Abstract
Quaternary glasses with the chemical composition 50SiO2-25TiO2-5La2O3- (20-x) Na2O-xY2O3 by use the melt-quench method. The FT-IR spectroscopy investigated the structural change in these glasses. XRD examined the nature of these glasses. While the density is increased, the molar volume of the glass system is reduced. Ultrasonic velocities and elastic modulus of these glasses were experimentally and theoretically calculated based on the Makishima-Mackenzie model. Moreover, the radiation shielding capacity was evaluated for the studied glasses. The mass attenuation coefficient (μ/ρ), half value layer(HVL), tenth value layer (TVL), mean free path (MFP), effective atomic number (Zeff), electron density (Neff), equivalent atomic number Zeq, and effective removal cross section (ΣR) of prepared glasses were simulated for gamma photon energies between 0.015 and 15 MeV. Exposure build-up factor (EBF) and (EABF) of prepared glasses were evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glass is a transparent, amorphous material and melts without crystallisation of an inorganic material (Ref 1,2,3,4,5,6). Sodium titanium silicate glasses have high importance in optical and electronic instruments as storage batteries. The sodium titanium silicate glasses are conventional glasses that have been developed because of their significant advantages in storage batteries (Ref 7,8,9,10,11,12,13,14,15). These features affected by the addition of modifiers as a transition metal and rare-earth ions. It is highly possible for applicants for UV optics and solid-state batteries because of the good ionic conductivity of these glasses (Ref 7,8,9,10,11,12,13,14,15,16). Due to the science and technology importance of sodium titanium silicate glasses, characteristics such as solid electrolytes in battery storage glasses modified with different oxides are strongly needed (Ref 17, 18). In addition, it enhances the properties of these glasses by introducing transitional oxides (Ref 19,20,21,22) or rare earth oxides (REs). Glasses containing rare-earth ions have attracted a great deal of interest because of their advantages. Lanthanum, with the symbol La and Atomic No.57. La2O3 has no color center in the glasses, so it is colorless. La2O3 enhances the chemical resistance and optical characteristics of the glass matrix. Today, the development of rare earth (RE) doped glasses for use in communication systems, such as fibers, amplifiers, and lasers, is one of the most important areas of research. Attempts were focused to improve the optics properties of RE ions in different oxides (Ref 23,24,25,26,27,28).
Depending on their concentration in the glass matrix, either as a glass modifier or a glass former, intermediate oxides such as Y2O3 can act. Y2O3 improves physical stability and other properties of doped glass matrix (Ref 29,30,31). The presence of TiO2 and Y2O3 in glass systems influences on UV-spectroscopic because of the TiO2 and Y2O3 act as an intermediate. The presence of Y2O3 improvement the glass-forming ability and decreases the devitrification. Because of higher density and its simple performance and lower melting temperature are several applicable titanium silicate glasses containing Y2O3. The photon energy of these glasses is smaller than other glasses and has a higher refractive index. Titanium borate glasses are therefore transparent, with successful optoelectronic, thermal, mechanical and chemical stability. Scientifically and technologically, the recent innovation of titanium silicate glasses containing Y2O3 and La2O3 is very significant. æTitanium silicate glasses can be regarded as an adaptable type of glass that is used in various applications because they have high thermal stability and mechanical properties. Besides, it is considered good for TMi, REi, and halides as host glasses. Due to their attractive structural, mechanical properties and infrared radiation shielding, there has been considerable interest in the study of 50SiO2-25TiO2-5La2O3- (20-x) Na2O-xY2O3 glasses over the last few years. Due to the increase in the concentration of Y2O3 in titanium lanthanum sodium silicate glasses, the attenuation, structural, and mechanical of these glasses can significantly increase. Furthermore, it is possible to use this glass in aircraft bodies, a shield from radiation in the x-ray canters, and facades of houses. It's the best study for the preparation of these glasses and their structural, mechanical, and shielding radiation. Thus, it is possible to find the prepared glasses are suitable for use in environments exposed to radiation. The purpose of this research is to identify the attenuation proficiency of prepared glasses by Phy-X/PSD (Ref 32) software and to identify the mechanical and structure of these glasses in order to determine their suitability as gamma-ray shielding materials (Table 1).
