Abstract
In the process of measuring the surface tension of Ga and Ga-based liquid metals by pendant-drop method, we found that at least two factors will lead to inaccurate measurement results. For one thing, it is due to the coating of the oxide film on the surface of the pendant-droplet, which shows the viscoelasticity. For another thing, the volume of pendant-drop does not exceed the critical value to ensure the accuracy of measurement. When the volume of the pendant-drops exceeds the critical value (the volume when Worthington number > 0.4), more accurate values can be obtained by using mechanical extrusion while rapidly forming pendant-drops. The surface tensions of Ga, E-GaIn, Galinstan, and GaInSnBiZn alloys are ~ 717.2, ~ 622.8, ~ 548.8 and ~ 545.7 mN/m, respectively, and the measured results are not sensitive to atmosphere. In this study, the dispersion component of GaInSnBiZn high-entropy alloy surface tension is ~ 327.81 mN/m, accounting for about 39.19%, which indicates that the proportion of dispersion component in the surface tension of multi-component alloys is close to that of elemental (Hg). This work will provide theoretical evidence for further development and application of low melting point high-entropy alloy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Gallium-based liquid metals have become one of the most popular emerging functional materials in recent years due to their high electrical conductivity, high surface tension, safety, non-toxicity and surface/interface properties that are easy to manipulate (Ref 1). Its application value is mainly reflected in the following two aspects. On the one hand, it is mainly used for flexible intelligent equipment, self-healing circuits, medical targeting materials with tensile deformation under electric field regulation (Ref 2,3,4). On the other hand, the wettability between the metal and the substrate is improved mainly using surface oxides, to achieve 3D printing, printing pressure plasticity, direct writing, preparation of two-dimensional thin film materials, etc. (Ref 5,6,7,8). Meanwhile, multi-component high-entropy alloys have unique properties such as typical creep resistance and sluggish diffusion effect, which complement the development of low-melting-point high-entropy alloys (such as SnBiInZn-based high-entropy alloys with melting points at 80 °C) as low-temperature solders (melting points around 100 °C), and provide important insights into their application to advanced electronic packaging (Ref 9,10,11,12). The application of such low melting point alloys and even alloys that are liquid at room temperature is based on a deep understanding of their surface/interfacial properties. Surface/interface tension is a fundamental physical property of liquid materials and its magnitude determines the conduct of many production processes, such as joining, electronic packaging, and forming. Understanding surface tension and its effect on interfaces is useful in a variety of applications, from joining dissimilar materials to the design of microfluidic devices. In addition, researchers can use techniques such as interfacial tension measurement to measure and study the surface tension of various systems (Ref 13, 14). The core task of surface and interfacial studies is the measurement of surface/interfacial tension (Ref 15).
Due to the high reactivity of gallium-based liquid metals, it is challenging to measure their surface tension accurately. Only a few methods are currently available for surface tension measurements on liquid metals, such as the sessile drop method, the pendant-drop method, the maximum bubble pressure method, the Wilhelmy method and the electromagnetic levitation oscillation method (Ref 16,17,18,19,20). The sessile drop method is the simplest technique to use for measuring metals. However, implementing the conventional method is not without its challenges. The metal to be measured is placed on the substrate surface and heated, but it’s challenging to remove the oxide film from the original metal surface. Even with computer-aided extraction of the droplet profile, it remains difficult. On the other side, a source of contamination by contact with the substrate material will be introduced, inevitably. Similarly, the Wilhelmy method also faces challenges. The test rod or ring must not react with the liquid metal for this method to work. As a result, it is impossible to obtain direct information about the meniscus profile from the optical system. The disadvantage of the maximum bubble pressure method is that the interfacial tension between two liquid media cannot be measured directly. The electromagnetic levitation method can measure the surface tension of the droplet under supercooling conditions. Although it can also avoid the impurities brought in by the droplet contacting the substrate and container, it is more difficult to achieve due to the rigorous experimental conditions and relatively large experimental errors. Sobczak et al. (Ref 21) proposed a new experimental study method of carrying out the sessile drop method and pendant-drop method simultaneously, and found that the surface tension measurements of highly reactive and oxidizable alloys obtained by this method had a good agreement. To sum up, the choice of the pendant-drop method, which squeezes the liquid metal during the droplet formation process to remove the original oxide film from the surface through narrow channels, combined with atmosphere control, offers the possibility to acquire the intrinsic value of surface tension.
