, W. Sha, S. Malinov, and A. Long, Enhancing the Microstructure and Properties of Titanium Alloys through Nitriding and Other Surface Engineering Methods, Surf. Coat. Technol., 2005, 200, p 2192–2207.
F. Zhang, M. Yan et al., Influence of Plasma Nitriding Temperature on Microstructures and Mechanical Properties of Ti-N/Ti-Al Multilayer Coatings on the Surface of 5083 Al Alloys, Surf. Coat. Technol., 2018, 335, p 80–87.
M. Gushchina, T. Carstensen et al., Plasma Nitriding Ti-6Al-4V with the Aid Non-Transmitted Plasma-Arc using Different Protection Atmosphere, Mater. Today Proc., 2020, 30, p 694–699.
T. Suzuki, D. Huang, and Y. Ikuhara, Microstructures and Grain Boundaries of (Ti, Al)N Films, Surf. Coat. Technol., 1998, 107, p 41–47.
S. Semboshi, S. Kimura, A. Iwase, and N. Ohtsu, Surface Hardening of Age-Hardenable Cu-Ti Dilute Alloys by Plasma Nitriding, Surf. Coat. Technol., 2014, 258, p 691–698.
M.N. Gardos, The Effect of Anion Vacancies on the Tribological Properties of Rutile (TiO2-x), Tribol. Trans., 2008, 31, p 427–436.
M.N. Gardos et al., The Effect of Anion Vacancies on the Tribological Properties of Rutile (TiO2-x), Part II: Experimental Evidence, Tribol. Trans., 2008, 33, p 209–220.
T.F.J. Quinn, J.L. Sullivan, and D.M. Rowson, Origins and development of oxidational wear at low ambient temperatures, Wear, 1984, 94, p 175–191.
L. Liu, H.H. Shen et al., Wear Resistance of TiN(Ti2N)/Ti Composite Layer Formed on C17200 Alloy by Plasma Surface Ti-Alloying and Nitriding, Appl. Surf. Sci., 2016, 388, p 103–108.
S.K. Yen, S.H. Chiou, S.J. Wu, C.C. Chang, S.P. Lin, and C.M. Lin, Characterization of Electrolytic HA/ZrO2 Double Layers Coatings on Ti-6Al-4V Implant Alloy, Mater. Sci. Eng., 2006, 26, p 65–77.
O.S. Adesina, B.A. Obadele et al., Influence of Phase Composition and Microstructure on Corrosion Behavior of Laser Based Ti-Co-Ni Ternary Coatings on Ti-6Al-4V Alloy, J. Alloys Compd., 2020, 827, p 154245.
S. Zhou, Y. Zhao et al., Enhanced Corrosion Resistance of Ti-5 wt.% TiN Composite Compared to Commercial Pure Ti Produced by Selective Laser Melting in HCl Solution, J. Alloys Compd., 2020, 820, p 153422.
R. Kumari and J.D. Majumdar, Studies on Corrosion Resistance and Bio-Activity of Plasma Spray Deposited Hydroxylapatite (HA) Based TiO2 and ZrO2 Dispersed Composite Coatings on Titanium Alloy (Ti-6Al-4V) and the Same After Post Spray Heat Treatment, Appl. Surf. Sci., 2017, 420, p 935–943.
C. Haixiang and K. Dejun, Comparison on Electrochemical Corrosion Performances of Arc and Laser Thermal Sprayed Al-Ti-Ni Coatings in Marine Environment, Mater. Chem. Phys., 2020, 251, p 12320.
Y. Chen, X. Nie, and D.O. Northwood, Plasma Electrolytic Oxidation (PEO) Coatings on a Zirconium Alloy for Improved Wear and Corrosion Resistance, WIT Trans. Eng. Sci., 2010, 66, p 183.
D.D. Macdonald, The History of the Point Defect Model for the Passive State: A Brief Review of Film Growth Aspects, Electrochim. Acta, 2001, 56, p 1761–1772.
J. Geringer and D.D. Macdonald, Modeling Fretting-Corrosion Wear of 316L SS Against poly(methyl methacrylate) with the Point Defect Model: Fundamental Theory, Assessment, and Outlook, Electrochim. Acta, 2012, 79, p 17–30.
D. Digby, Macdonald, Some Personal Adventures in Passivity—a Review of the Point Defect Model for Film Growth, Russ. J. Electrochem., 2012, 48, p 235–258.
A.M. Fekry, The Influence of Chloride and Sulphate Ions on the Corrosion Behavior of Ti and Ti-6Al-4V Alloy in Oxalic Acid, Electrochim. Acta, 2009, 54, p 3480–3489.
D. Sazou, K. Saltidou, and M. Pagitsas, Understanding the Effect of Bromides on the Stability of Titanium Oxide Films Based on a Point Defect Model, Electrochim. Acta, 2012, 76, p 48–61.
R. Narayanan and S.K. Seshadri, Point Defect Model and Corrosion of Anodic Oxide Coatings on Ti-6Al-4V, Corros. Sci., 2008, 50, p 1521–1529.
Z. Jiang, X. Dai, T. Norby, and H. Middleton, Investigation of Pitting Resistance of Titanium Based on a Modified Point Defect Model, Corros. Sci., 2011, 53, p 815–821.
G. Ansari and A. Fattah-Alhosseini, On the Passive and Semiconducting Behavior of Severely Deformed Pure Titanium in Ringer’s Physiological Solution at 37 °C: A Trial of the Point Defect Model, Mater. Sci. Eng. C, 2017, 75, p 64–71.
Acknowledgments
This work was supported by the NSFC (Grant no.51531005/51571174).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, J.W., Ai, X.Y., Zhang, B. et al. Hydrogen-Free Plasma Nitriding to Enhance Wear and Corrosion Resistance of TiZrAlV Alloy. J. of Materi Eng and Perform 33, 54–63 (2024). https://doi.org/10.1007/s11665-023-08151-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-023-08151-6