Abstract
This study addresses the use of activated flux (MoO3, SiO2, TiO2, and CaO) for the TIG welding of chrome-manganese (Cr-Mn SSs) stainless steel. Bead-on-plate welding trials were performed to investigate the weld bead microstructure, mechanical, and electrochemical properties. The behavior of activated flux powder was studied with the orientation of fluctuations of arc voltage and dissolved oxygen in the weld bead. It was observed δ-ferrite phase evolution on employing activated flux. TiO2-assisted weld was found to have the highest mechanical properties than other TIG and ATIG welds. The presence of secondary phases (Cr23C6) in the heat-affected zone was confirmed with x-ray diffraction (XRD) and energy-dispersive spectroscopy (EDS) technique. The tensile and impact test failures were observed in all TIG and ATIG welding processes at the weld zone. Numerous ductile ridges, voids, and dimples were seen on all tensile and impact fracture surfaces. Improvement in the hardness value was observed in the weld zone of ATIG weldment than No flux. CaO-assisted weldment offers excellent pitting corrosion resistance \((\mathrm{Epitt}=-130.164\mathrm{ mv})\) than other TIG and ATIG weldments. The degree of sensitization (DOS) of the ATIG welded sample was observed higher than the No flux TIG weld.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Welding is highly prevalent in different sectors such as railways, defense, chemical, nuclear industries, marine industry, and power plants to supply demand of higher strength at minimal cost (Ref 1). Austenitic stainless steel (ASS) is one of the most popular stainless steels in the domestic and commercial markets among preceding industries. Further, it is subcategorized as low-nickel high-manganese 200-series ASS and high-nickel 300-series ASS. Due to the high cost of nickel, the inadequate supply of nickel in India and its allergies to mankind is the main reason for the increase in demand for 200-series ASS. Although 200 series ASS is becoming more popular as a cost-effective material, its complexities in terms of microstructural and mechanical properties during welding have yet to be fully explored. In the 200 series ASS, nickel is replaced by Mn and N (Ref 2,3,, 3). To stabilize the austenitic phase, Mn also plays a vital role instead of Ni, while nitrogen improves the impact energy of steel, so it is also known to be chrome-manganese (Cr-Mn SSs) stainless steel. Nowadays, regional manufacturers have approached Cr-Mn stainless steel for applications like construction, home accessories, office appliances, boiler tubes, condenser tubes, etc. This low-nickel chrome-manganese SSs steel is readily weldable, unlike different steel grades. Different welding methods can be used to weld Cr-Mn stainless steel like a laser beam, electron beam, arc welding, tungsten inert gas welding, etc. Higher depth-to-width (aspect ratio) can be achieved by employing laser beam welding and electron beam welding. However, their application is restricted in a limited area of welding technology. In lieu of these techniques, the most effective tungsten inert gas (TIG) welding technique is used to produce high-quality welds. Compared to other welding processes, a poor joint penetration problem is associated with TIG welding (Ref 4,5,6). Usually, TIG welding is limited to 3 mm depth for stainless steel to make the butt joint. Therefore, to improve the penetration capacity for manufacturing productivity, it is necessary to learn novel TIG welding techniques.
Keyhole TIG welding, electromagnetic TIG welding, hybrid TIG welding, and activated tungsten inert gas (ATIG) welding are some of the advanced TIG welding variants available where the researchers are doing works. Among the above techniques, ATIG welding is a low-cost process that improves productivity by addressing the penetration problem associated with TIG welding. The Paton Welding Institute (Ukraine) was the first welding institute to develop this method in 1960 (Ref 7, 8). However, it wasn't until the 1980s that a number of researchers sparked a genuine interest. Adding active chemical components (activated flux) to the molten pool, such as sulfur, fluorine, or oxygen, could enhance weld depth by altering the molten pool's orientation from outward to inward (Ref 9,10,11). Before the welding procedure, an activating flux (commonly powdered metal oxides) is mixed with an appropriate career medium and applied to the base plate surface. The thin flux coating deposited on the plate surface gets melted and vaporized due to the arc temperature during the welding operation. This revolutionary TIG approach may address other TIG welding challenges such as multi-pass welding, a broad heat-affected zone (HAZ), and edge preparation (Ref 1, 12, 13). Sujai et al. (Ref 14) observed that SiO2 flux could reach a complete penetration depth and the maximum depth-to-width ratio during welding of Incoloy 625. Patel et al. (Ref 15) revealed a lathy martensitic microstructure after employing oxide fluxes during welding, which led to a higher hardness value in the weld bead. Sharma et al. (Ref 16) observed full penetration depth in a single pass dissimilar welding of P92 steel-304H ASS joints using oxide-based (Cr2O3, MoO3, SiO2, and TiO2) TIG welding. According to Ramkumar et al. (Ref 17), improved corrosion resistance was observed during super duplex stainless steel NiO-based TIG welding.
However, selecting proper flux during ATIG welding of various grades of stainless steel is one of the most critical concerns. The ATIG weld bead was formed during dissimilar welding of stainless steel (SS) to carbon steel (CS); their microstructure was observed to contain carbide development at the SS weld zone, resulting in tensile test specimens being fractured from the CS side, according to Nayee et al. (Ref 1). The microstructure of ATIG welded 321 stainless steel revealed the equiaxed dendritic structure with higher delta ferrite, leading to an increase in tensile strength, according to Mohan Kumar et al. (Ref 18). Ramkumar et al. (Ref 19) evaluated the corrosion resistance performance of ATIG weldment of Inconel X750 employing tricomponent flux. In their literature, the authors reported that on utilizing flux, the production of precipitation (Cr23C6 and NbC) at the weld zone increased, which helps to enhance the corrosion resistance of the weldments.
Based on the existing literature on the ATIG welding technique, the weldability of certain steels has been shown earlier (Ref 20,21,22,23). On the other hand, the impact of ATIG welding on Cr-Mn stainless steel has not been studied yet. In light of this, The ATIG welded Cr-Mn stainless steel weldments have been systematically characterized for their metallurgical mechanical and electrochemical properties. The following chapters will give a detailed outline of the experimental methodology and the outcomes of the work.