Nevertheless, the experimental measurement of surface tension of gallium-based liquid metals still faces many challenges and is always subjects with many sources of errors. The measurement difficulties arise mainly from the obstruction of the original oxide film removal of gallium-based liquid metals and the extremely fast oxidation rate of the fresh metal surface. This solid oxide layer will induce high yield stresses while reducing the surface tension of the metal (Ref 1). In order to avoid this situation, surface tension measurements of gallium-based liquid metals are often performed under controlled atmospheres, such as high vacuum (ultra-high vacuum), inert atmospheres (argon or helium), or even reducing atmospheres (hydrogen, hydrochloric acid vapor), which makes the implementation of surface tension measurements extremely inconvenient (Ref 22,23,24). However, only a few studies have been conducted on the measurement of surface tension of liquid metals by the pendant-drop method under air atmosphere, especially whether the metal oxide film can be removed by physical means before measurement, and then formed the pendant-drop quickly for procuring the acceptable values of surface tension.
In this work, we present the characteristics of the apparent value of surface tension of liquid metals under the interference of oxide film. Further, the surface tensions of pure Ga, E-GaIn, Galinstan, and the GaInSnBiZn high-entropy alloy were systematically investigated by selecting needles of different diameters. To remove oxide film from the surface of liquid metals through mechanical compression, we designed a small extrusion hole within the needle. Using this approach, we conducted experiments to measure the surface tension of liquid metals. The experiments were carried out in both air and hydrogen atmospheres to determine the feasibility of this method. Finally, the dispersive contribution of GaInSnBiZn high-entropy alloy was determined by measuring the interfacial tension of liquid metal in organic solvent according to Fowkes’s two-component method (Ref 25). The results of this work are expected to enhance the potential applications of liquid metals in chip, thermal management devices and printed circuits.
2 Experimental
The metals used in the experiments were provided by Dongguan Dingguan Metal Technology Co., LTD. The alloys were produced by melting raw materials of analytical purity grade (purity > 99.999%), E-GaIn (85.8 at.% Ga, 14.2 at.% In), Galinstan (77.2 at.%Ga, In, and 8.4 at.% Sn), and GaInSnBiZn (isoatomic ratio components) high-entropy alloys. The physical properties of these metals are shown in Table 1. The organic solvents used, the density, the surface tension and the components of surface tension, are shown in Table 2.
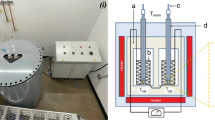
The surface tensions of Ga, E-GaIn and Galinstan alloys at 33 °C, GaInSnBiZn HEA at 70 °C, and their interfacial tensions in organic solvents were measured using an optical contact angle and interfacial tension measurement system (OSA60, NBSI, China) based on the standard pendant-drop method. During the surface tension measurements, the droplets were formed rapidly (~ 0.1 s per droplet) by an autosampler. To analyze the surface/interfacial tensions of liquid droplets, we utilized the Young–Laplace equation. Specifically, we recorded droplet formation using a frame rate of 63 fps and then used the Surface Meter software to fit the droplet profiles in the video. By doing this, we were able to accurately calculate the surface and interfacial tensions of the liquid droplets. In order to investigate the effect of mechanical squeezing of liquid metal with different diameter needles to remove the surface oxide film and further analyze the influence on surface tension measurements, the stainless-steel needles with 0.46 mm outer diameter (O.D.) (0.24 mm inner diameter (I.D.)), 1.806 mm O.D. (1.36 mm I.D.), 3.996 mm O.D. (3.50 mm I.D.) with a 1 mm extrusion hole were used in the experiments. In order to compare the impact of oxide film and surfactants on surface tension measurements, we utilized water droplets covered with PDMS. This approach was used to simulate the conditions experienced by liquid metals that are covered with oxide film. By using this method, we were able to examine the effects of these variables on surface tension measurements in a controlled environment. Their rheological properties were measured by introducing a sinusoidal oscillation mode. To investigate the effect of different atmospheres (air, hydrogen) on the measurement results, the formation of pendant-drops was carried out in a sealed quartz cuvette injected with the corresponding gas, where the purity of hydrogen was ~ 99.99% (O2 < 2 ppm). The variation of the ellipsometry angle before and after the formation of the oxide film of E-GaIn alloy was measured using a laser ellipsometer (SE 400adv PV, SENTECH, Germany). The kinetics of the oxide film formation was deduced from this, with a data acquisition frequency of 2 Hz.