Experimental Processes and Techniques
The glasses in this study were synthesized from 50SiO2-25TiO2-5La2O3- (20-x) Na2O-xY2O3 using the melt-quench technique method where \(x\) = (0,2,4,6,8,10 \(\mathrm{0,5},\mathrm{10,15,20,25}\)). The starting materials to obtain these glasses are SiO2, Na2CO3, La2O3, Y2O3 and TiO2 with high purity. All chemicals used for the glass preparation obtained from Sigma-Aldrich. The starting materials were mixed together by grinding the mixture repeatedly to obtain a fine powder. Firstly, the starting materials have been heated to 450°C for 1 h to eliminate H2O, and CO2. The temperature has been raised to 1200°C for 30 min. The glasses were annealed at 450 °C for 2 h to relieve the internal stresses and allowed to cool gradually to room temperature at a rate of about 30 °C h-1. The weight losses were found to be less than 1%.
The amorphous state of the glasses was checked using X-ray diffraction. A Philips X-ray diffractometer PW/1710 with Ni-filtered Cu-Kα radiation (λ = 1.542 Å) powered at 40 kV and 30 mA was used.
FTIR spectra of the as-quenched glasses (after crushing them into powder form) were obtained with a Fourier transform IR spectrometer (JASCO, FT/IR–430, Japan). For this purpose, each glass powder was mixed with KBr in the proportion of 1:100 (by weight) for 20 min and pressed into a pellet using a hand press. In the wavenumber range of 4000–400 cm−1 with a resolution of 4 cm−1, corrected for dark-current noise and normalized. The resulting spectra were curve fitted to get quantitative values for the band areas of heavily overlapped bands using a computer program Origin 8.5. Estimated error limit in the fitting process is about ± 2 cm−1.
The density of each sample was measured by Archimedes’ principle by using toluene as the immersion fluid. Four samples of each glass were used to determine the density (ρ). A random error in the density values was found as ± 0.025 g cm-1.
The prepared samples were grinded and polished with different grades of SiC emery powder on a soft leather piece fixed on a flat platform for the ultrasonic velocity measurements. Non-parallelism of the two opposite side faces was measured with a micrometer, which could measure down to 0.01 mm. The ultrasonic velocities, longitudinal (vL) and shear (vT), at room temperature (~300 K) were obtained using the pulse-echo method. In this method, x-cut and y-cut transducers (KARL DEUTSCH) operated at a fundamental frequency 4 MHz along with a digital ultrasonic flaw detector (KARL DEUTSCH Echograph model 1085) were used. The uncertainty in the measurement of the ultrasonic velocity is ±10 m s-1. The two velocities besides the density were utilized to determine two independent second-order elastic constants. (See revised paper).
Results and Discussion
XRD and, FT-IR
In Fig. 1, the XRD did not demonstrate intense peak that demonstrate the high glass status of the glass samples were tested.
The FT-IR spectrum of the yttrium lanthanum titanium silicate glasses is shown in Fig. 2. Figures 3 shows the Gaussian fit of the FT-IR spectrum of these samples, respectively, are shown in Table 2. The network structural units in these glasses detected at ~1440, ~1180, ~1040, ~940, ~850, ~715 and ~485 cm-1 (Ref 33,34,35). Bands in the 1442-1433 cm-1 region had been ascribed to antisymmetric vibrations of bridging oxygen from Si-O-Si. Bands in the 1181-1142 cm-1 region had been ascribed to Si-O non-bridging oxygen stretching mode. Bands in the 1058-1042 cm-1 region had been ascribed to Si-O-Si unit-asymmetric bridging stretching. Bands in the 873-843 cm-1 region had been ascribed to O-Si-O bonds' symmetric stretching vibration. Bands in the 720-712 cm-1 region had been ascribed to (LaO7), (YO8) and (TiO6) vibrations and they overlap with the O-Si-O unit bending vibrations.