2 Method and Experiment
2.1 ATIG Welding Sample Preparation and Welding Methodology
TIG welding was done with a low nickel chrome-manganese austenitic stainless steel (Cr-Mn SSs). The chemical compositions of the base metal Cr-Mn were confirmed by the spectroscopy method listed in Table 1. Wire-cut electric discharge machine (Model:3240 NXG, Ratnaparkhi Electronics Industries Private Limited, Nasik, Maharashtra) was used to cut the steel samples into 150 x 120 x 6 mm (India). Before performing the welding operation, the edges of these samples were rough polished with silicon carbide abrasive paper of 400 grit. After that, samples were annealed at 1050°C for 90 minutes, after which they were immediately water quenched to prevent any new phase transformations and keep the sample homogeneous throughout. After solution annealing on the specimen surface, acid pickling was used to remove the formed oxide layer by mixing concentrated HNO3 and concentrated HF in a 4:1 ratio (Ref 24). The heat input generated during TIG welding is calculated using the formula listed below by considering an efficiency \((\eta\)) of 0.7 (Ref 25, 26).
where
V— voltage in volts (V),
I—welding current in amperes (A) and
S—welding speed in (mm/s).
The commercial grade of activated flux powder MoO3, SiO2, TiO2, and CaO was picked for the current investigation purchased from Sigma-Aldrich. Acetone, methanol, and water can be used as solvents in ATIG welding for a thin flux coating on the plate. However, several researchers reported that methanol and water take more time to evaporate, so acetone was used as a solvent (Ref 19, 27, 28). Before the coating operation, the powdered flux was baked and mixed with acetone by a glass rod until the mixture turned into a paint-like uniformity. This mixture was coated manually on the surface of plates using a 10-mm-wide paintbrush. The acetone evaporated after a few seconds, but a tiny layer of regular coating remained on the plates' surface.
Note To avoid any form of impurities and to get good results, the coating was done just before the welding operation.
All welding was done together with the co-operation of the WeldFast Industries Pvt. Ltd., Nagpur, India. Bead-on-plate, TIG (No flux used), and ATIG (TIG with flux) welding was performed on the plates. Welding operation was done with an AC/DC inverter-controlled TIG welding power source (VIRGO PLUS: Model TIG-315P). All welding worked out using the workshop-developed mechanized system to maintain a torch at a constant speed and arc length. The photograph of the actual TIG setup is shown in Fig. 1.
Tungsten electrode with a tip configuration of 45° included angle and 3.15 mm diameter (2% thoriated) was used for all welding operations. The welding machine was connected with the direct current electrode negative (straight polarity) by keeping the TIG torch at 90° with the base plate. After several trials on the welding plate, the current value of 120 A was analyzed at a constant speed of 150 mm/min with an accurate electrode gap of 2 mm. In all TIG and ATIG welding, the flow rate of 16 liters/minute argon gas with 99.99% purity was kept. The weld parameters chosen for the TIG investigation are listed in Table 2.
2.2 Metallography and Microscopy
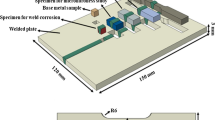
The welded samples were polished using a series of 400-, 800-, 1200- up to 2000-grit size silicon carbide polishing paper, followed by a velvet cloth using 0.75 µm alumina powder. After polishing, samples were cleaned by the ultrasonic method. Etching was done with an electrolytic solution (10 gm chromic acid in 100 ml distilled water) at 1 A for 180 seconds. An optical microscope (Zeiss Axio Lab A1, Carl Zeiss GmbH, Göttingen, Germany) was used to capture the microstructures of different zones of welded joints at various magnifications. A scanning electron microscope (SEM) was used to examine the morphology of weld beads in terms of depth and width. Energy-dispersive spectrometry (EDS) analysis of fusion zones was conducted in various welded joint zones to evaluate alloying elements' micro-segregation. To measure the welded sample's microhardness, a Vickers microhardness testing machine (Shimadzu microhardness tester, Shimadzu Corp., Kyoto, Japan) was used by applying 200 gm of force for 15 sec dwell time. A schematic diagram showing the sample preparation from TIG and ATIG welded plate is shown in Fig. 2.
3 Result and Discussion
According to Heiple and Roper (Ref 29), the reverse Marangoni effect is the crucial mechanism for ATIG welding. According to their literature, the amplitude of the Marangoni effect is governed by the combined resistance of the surface tension and temperature gradient of the molten pool. As the temperature rises, the surface tension of metals and alloys reduces, \(\partial \sigma /\partial \mathrm{T}<0\) (Ref 30, 31), and the weld morphology is wide and shallow (Fig. 3a). When the surface-active elements (sulfur or oxygen) in stainless steel surpasses a specified value, the surface tension temperature coefficient \(\partial \sigma /\partial \mathrm{T}\) shifts from a positive to a negative \(\partial \sigma /\partial \mathrm{T}>0\), and the Marangoni convection on the molten pool changes its direction (Fig. 3b). Arc constriction, which is encouraged by flux molecules (typically oxides, sulfides, and halides) with electron attachment cross section by giving a greater number of vaporized molecules and atoms in the arc's outer regions (Fig. 3c), is another method for increasing weld depth (Ref 32,33,34). Figure 3 depicts the Marangoni and arc constriction effects schematically.
3.1 Morphology Study of the Weld Bead
After welding, samples were cut with 10 x 10 x 6 (mm) dimensions to analyze the microstructural study. The geometrical characteristics of the TIG and ATIG welding trials were detected with scanning electron microscopy (JEOL 6380A, JEOL Ltd., Tokyo, Japan), as shown in Fig. 4.
The value of the bead width (W), depth of penetration (DOP), and depth-to-width (D/W) ratio detailed in Table 3. A 15 kV accelerating voltage was kept for taking microstructure. The cross-sectional profiles of the weld beads indicate an increase in weld penetration after the transition from TIG to ATIG welding. On the other hand, the weld bead geometries changes in each scenario. With a depth of penetration of 3.201 mm and a depth to width ratio of 0.8079, welding without flux produced a shallower and broader bead.
However, SiO2 and TiO2 weld resulted in excellent thickness penetration of 4.667 and 4.713 mm, respectively. SiO2 weld resulted in an inverted bell-shaped weld bead with a larger depth-to-width ratio of 0.8420. While adopting a flux MoO3, a maximum DOP (4.713 mm) is achieved. Weld bead with CaO flux had found increased bead width (5.107 mm) than bead depth (3.853 mm). In any of these weldments, there were no physical surface flaws such as undercut, cracks, or arc strike. As a result, it can be shown that the oxide fluxes and processing parameters used for TIG welding are compatible for all instances.