3 Results and Discussion
The surface tension of E-GaIn alloy was tested under atmosphere using a stainless-steel needle with an outer diameter of 0.46 mm, as shown in Fig. 1. Despite using liquid E-GaIn alloy and entering it through the narrow channel during the continuous formation of pendant-drops, we were unable to obtain stable surface tensions. Despite our efforts, we were not able to achieve the desired results using this method. Surface tensions close to ~ 590-650 mN/m were obtained only at the instant of separation of the pendant-drop from the needle, as shown in Fig. 1(a). The surface tension shows a dependence on the volume, as shown in Fig. 1(b). It seems that the larger the droplet volume is, the closer surface tension is to the intrinsic value. There are at least two possible factors that could contribute to the inaccuracy of droplet surface tension measurements: 1. the droplet size was not sufficient to form a shape conducive to measurement; 2. the rapid formation of surface oxide film, which makes the actual measured surface tension is the apparent value under the oxide film coverage. In the first case, since the surface tension of liquid metals is typically greater than 500 mN/m, which requires that the shape of the formed droplet deviate as much as possible from a perfect sphere, and at the same time requires a larger droplet mass (or volume) to achieve accurate measurements (Ref 28). As for the latter, the liquid metal surface behaves viscoelastic once an oxide film is formed. Just as Xu et al. (Ref 29) argued that the measured surface tension should be surface stress after the oxide film has formed on the surface of E-GaIn alloy. It’s possible that the viscous properties of the E-GaIn alloy deviated from the typical Newtonian fluid characteristics of liquid metals. This deviation could potentially contribute to the inaccuracy of our droplet surface tension measurements.
Typically, the surface tension of oxide film is lower than that of the metal it covers. Especially for E-GaIn alloy, the surface tension of the oxide film (Ga2O3, Ga2O) formed on E-GaIn surface is only 350-365 mN/m (~ 337.0 mN/m at 2228 K for Ga2O3) (Ref 30, 31). Since the oxide film may reduce the apparent surface tension in the form of surfactant, polydimethylsiloxane (PDMS) was used to cover the water surface to simulate this characteristic (surface tension and viscosity of PDMS were 19 mN/m, 200 mPa.s, respectively). In the first step of the experiment, we adhered approximately 2 μl of PDMS under a 0.8 mm O.D. needle. We then utilized a syringe feed system to inject water (which has a surface tension of 72.8 mN/m) into the PDMS in a sinusoidal pattern with step-backs, as depicted in Fig. 2(a). The obtained apparent surface tension, shown in Fig. 2(b), which also exhibit a strong dependence on volume, as shown in Fig. 2(c). This phenomenon confirms the inference that the oxide film may act as a surfactant to reduce the surface tension. It’s important to note that the high viscosity of PDMS can result in the droplet exhibiting significant viscoelastic properties that should not be overlooked. The Fourier transform of the real time variation data of the droplet area or surface tension gives the surface expansion modulus (G) of 63.584 mN/m and the modulus angle (ϕ) of 0.5265°. It can be deduced that the surface elastic modulus (G′ = Gcosϕ) is 63.546 mN/m and the surface loss modulus (G′′ = Gsinϕ) is only 0.584 mN/m. G′ > > G′′, which indicates that the surface properties of the fluid are closer to the solid state than the liquid state. When the same method is applied to measure the viscoelasticity of E-GaIn droplets wrapped with oxide film, the droplets can only expand in one direction but not retract on account of both the low shear between the oxide film and the inner E-GaIn alloy and the incompressible nature of the oxide film (Ref 32). Put simply, when the droplet is extracted in a sinusoidal stepwise manner to reduce the volume, the droplet shape shows an asymmetric characteristic just like the liquid metal reducing its volume in a dense solid-phase slag shell. Therefore, the apparent surface tension at the droplet volume retraction stage cannot be accurately measured, and hence the corresponding modulus value cannot be calculated.