FT-IR spectral causes a shift to higher wavenumbers with an increase in yttrium concentration. In addition, it is pointed out with the increase in the concentration of yttrium, there is a high increase in the strength of the bond associated with increasing the settled glass arrangement to provide more stable structures in cooperation with yttrium in the structural network. In Table 3, the peak assignments are shown.
Physical Features
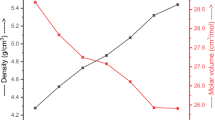
The density of these glasses increases, and the molar volume reduces Fig. 4. The density decreased because of the difference of molecular masses between Na2O and Y2O3 [61.979 & 225.81] and densities [2.27& 5.03 g/cm3]. The reduce in molar volume because of the rise in density (Ref 21, 22).
Y+3 concentration was calculated as \(Yi=\left(\frac{6.023\times {10}^{23}x mol fraction of cation\times valency of cation}{Vm}\right)\). The concentration of Y+3 is well known to increase. Inter-ionic distance (\({R}_{i}\)) was considered between two Y+3 - Y+3 as \({R}_{i}= {\left(\frac{1}{\mathrm{content of Y}}\right)}^\frac{1}{3}\) , It is well known that with the Y+3 concentration, Ri decreased because of the molar volume decrease. Y+3– Y+3 separation (dY-Y) of glasses projected \((\mathrm{dY}-\mathrm{Y}) = {\left(\frac{{V}_{m}^{B}}{N }\right)}^\frac{1}{3}\) and \({V}_{m}^{B}\text{ } = \frac{Vm}{2\left(1- 2\mathrm{Xn}\right)}\) . As molar volume has decreased, (dY-Y) values had also decreased. Polaron radius rp and inter-nuclear distance ri were estimated as \(rp=\frac{1}{2}\) \({\left(\frac{\pi }{6N }\right)}^\frac{1}{3}\) , \(ri={\left(\frac{1}{N }\right)}^\frac{1}{3}.\) Because of the reduction in molar volume when Y2O3 has increased, these characteristics decrease. Oxygen molar volume (Vo), is estimated as, \({V}_{o}=\left(\frac{M}{\rho }\right) \left(\frac{1}{\sum xini}\right)\) and, oxygen packing density (OPD) is estimated as. \(OPD=\left(\frac{1000 C}{Vm}\right)\left(\frac{Mol}{L}\right)\). It is noted that (Vo), decreased, and (OPD) increased. These observations may be due to molar volume and glass density. Packing density estimated as \({V}_{i,}=\left(\frac{3\pi }{4}\right)NA \{ m\mathrm{R}{}_{\mathrm{m}}{}^{3}+ n \mathrm{R}{}_{\mathrm{i }}{}^{3}\} (\frac{m{}_{ }{}^{3} }{mol})\), where Rm and Ri are Pauling radii of M and O. Dissociation energy \(G={({V}_{i}}^{2}{G}_{i})/\left({V}_{i}\right){}_{ }{}^{-1}\). Poisson's ratio is estimated as\(\sigma = \frac{1}{2} - \left( {\frac{1}{7.2 * Vi}} \right)\) , Micro-hardness is estimated as \(\mathrm{H}=\frac{\left(1-2\sigma \right)\mathrm{Y}}{6\left(1+\sigma \right)}\) ,and, Debye Temperature is assessed as \({\theta }_{D}= \frac{h}{k}{\left(\frac{9N}{4\pi {V}_{m}}\right)}^\frac{1}{3}{M}_{s}\). Average of ultrasonic velocities (Ms) is estimated as \({M}_{s}= \frac{1}{3}{\left(\frac{\frac{2}{{v}_{T}^{3}}}{\frac{1}{{v}_{l}^{3}}}\right)}^\frac{1}{3} ,\) thermal Expansion (αP), \({\alpha }_{P =23.2 \left({v}_{L}-0.57457\right)}\). The mechanical constraints as (Vi), (Gi), (H), (αP), (Z), and the connectivity to fractal bond (d) will be strongminded, these constraints are described in Table 4. This activity can be linked to the glass structure network. With the concentration of yttrium, the value of (Vi), (H), (αP), (Z), and (σ) is increased. Yet with the attentiveness of yttrium, this is correlated with the glass structure network, the importance of (Gi) is decreased. The fractal bond connectivity (d) is constant in two dimensional. Table 4 shows these values.