3.2 Dissolved Oxygen in the Weld Bead
An oxygen/hydrogen/nitrogen analyzer (LECO TCH-600) was used to determine the dissolved oxygen content in each weld. The samples were cut as per the ASTM E-1019 standard to carry out the dissolved oxygen standard (Ref 14). Table 3 shows the dissolved oxygen (PPM) in the weld bead. Higher oxygen concentration was observed in the ATIG weld zone than the TIG weld zone, resulting in improved penetration in the ATIG weld bead. It was observed that more oxygen (135 PPM) was dissolved in the MoO3 weld bead, followed by TiO2 (121 PPM), SiO2 (112 PPM), and CaO (81 PPM). The more dissolved oxygen in the MoO3 flux weld bead could be due to its lower melting point than other flux weld beads, requiring less heat to decompose it into the number of oxygen ions (O−2 and O− ions)(Ref 38). Dissolved O−2 and O− ions changed the surface tension gradient of the molten weld to a positive value, resulting in an increased weight percentage of oxygen in the weld bead (Ref 33, 39). As the dissolved oxygen in the weld bead increased, the reverse Marangoni effect increased, resulting in improved weld bead depth of penetration (Ref 40).
3.3 Effect on Arc Voltage
The arc voltage fluctuation for TIG and ATIG welding approaches is shown in Table 3. A high-speed digital video recorder was continuously monitored on the voltage display screen to record the arc voltage data. A video was recorded at a sampling rate of 250 frames per second. Every second arc voltage fluctuation was viewed using Camtasia (2019) video editing software. According to research, the arc voltage increases when the ATIG welding process is used while maintaining a constant current value. Compared to TIG welding, MoO3-assisted TIG caused a 1.01 V voltage change [ΔVMoO3 =VMoO3−VNo flux=15.01 V−14 V=1.01 V], whereas CaO had a less pronounced effect with an average voltage change of about 0.1 V. Several researchers have reported the same increase in voltage in previous studies (Ref 28,29,30). This increased voltage causes an increase in heat input, resulting in extremely high melting of workpieces where liquid metal flows beyond the penetration depth of TIG with No flux (Ref 41, 42).
3.4 Examination of the Microstructure
To assess the microstructural examinations, samples were cut with 10 mm x 10 mm x 6 mm dimensions after welding. All the interface and weld zone microstructures of TIG and ATIG welding are shown at 20 µm and 10 µm magnification, respectively, as shown in Fig. 5.
In almost all the Welded cases, a microstructure of austenite (γ) and ferrite (δ) veins was observed. The interface microstructure between weld zone and heat-affected zone is fully visible the no un-mixed zone which is depicted in Fig. 5. This could be acquainted with the null effect of the flux and base metal, which doesn’t affect the dilution in the re-solidification stage. It is also noticed the larger grain growth in the HAZ of No flux weldment due to the divergence of arc during welding passes (Fig. 5a). In all instances, the heat-affected zones were observed to have higher grain boundary precipitation (GBP) and grain coarsening. The precipitation in the HAZ was scanned in EDS to reveal precipitation formed. SEM/EDS analysis (Fig. 6) unveiled the unnormalized enriched region of the chromium, which could be the existence of intragranular chromium carbide (Cr23C6) precipitates in the grain boundary of the heat-affected zone. The precipitations and HAZ were analyzed using XRD Diffraction techniques recorded from 40° incident angle to 100° with the silicon photodiode point detector to confirm chromium carbide existence. This precipitation predominantly comprised of chromium and carbon and was identified as the Cr23C6 phase by x-ray diffraction, as depicted in Fig. 7. It is also observed that the width of HAZ decreased after employing the activated flux. This is due to more heat transmission by molten pool flowing from the periphery to the center of the weld. The decrease in the width of HAZ causes lesser grain growth in that region. From optical microscopy, the estimated average width of HAZ was 60 µm for No flux, 45 µm for MoO3,42 µm for SiO2, 40 µm for TiO2, and 38 µm for CaO welds. For all the welded specimens, the fusion zone's microstructure was seen to have dendritic skeletal, lathy, and vermicular ferrite. As reported by Brooks et al., the formation of a vermicular ferrite microstructure in the center of bead on welds shall be due to the rejection of chromium from the austenite phase, which offers the ideal thermal environment for the development of lathy (lacy) ferrite (Ref 43). It was seen that more lathy structures were found after employing activated flux. The same result has reported by Vora et al. in their research (Ref 44). From the microstructure, it is observed that inter-dendritic distance increases on employing flux. This increased interdendritic spacing could be the region for increasing the arc voltage, which ultimately affects heat input.
3.5 Energy-Dispersive Spectroscopy (EDS) Analysis
All EDS results are quantified in the unnormalized concentration in weight percent of the element. Analysis of No flux TIG weld zone shows enriched weight percentage concentration of Ni, Cr, Fe, Ni elements compared to base metal as shown in Fig. 8a. The EDS analysis of the weld zone of MoO3 found that compared to No flux, near the dendrites, and interdendritic region, enrichment of Mo and Fe element and impoverishment of Cr, Mn, and Ni elements are observed (Fig. 8c), while in the case of the SiO2 weld zone region, enrichment of Fe, Cr, Mn, and Si elements has been observed (Fig. 8d). The Fe and Ti element enrichment was analyzed in TiO2 weld compared to other elements (Fig. 8e), while in the case of CaO weldment, enrichment of Cr and Ca elements has been observed (Fig. 8f). This enrichment and impoverishment of elements may be clubbed and develop different secondary phases. A high characterization technique such as electron backscattered electron (EBSD) and transmission electron microscopy (TEM) is required to identify these secondary phases.
3.6 XRD Analysis
XRD diffraction techniques were used to investigate new phase evolution in the weld bead, which was recorded from 40° incidence angle to 100° with a silicon photodiode point detector. Initially, an XRD study was carried out on the base metal to compare the weld bead study of different welded joints. It was observed that base metal shows purely austenitic (γ) peaks having (111), (200), (220), (311), and (222). XRD analysis of the fusion zone of SiO2, TiO2, and CaO welded sample confirmed the evolution of the δ phase, as shown in Fig. 9. It is observed that TiO2 shows more peaks of δ (ferrite) phase evolution having (110), (200), and (211) planes. This could be due to the faster cooling of the TiO2 weldment, which retains more delta ferrite in the weldment (Ref 45). However, in SiO2 and CaO welded samples, peaks of (110) and (211) planes revealed and indicated the δ phase evolution (Ref 46). Further, the MoO3 and No flux weld did not show any new phase evolution.