Because the oxide film on the surface of the E-GaIn alloy has strong adhesion, we first adhered it to the sapphire substrate before subsequently scraping it off. The variation of the ellipsometry angle was measured in situ using a laser ellipsometer. The kinetic process of the oxide film formation was thus obtained in parallel, as shown in Fig. 3(a). According to the thermodynamic calculations, the oxygen partial pressure to prevent the formation of Ga oxide film at room temperature should be lower than ~ 10−45 Pa (Ref 33). Almost no existing equipment can achieve such low oxygen partial pressure, indicating that the form of oxide film on the alloys surface seems inevitable, which embodies the high reactivity of the liquid metals. The adsorption of oxygen atom on the surface of liquid metal can be completed within 0.02 s (Ref 34). Nonetheless, due to the self-limiting thickening properties of the oxide film, the thickening process of the oxide film on the liquid metal exposed to air occurs very gradually, resulting in an average thickness of around 25 nm, as depicted in Fig. 3(a). When wrapped with oxide film, the pendant-drops exhibit low elasticity and great shear modulus (Ref 30). In most cases, the measured apparent surface tensions are affected by this, and the strong dependence of it on volume should actually be characterized by a linear relationship between surface tension and the maximum diameter of the pendant-droplet after oxide film generation (Ref 35).
where γapp is the apparent surface tension, τ0 is the yield stress, l is the maximum diameter of the pendant-drop, and γ0 is the intrinsic surface tension of the alloy in the absence of oxide film action. The yield stress τ0 is only a surface effect of the oxide layer, not an intrinsic property. As an inherent property of the alloy droplet, γ0 is a fixed value. The apparent surface tension (γapp) obtained by the pendant-drop method is proportional to l, as shown in Fig. 3(b). However, the intercept obtained from the linear fit in Fig. 3(b) is not γ0, which may be related to the diameter of the needle used in the test.
As previously mentioned, due to the high surface tension of liquid metals (> 500 mN/m, typically), which requires the use of larger O.D. needles to obtain sufficiently large droplet in order to ensure measurement accuracy. The Worthington number (Wo) can serve as an indicator to describe the measurement accuracy. Surface/interfacial tension values with good reproducibility can only be obtained when the Worthington number (Wo) is above 0.4 (Ref 36).
According to the Worthington number expression:
where Δρ is the density difference between the test droplet and its surroundings, g is the acceleration due to gravity, Vd is the volume of the droplet, γ is the interfacial tension, and Dn is the diameter of the needle. To achieve a Worthington number (Wo) above 0.4, a drop volume of at least ~ 5.6 μl is required when using a 0.46 mm O.D. needle. However, Fig. 1(b) demonstrates that even without the oxide film effect, stable and accurate surface tension values cannot be obtained with this needle. If a typical surface tension value of 550 mN/m is assumed, then using a needle with 1.806 mm O.D., experiment results with good reproducibility can only be achieved if the droplet volume is at least ~ 25.3 μl. Figure 4(a) shows that a linear fit of the pure Ga surface tension data measured above 25 μl (above ~ 62.5% of the maximum volume) yields a surface tension value of ~ 717.2 mN/m at 33 °C, consistent with the data reported in the handbook (Ref 28). It is noteworthy that the values of the surface tension measured under hydrogen atmosphere are comparable to those under air atmosphere. Furthermore, the surface tensions of E-GaIn alloy and Galinstan alloy under air and hydrogen atmospheres at 33 °C were compared and tested, as shown in Fig. 4(b) and (c). Both the results showed good consistency. The surface tension of the two is ~ 622.8 and ~ 548.8 mN/m, respectively, which is matching to the values reported in the literature (Ref 36, 37). Indicating that the rapidly formed pendant-drops after mechanical extrusion can obtain a fresh surface and then procure a surface tension close to the intrinsic value (Ref 35, 36). To ensure Wo > 0.4, a droplet volume greater than or equal to 50 μl is required when using the 3.996 mm O.D. needle to test surface tension, as depicted in Fig. 4(b). The stable surface tensions were obtained for droplets with more than 50% of the maximum volume. However, the choice of a 3.996 mm O.D. needle for testing inevitably leads to a decline of the squeezing effect on the liquid metal, which deteriorates the oxide film removal effect. Therefore, a dropping device with a 1 mm embedded extrusion hole was designed, as shown in Fig. 4(e). The measured surface tension of E-GaIn alloy by this device has good stability and small error, which matches the experimental requirements for accuracy, as shown in Fig. 4(c). The surface tension of GaInSnBiZn was measured at 70 °C using a dropping device in combination with external tube heating, within a constant temperature chamber, as shown in Fig. 4(d), which is ~ 545.7 mN/m.