Using the equation, the mean number of coordinates (m) is computed \(m=\sum {n}_{ci }{X}_{i}\) co-ordination of cations is \({n}_{ci}\). It is observed that the value of \(m\) increases with the increase in Y2O3. The number of bonds computed by volume of units is\({n}_{b}=\frac{{N}_{A}}{{V}_{m}}\) \(\sum {n}_{ci }{X}_{i}\) where \({N}_{A}\)is the Avogadro number. As the content of Y2O3 increased, our analysis indicates that \({n}_{b}\) was rising. There is strong evidence the role of a Y2O3 modifier in the glass network. Table 4 shows these values.
Mechanical Properties
Figure 5 and Table 5 represented sound velocities of prepared glasses. This found that Y2O3 increase those velocities, because of increased density and cross-link density (Ref 33,34,35,36). vL ranges between 5370 and 5510 m/s and vT ranges between 2850 and 2935m/s. According to the previous FTIR analysis, with an increasing in Y2O3 contented, band position shifted to a higher wavenumber, this shift caused the composition change in the glass network and increased the glass network connectivity.
To estimate elastic-modules as,\(L=\rho {v}_{l}^{2}\), \(G=\rho {v}_{s}^{2}\), \(Y=\left(1+\sigma \right)2G , and\), \(K=L-\left(\frac{4}{3}\right)G.\) Elastic-modulus of glasses have been estimated and represented in Fig. 6 (A,B) and Table 5. It indicated that, are increasing with the increasing of Y2O3 content. It is because of the transformation of Si-O-Na into Si-O-Y \(,\) and Na-O bond strength (20KCal/mol) is much lower than Y-O (50KCal/mol) (Ref 37). This behavior is linked to the modification of the coordination number with Y2O3 increase, the growth in average constant force and cross-link density. As Y2O3 increases at the expense of Na2O, the molar volume decreases, and the density increases, making the glass structure more compact.
Mass Attenuation Coefficient (μ/ρ).
The (μ/ρ) has been estimated as \(\left(\frac{\mu }{\uprho }\right)=\sum_{i}{w}_{i }{\left(\frac{\mu }{\rho }\right)}_{i}.\) Figure 7 represented the (μ/ρ) as a function of photon energy, and yttrium concentrations . It is shown that the (μ/ρ) of prepared samples increased at small energy, then it has remained constant at higher energy (Ref 38,39,40,41,42,43,44). It designated that the Y2O3 increases when (μ/ρ) increases. This increase is associated to density. Thus, the adding of Y2O3 enhances γ- radiation attenuation. Table 6 represents the comparison the (μ/ρ) of sample number G6 with the other glasses.
(HVL), (TVL) and (MFP)
The mean free path (MFP) has been projected \(MEP=\left(\frac{1}{\mu }\right).\) The tenth (TVL) and half-value layer (HVL) determined by \(\mathrm{TVL}= \left(\frac{\mathrm{ln}10}{\upmu }\right) ,\) \(\mathrm{HVL}=\left(\frac{\mathrm{ln}2}{\upmu }\right).\) Fig. 8, 9 and 10 shows values of these parameters of prepared samples. These values are increased at the photon energy increased rendering to achieve results. As Y2O3 rise, these parameters values are reducing as well. Hence the addition of Y2O3 enhances γ- radiation attenuation. The comparison of (HVL) and (MFP) with standard materials was represented in Fig. 11(A, B). These glasses have been found to have a high radiation absorption factor of γ- over the other glasses (Ref 38,39,40,41,42,43,44).