3.7 Mechanical Properties of Welded Joint
3.7.1 Tensile Testing
The tensile test is performed in accordance with ASTM E8/8M standards using an Instron universal testing machine at room temperature at a strain rate of 1.5 mm/min. Each test was performed two times to confirm the data's reproducibility. Figure 10 shows the stress-strain relationship of the tensile tested sample (Fig. 10a) and photographs (Fig. 10b) of the fractured specimen. The weld zone fracture was discovered in all of the tensile specimens. It is appropriate to analyze weld strength since all-welded samples were broken in the welded zone; however, discrepancies in weld strength may solely be attributable to solid solution strengthening of different flux compositions employed during TIG. The ultimate tensile strength of base metal was claimed to be 855 MPa which is highest than welded sample due to well defined austenitic structure (Ref 47, 48). In comparison with welded joints, the highest ultimate tensile strength (825 MPa) was observed in TiO2-assisted TIG welded joint, which is satisfactory as the values are similar or nearer to the base metal, followed by No flux (821 MPa), SiO2 (812 MPa), MoO3 (793 MPa), and CaO (770 MPa) (Table 4). This improved joint efficiency of TiO2 \(\left(\frac{\mathrm{ultimate\; tensile \;strenghth\; of \;TiO}2\mathrm{ weldment}}{\mathrm{ultimate\; tensile\; strenghth \;of \;Base\; metal }}\times 100=96.49\% \right)\) weldment is due to increased delta ferrite content confirmed by the XRD plot (Fig. 9) (Ref 49). MoO3 flux weld exhibits deteriorating joint efficiency; this could be due to increased dissolved oxygen in the weld bead. (Ref 11, 50). It is evident from the tensile result CaO weldment shows the lowest tensile strength compared to other weldments. After analyzing the CaO weld bead in EDS (Fig. 8f), calcium and sulfur segregation in the weld bead was noticed. This segregation could form calcium sulfide as an impurity that deteriorates the joint's tensile strength (Ref 51). Several researchers reported that the mechanical properties of the welded joint deteriorated dramatically as a result of the oxygen dissolvent (Ref 11, 52). To better understand failure behavior, the fracture surface of TIG and ATIG weldment was examined under SEM (Fig. 11). Furthermore, tensile fractography revealed numerous larger voids with ductile tearing edges in flux and No flux weldments, indicating a ductile fracture mechanism.
3.7.2 Impact Testing
The impact testing specimen was made in accordance with ASTM E23. A pendulum-type testing machine with a 300 J capacity was used to conduct the impact test. Two trials of impact tests of each type of welding were conducted at room temperature to ensure the reproducibility of the results. The impact strength of base metal was claimed to be 130 J which is highest than welded sample due to well organized austenitic structure and less entrapped oxygen (Ref 53, 54). In comparison with flux-assisted welded joint, the highest impact strength (124 J) was observed in TiO2-assisted TIG welded joint, followed by SiO2 (122 J), MoO3 (112 J MPa), and CaO (90 J) (Table 5). It was discovered that a base metal requires more energy to fracture due to well-organized austenitic structure and less entrapped oxygen, followed by No flux welding (128 J). It is to be thought that that entrapped oxygen deteriorates the weld metal's impact toughness (Ref 13). The ATIG weld comparison noticed that CaO weldments absorb less energy before fracture due to deprived Mn and Ni content and increment in chromium content in the matrix of fusion zones. It was widely assumed that increasing the chromium (ferrite former) and decreasing the Ni (austenitic former) reduce the impact energy value (Ref 25, 26, 55). The more absorbed energy of the TiO2 weldment before fracture could be due to extensive delta peaks found, which increases its impact value (Ref 56, 57). Thus, the result concludes that the austenitic and ferritic phase content should be optimized to obtain excellent impact strength. Further, a fractured specimen of ATIG weldment and No flux weldment was analyzed in SEM to understand welded samples' failure mode better. Figure 12 shows the fractured image of base metal and weldments. From fractography, it was noticed that activated TIG welds reveal more dimple structure, voids, and a honeycomb-like structure with a fibrous network uniformly distributed all over the surface, indicating that the failure mode is ductile in nature (Ref 18, 57). Generally, such structures are revealed due to the predominant austenite in the weld region (Ref 58).
3.7.3 Micro Hardness Testing
At a constant load of 200 gf and a dwell time of 15 s, the hardness value was measured parallel to the welded surface and 1 mm below the top weld bead. Hardness reading measurements were taken in the transverse to the welding direction, at a consistent indentation of every 0.25 mm distance from the weld centerline up to the base metal, using the same methods as used for microstructural analysis. Figure 13 depicts the hardness distribution of TIG and ATIG weld. According to the findings, the fusion zone of the flux-assisted TIG joint has more hardness than No flux TIG. On the other hand, there is lower measured hardness compared to all TIG and ATIG weld joints in the heat-affected zone. This lower hardness value in the heat-affected zone might be owing to heat dissipation, which led to a higher peak temperature (due to greater heat input) in the HAZ area, coarsening the grain and lowering hardness marginally (Ref 11, 27). During TiO2-assisted ATIG welding, the fusion zone region achieved the highest average hardness of \(240 \pm 0.2\mathrm{ HV}\). However, SiO2 \((239.5 \pm 0.20\mathrm{ HV})\), MoO3 \((239.5 \pm 0.10\mathrm{ HV})\), and CaO \((239.5 \pm 0.15\mathrm{ HV})\) weldment found almost near hardness value. The increased delta ferrite phase in the TiO2 weld bead acts as a strengthening phase (Fig. 9) (Ref 36). As we know, δ-ferrite exhibits BCC stronger and harder than FCC (austenitic phase), supporting mechanical properties such as hardness. On the other side, base metal Cr-Mn SSs showed the lowest average hardness \((237 \pm 0.3\mathrm{ HV})\) followed by No flux \((238 \pm 0.2\mathrm{ HV})\) weld bead because of their purely austenitic characteristics (FCC) and no other alloying effect on the microstructure. This discrepancy in hardness might also be related to differences in the TIG weld bead's cooling rate. The high heat generated by the use of oxide fluxes, which might result in more tungsten electrode melting during ATIG welding, also could be a reason for the improvement in the hardness of the ATIG weld bead (Ref 59).