The main contribution to the surface tension of the liquid metal should be attributed to the chemical bonding within the metal, i.e., metallic bonding. Fowkes took Hg as the research object and proposed that the two main contributions to the surface tension of liquid metals are the London dispersive contribution (\(\gamma_{{{\text{LM}}}}^{d}\)) and the contribution due to metallic bond (\(\gamma_{{{\text{LM}}}}^{m}\)) (Ref 25, 38):
The surface tension of Hg at 20 °C is 484 mN/m, of which the dispersive contribution is 200 ± 7 mN/m (about 41.3%) (Ref 25). Gaining insight into the universality of surface tension components of liquid metals is challenging due to the limited number of metals that exist in a liquid state at or near room temperature. This is especially true for multi-component high-entropy alloys, which have rarely been reported on. Wang et al. (Ref 36) derived that \(\gamma_{{{\text{LM}}}}^{d}\) = 239.7 ± 9.1 mN/m by measuring the interfacial tension of Galinstan alloys in typical nonpolar organic solvents (n-hexane, benzene, and o-xylene) (~ 533 mN/m in their work for ~ 39.6% of the surface tension of Galinstan alloys).
The components of the GaInSnBiZn high-entropy alloy studied in this work are more complex than those of the Galinstan alloy. As a result, the proportion of the dispersive contribution in the surface tension is less understood. Therefore, the above embedded pore dropping device was used to measure its interfacial tension in water, ethylene glycol, glycerol, n-butanol and 1,4-dioxane (pure dispersive contribution to the surface tension in the liquid phase, as shown in Table 2, without the polar contribution in the surface tension) at 70 °C. During successive droplet formation, the initially formed droplets did not acquire stable values of interfacial tension, but instead showed a dependence on volume, as shown in Fig. 5. According to the Worthington number, the condition of Wo > 0.4 is satisfied when the volume of the pendant-drop is above ~ 28.5 μl. This dependence of the interfacial tension on the volume may be attributed to the introduction of the initial oxide film, and thus the average values of the interfacial tension corresponding to the volume interval when the drop is in the steady state is taken as the stable value, such as 30-35 μl in 1, 4-dioxane, 55-65 μl in n-butanol, 30-35 μl in the fourth droplet of ethylene glycol, 30-35 μl in the third droplet of glycerol, 45-50 μl in the fourth droplet of water, as shown in Table 3. Good and Girifalco (Ref 39) and Fowkes (Ref 38) concluded that when only dispersion interaction forces are present, the interfacial tension between the two condensed phases can be expressed as:
If the intermolecular attraction at the alcohol-mercury interface is solely due to the London dispersion force, Fowkes’s formula is appropriate. However, if one of the molecules has a permanent dipole, such as in the case of the alcohol-mercury interface, other forces are at play besides the dispersion force. In such a system, the permanent dipole contribution of the alcohol must also be considered. As a result, Matsumo modified the equation for the alcohol-mercury system to account for this additional contribution (Ref 40). Considering that the liquid phase in the test environment contains polar components, then Eq (4) would be rewritten as:
where the \({\gamma }_{\mathrm{solvent}}^{\mathrm{pd}}\) is the permanent dipole contribution to the surface energy of alcohol. However, when the interfacial tension value of GaInSnBiZn high-entropy alloy in 1,4-dioxane is substituted into the formula, the dispersion component of the surface tension is derived as 570.84 mN/m. Even if n-butanol is approximated as a condensed phase with pure dispersion component, the calculated dispersion component of surface tension in GaInSnBiZn high-entropy alloy is still as high as 643.12 mN/m. Based on the measured interfacial tension values in these two organic solvents, the calculated dispersion component clearly exceeds the surface tension of the GaInSnBiZn high-entropy alloy, which is not a characteristic surface property of the high-entropy alloy. From Fig. 5(a), (a-1), (b) and (b-1), it is easy to see that the interfacial tension hardly converges to a stable value actually and there is a weak dependence