3.8 Potentiodynamic Polarization (PDP) Study
Metal chloride is formed when a solution containing chloride ions (Cl−) interacts with a metal surface, generating a discontinuity in the passive layer in contact with the metal surface, pitting on the metal. The resistance to the pitting has been evaluated by (potentiodynamic polarization test) PDP test. In the PDP test, the samples were cut to the adopted weld surface area of 0.40 cm2 and polished to 1500-grit size silicon carbide paper, followed by ultrasonic treatment. The test was performed in the 3.5 weight percentage of NaCl environment by maintaining the scan rate of 1.67 mV/s. For the sake of data reproducibility, the tests were repeated three times. A fresh electrolytic solution (maintained at room temperature) was used after each PDP test.
Figure 14 shows polarization plots of base metal and welded fusion zones. These plots were subjected to Tafel exploration for getting electrochemical data. Table 6 shows summarized electrochemical data obtained from a polarized plot. The curve observed that the base metal had not shown any sign of pitting potential. The Cr-Mn base metal was ascribed to a symbiosis of consistent Ni, Cr, and Mn content, making it more prone to corrosion attack than other TIG and ATIG welded joints. Stable pits were observed from the SEM micrograph (Figure 15) of Cr-Mn BM. The next most resistant was CaO weldment \((\mathrm{Epitt}=-130.164\mathrm{ mv})\) followed by MoO3 weld \((\mathrm{Epitt}=-195.448\mathrm{ mv})\), TiO2 \((\mathrm{Epitt}=-196.547\mathrm{ mv})\) weld, No flux weld \((\mathrm{Epitt}=-201.839\mathrm{ mv})\), and SiO2 \((\mathrm{Epitt}=-262.931\mathrm{ mv})\). It is well known that the Cr-enriched ferrite phase is suitable for excellent pitting corrosion resistance. Thus, excellent pitting corrosion resistance of CaO welded joint could be due to the chromium enrichment in the weld bead. We know that Cr, Mo, and Mn are the ferrite formers elements necessary to maintain the best pitting corrosion resistance if their content is optimal. TiO2-assisted weld was also attributed to the good immune for pitting corrosion which had the highest δ-ferrite content and enriched Ti element; therefore, chloride ions do not quickly accumulate. However, heavy pits were observed in all-welded joints excluding CaO, and No flux TIG welded joint, as it is shown in Fig. 15. This deterioration of surface properties may be due to encountered dissolved oxygen or deprived chromium (Ref 17, 26). Figure 15 shows the pitting images of base metal and welded sample under a chloride environment.
3.9 Double-Loop Potentiokinetic Reactivation (DLEPR) Test
Due to the elevated temperatures generated during welding, the Cr element is depleted from its adjacent portion, resulting in chromium carbide formation at the grain boundary, also known as intergranular corrosion (IGC). To determine the susceptibility of TIG and ATIG weld beads toward intergranular corrosion, a double-loop electrochemical potentiokinetic reactivation (DLEPR) test using a 0.5 M H2SO4 + 0.01 M KSCN electrolytic solution was carried out in a potentiostat (Solartron-1285). Forward and reverse scans are implemented at a constant scan rate of 1.667 mV/second. Three samples of each welding were tested to ensure high reproducibility (error was less than 5%) of the DLEPR results. A fresh electrolytic solution (maintained at room temperature) was used after every DLEPR test. A forward scan was performed with a potential range of − 0.7 to 0.5 V (standard calomel electrode), and the reverse scan was performed with a potential range of 0.5 to − 0.7 V (standard calomel electrode). Prior to that, the oxide layer on the working electrode's surface is removed for 2 minutes at 0.5 V in the same solution; then, the working electrode is stabilized for 20 minutes using open-circuit voltage (OCP). After reaching maximum current density, the passive layer covers depleted Cr parts in the forward scan (Ia). In reverse scan, the KSCN agent breaks this passive layer, and parts begin to sensitize until the reactivation peak current density is reached (Ir). The percentage degree of sensitization (% DOS) provides a quantitative measurement of the affected part.
Figure 16 shows DLEPR curves for Base Metal, TIG, and ATIG weld. The weld obtained from MoO3 flux showed the highest % DOS (6.49%) followed by TiO2 (6.18%), CaO (4.82%) SiO2 (4.17%), No flux (2.28%) and base metal (0.97%). The dissolved oxygen in the weld bead could be expedited the degree of sensitization in the case of MoO3 weldment (Ref 16), while impoverishment in the chromium element in the solidified grain matrix is also more prone to sensitization (Ref 55, 60). The presence of precipitation (Cr23C6) also expedites the % DOS rapidly (Ref 26). Data obtained from DLEPR curves for base metal, TIG, and ATIG welded samples are detailed in Table 7.
4 Conclusions
The findings of this study show that using flux in Cr-Mn SSs TIG welding can significantly increase weld penetration. Without edge preparation and currents as low as 120 A, penetration of 4.713 mm thick austenitic stainless steel plates was achieved. The based-on understanding welding experiments for TIG and ATIG, the following conclusions were drawn.
-
(1)
In the present work, the ATIG technique was employed to weld Cr-Mn stainless steel with varying oxide fluxes (MoO3, SiO2, TiO2, CaO). Among the above MoO3 activated flux achieved the highest penetration with reduced HAZ, attributed to the weld pool's centripetal flow (Reverse Marangoni mechanism) caused by a higher weight percentage of dissolved oxygen in the fusion zone.
-
(2)
The microstructure developed by welding with varied ATIG fluxes showed scattered vermicular, lathy, and skeletal ferrite in the austenite matrix. XRD analysis study found δ phase evolution after employing flux which helps to cool the weld bead faster causes retention of ferrite in the austenitic phase.
-
(3)
There is a slight improvement in hardness when comparing the fusion zone of an active TIG weld to the No flux TIG weld zone owing to heat dissipation, which led to a higher peak temperature (due to more significant heat input) in the HAZ area, coarsening the grain and lowering hardness marginally.
-
(4)
It is observed that TiO2-assisted Cr-Mn stainless steel weld is comprised of good tensile impact hardness and corrosion resistance properties.
-
(5)
The activating flux had a considerable impact on the corrosion resistance of the A-TIG weld fusion zone. The CaO weld showed better pitting corrosion potential \((\mathrm{Epitt}=-130.164\mathrm{ mv})\) than all other welded joints due to enriched chromium in the weld bead.
References
S.G. Nayee and V.J. Badheka, Effect of Oxide-Based Fluxes on Mechanical and Metallurgical Properties of Dissimilar Activating Flux Assisted-Tungsten Inert Gas Welds, J. Manuf. Process., The Society of Manufacturing Engineers, 2014, 16(1), p 137–143, Doi: https://doi.org/10.1016/j.jmapro.2013.11.001.