on the volume. This situation suggests that the formation of the interfacial region may not be biphasic, but rather a combination of ambient liquid phase/oxide film/liquid phase metal. To the best of our knowledge, the surface tension component of Ga2O3 or Ga2O has not been reported in the literature. If we assume that the surface/interfacial tension only depends on several layers of atoms or molecules in the surface/interfacial layer, replacing γLM in Eq (4) with the surface tension of the oxide film (350-365 mN/m as described above), we can obtain the dispersion component of the oxide as 47.04-86.60 mN/m and accounting for about 13.44-23.73%. These values are comparable to the proportion of dispersion components in the oxides of aluminum. Al and gallium Ga belong to the same group in the periodic table. For Al2O3, the dispersion component is approximately 75.5 mN/m, accounting for roughly 13% of the total surface tension of approximately 580 mN/m (Ref 41). Analyzing pure grade water, ethylene glycol and glycerol, it is worth noting that the oxygen content in pure water is much lower than that of 1,4-dioxane and n-butanol (only 10−5) (Ref 42). It takes some time to form a complete oxide film even if the conditions for oxide film formation are met, and thus it is expected to establish a pure interface between liquid high-entropy alloy and solvents after removing the original oxide film by rapid mechanical extrusion within the test environment. Using Eq (5), the interfacial tensions of GaInSnBiZn high-entropy alloy in water, ethylene glycol, and glycerol are inputted to determine the components of surface tension, as shown in Table 3. The dispersion component of the GaInSnBiZn high-entropy alloy is approximately 327.81 mN/m, constituting roughly 39.19% of the total surface tension. This is close to the results of Wang et al. (39.6%) and Fowkes’s (41.3%) work.
4 Conclusions
In this study, we utilized the pendant-drop method to measure the surface/interfacial tension of pure Ga, E-GaIn, Galinstan, and the GaInSnBiZn high-entropy alloy. Based on our findings, we have reached the following conclusions:
-
(1)
When the surface tension of gallium-based liquid metals shows a significant volume dependence, there are at least two reasons for this phenomenon. From one side, it is due to the viscoelasticity exhibited by the oxide film covering the surface of the metallic droplet. On the other side, the volume of the pendant-droplet does not reach the critical value to ensure the accuracy of the measurement.
-
(2)
The rapid formation of the droplet after the oxide film removal by mechanical squeezing, while ensuring the droplet volume above the critical value mentioned. Nearly consistent results were obtained under air and hydrogen atmospheres, with surface tensions of ~ 717.2, ~ 622.8, ~ 548.8, and ~ 545.7 mN/m for Ga, E-GaIn, Galinstan, and GaInSnBiZn alloys, respectively.
-
(3)
The dispersion component of the surface tension of the GaInSnBiZn high-entropy alloy is ~ 327.81 mN/m, accounting for about 39.19%. When the oxygen content in the solvent is high, the differentiation of the interfacial region (i.e., solvent/oxide film/liquid metal interface) should be considered.
References
M.D. Dickey, Emerging Applications of Liquid Metals Featuring Surface Oxides, ACS Appl. Mater. Interfaces, 2014, 6(21), p 18369–18379.
C.B. Cooper, K. Arutselvan, Y. Liu, D. Armstrong, Y. Lin, M.R. Khan, and M.D. Dickey, Stretchable Capacitive Sensors of Torsion, Strain, and Touch Using Double Helix Liquid Metal Fibers, Adv. Funct. Mater., 2017, 27(20), p 1605630.
B.J. Blaiszik, S.L. Kramer, M.E. Grady, D.A. McIlroy, J.S. Moore, N.R. Sottos, and S.R. White, Autonomic Restoration of Electrical Conductivity, Adv. Mater., 2012, 24(3), p 398–401.
Y. Lu, Q. Hu, Y. Lin, D.B. Pacardo, C. Wang, W. Sun, F.S. Ligler, M.D. Dickey, and Z. Gu, Transformable Liquid-Metal Nanomedicine, Nat. Commun., 2015, 6, p 10066.
Y. Yu, F. Liu, and J. Liu, Direct 3D Printing of Low Melting Point Alloy Via Adhesion Mechanism, Rapid Prototyp. J., 2017, 23(3), p 642–650.
L. Wang, M. Wang, J. Lu, R.E.A. Ardhi, J. Liu, G. Liu, and J.K. Lee, Enhanced Adhesion Between Liquid Metal Ink and the Wetted Printer Paper for Direct Writing Electronic Circuits, J. Taiwan Inst. Chem. Eng., 2019, 95, p 202–207.
L. Wang and J. Liu, Printing Low-Melting-Point Alloy Ink to Directly Make a Solidified Circuit or Functional Device with a Heating Pen, Proc. Math. Phys. Eng. Sci., 2014, 470(2172), p 20140609.
A. Zavabeti, J.Z. Ou, B.J. Carey, N. Syed, R. Orrell-Trigg, E.L.H. Mayes, C. Xu, O. Kavehei, A.P. O’Mullane, and R.B. Kaner, A Liquid Metal Reaction Environment for the Room-Temperature Synthesis of Atomically Thin Metal Oxides, Science, 2017, 358(6361), p 332–335.
Y.L. Chou, J.W. Yeh, and H.C. Shih, The Effect of Molybdenum on the Corrosion Behaviour of the High-Entropy Alloys Co1.5CrFeNi1.5Ti0.5Mox in Aqueous Environments, Corros. Sci., 2010, 52(8), p 2571–2581.
Y.E.H. Jien-Wei, Recent Progress in High Entropy Alloys, Ann. Chim. Sci. Mater., 2006, 31(6), p 633–648.
S. Wang, J. Feng, S. Wang, K. Wang, M. Yu, and Y. Tian, Interfacial Reaction Between Novel High Entropy Alloy SnPbInBiSb and Cu Substrate, Mater. Lett., 2022, 325, p 132901.
Y. Liu, L. Pu, Y. Yang, Q. He, Z. Zhou, C. Tan, X. Zhao, Q. Zhang, and K.N. Tu, A High-Entropy Alloy as Very Low Melting Point Solder for Advanced Electronic Packaging, Mater. Today Adv., 2020, 7, p 100101.
X. Wang, R. Guo, and J. Liu, Liquid Metal Based Soft Robotics: Materials, Designs, and Applications, Adv. Mater. Technol., 2019, 4(2), p 1800549.
X. Zhao, S. Xu, and J. Liu, Surface Tension of Liquid Metal: Role Mechanism and Application, Front. Energy, 2017, 11, p 535–567.
I. Egry, E. Ricci, R. Novakovic, and S. Ozawa, Surface Tension of Liquid Metals and Alloys—Recent Developments, Adv. Colloid Interface Sci., 2010, 159(2), p 198–212.
M.A. Duchesne and R.W. Hughes, Slag Density and Surface Tension Measurements by the Constrained Sessile Drop Method, Fuel, 2017, 188, p 173–181.
S.M.I. Saad, Z. Policova, and A.W. Neumann, Design and Accuracy of Pendant Drop Methods for Surface Tension Measurement, Colloid Surf. A, 2011, 384(1–3), p 442–452.
M. Fukuta, J. Sumiyama, M. Motozawa, and T. Yanagisawa, Surface Tension Measurement of Oil/Refrigerant Mixture by Maximum Bubble Pressure Method, Int. J. Refrig., 2017, 73, p 125–133.
N. Wu, J. Dai, and F.J. Micale, Dynamic Surface Tension Measurement with a Dynamic Wilhelmy Plate Technique, J. Colloid Interface Sci., 1999, 215(2), p 258–269.
H. Fujii, T. Matsumoto, S. Izutani, S. Kiguchi, and K. Nogi, Surface Tension of Molten Silicon Measured by Microgravity Oscillating Drop Method and Improved Sessile Drop Method, Acta Mater., 2006, 54(5), p 1221–1225.
N. Sobczak, R. Nowak, W. Radziwill, J. Budzioch, and A. Glenz, Experimental Complex for Investigations of High Temperature Capillarity Phenomena, Mater. Sci. Eng. A, 2008, 495(1), p 43–49.
S. Hardy, The Surface Tension of Liquid Gallium, J. Cryst. Growth, 1985, 71(3), p 602–606.
U. König and W. Keck, Measurement of the Surface Tension of Gallium and Indium in a Hydrogen Atmosphere by the Sessile Drop Method, J. Less Common Metals, 1983, 90(2), p 299–303.
A. Dobosz and T. Gancarz, Density, Viscosity and Surface Tension of Gallium Rich Al-Ga Alloys, Fluid Phase Equilib., 2021, 532, p 112923.
F.M. Fowkes, Additivity of Intermolecular Forces at Interfaces I. Determination of the Contribution to Surface and Interfacial Tensiions of Dispersion Forces in Various Liquid, J. Phys. Chem., 1963, 67(12), p 2538–2541.