H. Vashishtha, R.V. Taiwade, and R.K. Khatirkar, Effect of Thermal Treatment and Welding on Susceptibility to Intergranular Corrosion of 200-Series Stainless Steel, 2014, (February).
V. Tandon, A.P. Patil and R.C. Rathod, Enhanced Corrosion Resistance of Cr-Mn ASS by Low Temperature Salt Bath Nitriding Technique for the Replacement of Convectional Cr-Ni ASS, Anti-Corrosion Methods Mater., 2019, 66(4), p 439–445.
A.W. Fande, R.V. Taiwade, and L. Raut, Development of Activated Tungsten Inert Gas Welding and its Current Status: A Review, Mater. Manuf. Process., 2022. https://doi.org/10.1080/10426914.2022.2039695
K.D. Ramkumar, J.L.N. Varma, G. Chaitanya, S. Logesh, M. Krishnan, N. Arivazhagan and N.S. Shanmugam, Experimental Investigations on the SiO2 Flux-Assisted GTA Welding of Super-Austenitic Stainless Steels, Int. J. Adv. Manuf. Technol., 2017, 93(1–4), p 129–140.
T.S. Chern, K.H. Tseng, and H.L. Tsai, Study of the Characteristics of Duplex Stainless Steel Activated Tungsten Inert Gas Welds, Mater. Des., Elsevier Ltd, 2011, 32(1), p 255–263, Doi:https://doi.org/10.1016/j.matdes.2010.05.056.
H.L. Lin, T.M. Wu and C.M. Cheng, Effects of Flux Precoating and Process Parameter on Welding Performance of Inconel 718 Alloy TIG Welds, J. Mater. Eng. Perform., 2014, 23(1), p 125–132.
P.J. Modenesi, E.R. Apolinário and I.M. Pereira, TIG Welding with Single-Component Fluxes, J. Mater. Process. Technol., 2000, 99(1), p 260–265.
Y. Zou, R. Ueji, and H. Fujii, Effect of Oxygen on Weld Shape and Crystallographic Orientation of Duplex Stainless Steel Weld Using Advanced A-TIG (AA-TIG) Welding Method, Mater. Charact., Elsevier Inc., 2014, 91, p 42–49, Doi: https://doi.org/10.1016/j.matchar.2014.02.006.
C. Li, Y. Shi, Y. Gu and F. Yang, Effect of Oxide on Surface Tension of Molten Metal, RSC Adv., 2017, 7(85), p 53941–53950. https://doi.org/10.1039/c7ra11185a
K.D. Ramkumar, A. Chandrasekhar, A.K. Singh, S. Ahuja, A. Agarwal, N. Arivazhagan, and A.M. Rabel, Comparative Studies on the Weldability, Microstructure and Tensile Properties of Autogeneous TIG Welded AISI 430 Ferritic Stainless Steel with and Without Flux, J. Manuf. Process., The Society of Manufacturing Engineers, 2015, 20, p 54–69, Doi:https://doi.org/10.1016/j.jmapro.2015.09.008.
R.S. Vidyarthy and D.K. Dwivedi, Activating Flux Tungsten Inert Gas Welding for Enhanced Weld Penetration, J. Manuf. Process., The Society of Manufacturing Engineers, 2016, 22, p 211–228, Doi:https://doi.org/10.1016/j.jmapro.2016.03.012.
P. Vasantharaja and M. Vasudevan, Studies on A-TIG Welding of Low Activation Ferritic/Martensitic (LAFM) Steel, J. Nucl. Mater., Elsevier B.V., 2012, 421(1–3), p 117–123, Doi: https://doi.org/10.1016/j.jnucmat.2011.11.062.
S. Sujai and K. Devendranath Ramkumar, Microstructure and Properties of Nano-SiO2 Activated Flux TIG (A-TIG) Welding of Incoloy 925 Joints, J. Manuf. Process., Elsevier Ltd, 2020, 58(September), p 998–1018, Doi:https://doi.org/10.1016/j.jmapro.2020.08.061.
N.P. Patel, V.J. Badheka, J.J. Vora, and G.H. Upadhyay, Effect of Oxide Fluxes in Activated TIG Welding of Stainless Steel 316LN to Low Activation Ferritic/Martensitic Steel (LAFM) Dissimilar Combination, Trans. Indian Inst. Met., Springer India, 2019, 72(10), p 2753–2761, Doi:https://doi.org/10.1007/s12666-019-01752-7.
P. Sharma and D.K. Dwivedi, Comparative Study of Activated Flux-GTAW and Multipass-GTAW Dissimilar P92 Steel-304H ASS Joints, Mater. Manuf. Process., Taylor & Francis, 2019, 0(0), p 1–10, Doi: https://doi.org/10.1080/10426914.2019.1605175.
K.D. Ramkumar, P.S. Goutham, V.S. Radhakrishna, A. Tiwari, and S. Anirudh, Studies on the Structure–Property Relationships and Corrosion Behaviour of the Activated Flux TIG Welding of UNS S32750, J. Manuf. Process., The Society of Manufacturing Engineers, 2016, 23, p 231–241, doi:https://doi.org/10.1016/j.jmapro.2016.05.006.
S.M. Kumar and N.S. Shanmugam, Studies on the Weldability, Mechanical Properties and Microstructural Characterization of Activated Flux TIG Welding of AISI 321 Austenitic Stainless Steel, Mater. Res. Express, 2018, 5(10), p 106524.
H. Rana, V. Badheka, P. Patel, V. Patel, W. Li, and J. Andersson, Augmentation of Weld Penetration by Flux Assisted TIG Welding and Its Distinct Variants for Oxygen Free Copper, J. Mater. Res. Technol., Elsevier Ltd, 2021, 10, p 138–151, Doi: https://doi.org/10.1016/j.jmrt.2020.12.009.
A. Surve, S. Bhosage, A. Mehta, A. Srikanth, M. Arivarasu, M. Manikandan, K. Gokulkumar, and D.N. Rajan, Investigation on Microstructure and Mechanical Properties of ATIG Welded Alloy C-276 with Fe2O3 Flux, IOP Conf. Ser. Mater. Sci. Eng., 2018, 310(1).
S. Saha, B.C. Paul, and S. Das, Productivity Improvement in Butt Joining of Thick Stainless Steel Plates through the Usage of Activated TIG Welding, SN Appl. Sci., Springer International Publishing, 2021, 3(4), p 1–14, doi:https://doi.org/10.1007/s42452-021-04409-7.
J. Sivakumar, M. Vasudevan and N.N. Korra, Effect of Activated Flux Tungsten Inert Gas (A-TIG) Welding on the Mechanical Properties and the Metallurgical and Corrosion Assessment of Inconel 625, Weld. World, 2021, 65(6), p 1061–1077.
A. Kulkarni, D.K. Dwivedi, and M. Vasudevan, Effect of Oxide Fluxes on Activated TIG Welding of AISI 316L Austenitic Stainless Steel, Mater. Today Proc., Elsevier Ltd., 2019, 18, p 4695–4702, Doi: https://doi.org/10.1016/j.matpr.2019.07.455.
L.F. Li and J.P. Celis, Pickling of Austenitic Stainless Steels (a Review), Can. Metall. Q., 2003, 42(3), p 365–376.
J. Verma and R. V. Taiwade, Effect of Austenitic and Austeno-Ferritic Electrodes on 2205 Duplex and 316L Austenitic Stainless Steel Dissimilar Welds, J. Mater. Eng. Perform., Springer US, 2016, 25(11), p 4706–4717.
C. Tembhurkar, R. Kataria, S. Ambade, J. Verma, A. Sharma, and S. Sarkar, Effect of Fillers and Autogenous Welding on Dissimilar Welded 316L Austenitic and 430 Ferritic Stainless Steels, J. Mater. Eng. Perform., Springer US, 2021, 30(2), p 1444–1453, Doi: https://doi.org/10.1007/s11665-020-05395-4.
K.H. Tseng and P.Y. Lin, UNS S31603 Stainless Steel Tungsten Inert Gas Welds Made with Microparticle and Nanoparticle Oxides, Materials (Basel), 2014, 7(6), p 4755–4772.
H. Gopal, J. Kleinsmidt, J. Case and P. Musonge, An Investigation of Tertiary Students’ Understanding of Evaporation, Condensation and Vapour Pressure, Int. J. Sci. Educ., 2004, 26(13), p 1597–1620.
C.R. Heiple, J.R. Rope, R.T. Stagner and R.J. Aden, Surface Active Element Effects on the Shape of Gta, Laser, and Electron Beam Welds, Weld. J. (Miami, Fla), 1983, 62(3), p 72–77.
P.J. Modenesi, The Chemistry of TIG Weld Bead Formation, Weld. Int., 2015, 29(10), p 771–782.
K.C. Mills and B.J. Keene, Factors Affecting Variable Weld Penetration, Int. Mater. Rev., 1990, 35(1), p 185–216.
L.M. Liu, D.H. Cai, and Z.D. Zhang, Magnesium Alloy Weld Using Manganese Chloride Coated Wire, Sci. Technol. Weld. Join., Taylor & Francis, 2008, 13(1), p 44–48.
Y. Zhao, H. Zhou and Y. Shi, The Study of Surface Active Element on Weld Pool Development in A-TIG Welding, Model. Simul. Mater. Sci. Eng., 2006, 14(3), p 331–349.
P.J. Modenesi, P. Colen Neto, E. Roberto Apolinário and K. Batista Dias, Effect of Flux Density and the Presence of Additives in ATIG Welding of Austenitic Stainless Steel, Weld. Int., 2015, 29(6), p 425–432.
S. Leconte, P. Paillard, P. Chapelle, G. Henrion and J. Saindrenan, Effect of Oxide Fluxes on Activation Mechanisms of Tungsten Inert Gas Process, Sci. Technol. Weld. Join., 2006, 11(4), p 389–397.
A. Kulkarni, D.K. Dwivedi, and M. Vasudevan, Study of Mechanism, Microstructure and Mechanical Properties of Activated Flux TIG Welded P91 Steel-P22 Steel Dissimilar Metal Joint, Mater. Sci. Eng. A, Elsevier B.V., 2018, 731(March), p 309–323, Doi:https://doi.org/10.1016/j.msea.2018.06.054.
S. Jayakrishnan, P. Chakravarthy, and A. Muhammed Rijas, Effect of Flux Gap and Particle Size on the Depth of Penetration in FBTIG Welding of Aluminium, Trans. Indian Inst. Met., Springer India, 2017, 70(5), p 1329–1335.
K.H. Tseng and C.Y. Hsu, Performance of Activated TIG Process in Austenitic Stainless Steel Welds, J. Mater. Process. Technol., Elsevier B.V., 2011, 211(3), p 503–512, Doi:https://doi.org/10.1016/j.jmatprotec.2010.11.003.
G. Chandrasekar, C. Kailasanathan, and M. Vasundara, Investigation on Un-Peened and Laser Shock Peened Dissimilar Weldments of Inconel 600 and AISI 316L Fabricated Using Activated-TIG Welding Technique, J. Manuf. Process., Elsevier, 2018, 35(September), p 466–478, doi:https://doi.org/10.1016/j.jmapro.2018.09.004.
J.J. Lowke, M. Tanaka and M. Ushio, Mechanisms Giving Increased Weld Depth Due to a Flux, J. Phys. D. Appl. Phys., 2005, 38(18), p 3438–3445.
R.H. Zhang, J.L. Pan and S. Katayama, The Mechanism of Penetration Increase in A-TIG Welding, Front. Mater. Sci., 2011, 5(2), p 109–118.
S. Jaypuria, S. Khandai, T.R. Mahapatra, and A. Singh, Development of Activated Flux for Deep Penetration in GTAW, Mater. Today Proc., Elsevier Ltd., 2019, 18, p 4703–4710, Doi:https://doi.org/10.1016/j.matpr.2019.07.456.
J.A. Brooks, J.C. Williams and A.W. Thompson, Microstructural Origin of the Skeletal Ferrite Morphology of Austenitic Stainless Steel Welds, Metall. Trans. A, 1983, 14(7), p 1271–1281.
J.J. Vora and V.J. Badheka, Experimental Investigation on Mechanism and Weld Morphology of Activated TIG Welded Bead-on-Plate Weldments of Reduced Activation Ferritic/Martensitic Steel Using Oxide Fluxes, J. Manuf. Process., The Society of Manufacturing Engineers, 2015, 20, p 224–233, doi:https://doi.org/10.1016/j.jmapro.2015.07.006.