S. Liu, K. Sweatman, S. McDonald, and K. Nogita, Ga-Based Alloys in Microelectronic Interconnects: A Review, Materials, 2018, 11, p 1384.
S. Zhu, L. Liu, and Q. Lin, Surface Tension of GaInSnBiZn Liquid High-entropy Alloy, J. Metall. Mater. Res., 2021, 4(2), p 1–6.
W.F. Gale and T.C. Totemeier, Smithells Metals Reference Book, Elsevier, Oxford, 2003.
Q. Xu, N. Oudalov, Q. Guo, H.M. Jaeger, and E. Brown, Effect of Oxidation on the Mechanical Properties of Liquid Gallium and Eutectic Gallium-Indium, Phys Fluids, 2012, 24(6), p 063101.
S. Handschuh-Wang, T. Gan, T. Wang, F.J. Stadler, and X. Zhou, Surface Tension of the Oxide Skin of Gallium-Based Liquid Metals, Langmuir, 2021, 37(30), p 9017–9025.
K. Yoshida, H. Kumagai, T. Yamane, A. Hayashi, C. Koyama, H. Oda, T. Ito, and T. Ishikawa, Thermophysical Properties of Molten Ga2O3 by Using the Electrostatic Levitation Furnace in the International Space Station, Appl. Phys. Express, 2022, 15(8), p 085503.
J. Liu, H. Ma, Y. Yang, W. Yang, Z. Jiao, Y. Yu, and X. Gui, Study on Direct Writing of Gallium Metal for the Flexible Sensor, Adv. Mater. Sci. Eng., 2021, 2021, p 1–10.
I. Barin, Thermochemical data of pure substances, Wiley, Weinheim, 1995.
Y. Ding, M. Zeng, and L. Fu, Surface Chemistry of Gallium-Based Liquid Metals, Matter, 2020, 3(5), p 1477–1506.
Q. Xu, N. Oudalov, Q. Guo, H.M. Jaeger, and E. Brown, Effect of Oxidation on the Mechanical Properties of Liquid Gallium and Eutectic Gallium-Indium, Phys. Fluids, 2012, 24(6), p 063101.
S. Handschuh-Wang, Y. Chen, L. Zhu, and X. Zhou, Analysis and Transformations of Room-Temperature Liquid Metal Interfaces—a Closer Look Through Interfacial Tension, Chem. Phys. Chem., 2018, 19(13), p 1584–1592.
Y. Plevachuk, V. Sklyarchuk, S. Eckert, G. Gerbeth, and R. Novakovic, Thermophysical Properties of the Liquid Ga-In-Sn Eutectic Alloy, J. Chem. Eng. Data, 2014, 59(3), p 757–763.
F.M. Fowkes, Attractive Forces at Interfaces, Ind. Eng. Chem., 1964, 56(12), p 40–52.
R.J. Good and L.A. Girifalco, A Theory for Estimation of Surface and Interficial Energies. Iii. Estimation of Surface Energies of Solids from Contact Angle Data, J. Phys. Chem., 1960, 64(5), p 561–565.
M. Matsumoto, A.G. Gaonkar, and T. Takenaka, The Estimation of Hamaker Constants of Alcohols and Interfacial Tensions at Alcohol-Mercury Interfaces, Bull. Inst. Chem. Res. Kyoto Univ., 1981, 58(5–6), p 523–533.
J. Drzymala, Hydrophobicityand Collectorless Flotation of Inorganic Materials, Adv. Colloid Interface Sci., 1994, 50, p 143–185.
G. Ryu, K. Park, and H. Kim, Interfacial Properties of Liquid Metal Immersed in Various Liquids, J. Colloid Interface Sci., 2022, 621, p 285–294.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 52165044).
Author information
Authors and Affiliations
Contributions
SZ: Writing- Reviewing and Editing. QL: Writing- Reviewing and Editing, Funding acquisition. RC: Writing- Reviewing and Editing, Funding acquisition. KX: Data curation, Methodology. JL: Data curation, Validation.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, S., Lin, Q., Cao, R. et al. Interfacial Tension of Ga, E-GaIn, Galinstan, and GaInSnBiZn High-Entropy Alloy. J. of Materi Eng and Perform 33, 2369–2378 (2024). https://doi.org/10.1007/s11665-023-08143-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-023-08143-6