H. Vashishtha, R.V. Taiwade, S. Sharma, and A.P. Patil, Effect of Welding Processes on Microstructural and Mechanical Properties of Dissimilar Weldments between Conventional Austenitic and High Nitrogen Austenitic Stainless Steels, J. Manuf. Process., The Society of Manufacturing Engineers, 2017, 25, p 49–59, Doi:https://doi.org/10.1016/j.jmapro.2016.10.008.
S. Shukla, A.P. Patil and A. Bansod, Effect of Thermal Aging on IGC of Cold Worked Cr-Mn Austenitic Stainless Steel, Anti-Corrosion Methods Mater., 2019, 66(6), p 757–767.
K. Devendranath Ramkumar, S. Singh, J.C. George, S. Anirudh, G. Brahadees, S. Goyal, S.K. Gupta, C. Vishnu, N.R. Sharan, and S. Kalainathan, Effect of Pulse Density and the Number of Shots on Hardness and Tensile Strength of Laser Shock Peened, Activated Flux TIG Welds of AISI 347, J. Manuf. Process., The Society of Manufacturing Engineers, 2017, 28, p 295–308, Doi:https://doi.org/10.1016/j.jmapro.2017.06.017.
V.J. Sutar and B.R. Jadhav, Implementation of TOPSIS Optimisation Technique in Comparative Analysis of Conventional TIG and Activated TIG Welding of Stainless Steel 304L, Int. J. Mater. Eng. Innov., Inderscience Publishers (IEL), 2021, 12(3), p 188–206.
V. Tandon, M.A. Thombre, A.P. Patil, R. V. Taiwade, and H. Vashishtha, Effect of Heat Input on the Microstructural, Mechanical, and Corrosion Properties of Dissimilar Weldment of Conventional Austenitic Stainless Steel and Low-Nickel Stainless Steel, Metallogr. Microstruct. Anal., Springer US, 2020, 9(5), p 668–677, doi:https://doi.org/10.1007/s13632-020-00681-y.
C.M. Lin, J.J. Liu, H.L. Tsai and C.M. Cheng, Evolution of Microstructures and Mechanical Properties of AZ31B Magnesium Alloy Weldment with Active Oxide Fluxes and GTAW Process, J. Chin. Inst. Eng. Trans. Chin. Inst. Eng. A, 2011, 34(8), p 1013–1023.
X. Li, X. Long, L. Wang, S. Tong, X. Wang, Y. Zhang, and Y. Li, Inclusion Characteristics in 95CrMo Steels with Different Calcium and Sulfur Contents, Materials (Basel)., 2020, 13(3).
X. Xie, J. Shen, L. Cheng, Y. Li, and Y. Pu, Effects of Nano-Particles Strengthening Activating Flux on the Microstructures and Mechanical Properties of TIG Welded AZ31 Magnesium Alloy Joints, Mater. Des., Elsevier Ltd, 2015, 81, p 31–38, Doi:https://doi.org/10.1016/j.matdes.2015.05.024.
J. Verma, R.V. Taiwade, R.K. Khatirkar, S.G. Sapate, and A.D. Gaikwad, Microstructure, Mechanical and Intergranular Corrosion Behavior of Dissimilar DSS 2205 and ASS 316L Shielded Metal Arc Welds, Trans. Indian Inst. Met., Springer India, 2017, 70(1), p 225–237.
J. Verma, R.V. Taiwade, C. Reddy and R.K. Khatirkar, Effect of Friction Stir Welding Process Parameters on Mg-AZ31B/Al-AA6061 Joints, Mater. Manuf. Process., 2018, 33(3), p 308–314.
W. Chuaiphan and L. Srijaroenpramong, Effect of Filler Alloy on Microstructure, Mechanical and Corrosion Behaviour of Dissimilar Weldment between AISI 201 Stainless Steel and Low Carbon Steel Sheets Produced by a Gas Tungsten Arc Welding, Adv. Mater. Res., 2012, 581–582(1), p 808–816.
S. Mohan Kumar, S. Sankarapandian, and N. Siva Shanmugam, Investigations on Mechanical Properties and Microstructural Examination of Activated TIG-Welded Nuclear Grade Stainless Steel, J. Brazilian Soc. Mech. Sci. Eng., Springer Berlin Heidelberg, 2020, 42(6), p 1–21, doi:https://doi.org/10.1007/s40430-020-02393-4.
Q. An, K.Y. Fan, Y.F. Ge, B.X. Liu, Y.C. Liu, S. Wang, C.X. Chen, P.G. Ji, and F.X. Yin, Microstructure and Mechanical Properties of Stainless Steel Clad Plate Joints Produced by TIG and MAG Hybrid Welding, J. Adhes. Sci. Technol., 2019, 4243.
A. Kulkarni, D.K. Dwivedi, and M. Vasudevan, Dissimilar Metal Welding of P91 Steel-AISI 316L SS with Incoloy 800 and Inconel 600 Interlayers by Using Activated TIG Welding Process and Its Effect on the Microstructure and Mechanical Properties, J. Mater. Process. Technol., Elsevier, 2019, 274(June), p 116280, Doi:https://doi.org/10.1016/j.jmatprotec.2019.116280.
H. Kumar, G.N. Ahmad, and N.K. Singh, Activated Flux TIG Welding of Inconel 718 Super Alloy in Presence of Tri-Component Flux, Mater. Manuf. Process., Taylor & Francis, 2019, 34(2), p 216–223, Doi:https://doi.org/10.1080/10426914.2018.1532581.
J. Verma, R. V. Taiwade, R. Kataria, and A. Kumar, Welding and Electrochemical Behavior of Ferritic AISI 430 and Austeno-Ferritic UNS 32205 Dissimilar Welds, J. Manuf. Process., Elsevier, 2018, 34(May), p 292–302, Doi:https://doi.org/10.1016/j.jmapro.2018.06.019.
Acknowledgments
To carry out this investigation and provide the necessary facilities to publish this work, the authors would like to thank Director Dr. P.M. Padole, VNIT Nagpur, for his constant encouragement. The authors would like to thank Mr. Ashwin Gaikwad, WeldFast Industries Nagpur, for carrying out TIG welding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fande, A.W., Taiwade, R.V. & Sekhar, P.C. Microstructure, Mechanical, and Electrochemical Evaluation of Chrome-Manganese Stainless Steel Activated Tungsten Inert Gas Welded Joint. J. of Materi Eng and Perform 31, 9431–9447 (2022). https://doi.org/10.1007/s11665-022-06894-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-022-06894-2