(Z eff) (Neff ) and Zeq of prepared glasses.
The effective atomic number (Zeff) estimated as \({Z}_{eff}=\left(\frac{{\sigma }_{a}}{{\sigma }_{e}}\right)\) where (σa) the atomic cross sections \({\sigma }_{a}{\upsigma }_{a} ={\sigma }_{m}\frac{1}{{\sum }_{i}{n}_{i}}={\left(\frac{\mu }{\rho }\right)}_{target}/{N}_{A}{\sum }_{i}\frac{{w}_{i}}{{A}_{i}}\), and σe the electronic cross sections \({\sigma }_{e} =\frac{1}{N} \sum_{i}{\left(\frac{\mu }{\rho }\right)}_{i}\frac{{f}_{i}{wA}_{i}}{{z}_{i}}\). Fig. 12 shows the Zeff of the studied glasses, which diverse with γ-energy and with the yttrium concentrations. Because of the communication of the photoelectric at this range, Zeff is suggested to have a greater value for these glasses with low energy. Because of X-ray K-edges, the Zeff values gradually increase at higher energy. Glass G 6, because of the replacement of Na2O by Y2O3, has a higher Zeff value. The inclusion of Y2O3 to glasses increases the attenuation rate for these glasses (Ref 38,39,40,41,42,43,44).
Electron density (Neff) was projected \({\mathrm{N}}_{\mathrm{eff}}=\mathrm{N }\frac{{\mathrm{Z}}_{\mathrm{eff}}}{{\sum }_{\mathrm{i}}{\mathrm{F}}_{\mathrm{i}}{\mathrm{A}}_{\mathrm{i}}}\). Figure 13 represented the (Neff) values of formulated glasses against energy and Y2O3 concentrations. At lower energy, it is noticed that (Neff) reduced and then increased slowly. The Compton scattering interaction is responsible for this decrease. Thus, the addition of Y2O3 enhances γ- radiation attenuation. This statement is associated to pair creation effect in higher energy and an increase in the content of Y2O3.
Equivalent atomic number Zeq was projected as \(Zeq= \frac{\mathrm{Z}1(\mathrm{logR}2-\mathrm{logR})+\mathrm{Z}2(\mathrm{logR}-\mathrm{logR}1)}{\mathrm{logR}2-\mathrm{logR}1}\). Fig. 14 symbolised the (Zeq) of glasses in range 0.015 and 15 MeV. It showed that (Zeq) enhanced with the photon energy incident and with the replacement of Y2O3 with Na2O. (Zeq) decreased with energy and Y2O3 content because of the interaction of Compton scattering. The highest (Zeq) value at 1 MeV. The (Zeq) value is reduced at higher energy than 1 MeV because of pair creation interaction (Ref 38,39,40,41,42,43,44).
Exposure Build-up Factor (EBF) and (EABF) of Prepared Glasses
G–P fitting parameters have been estimated as \(P= \frac{\mathrm{P}1(\mathrm{logZ}2-\mathrm{logZeq})+\mathrm{Z}2(\mathrm{logZeq}-\mathrm{logZ}1)}{\mathrm{logZ}2-\mathrm{logZ}1}\) where P1 and P2 are the G–P-fitting parameters. EABF and EBF have been projected by using G–P fitting \(B(E,X)=1+\frac{b -1}{K-1}\)(\({K}^{x}-1)\) for K \(\ne 1\), \(B(E,X)=1+(b-1)x\) K \(= 1\) where \(K(E,X)=c{x}^{a}+d\frac{tanh(\frac{x}{Xk} -2)-tanh(-2)}{1-tanh (-2)}\) . EBF and, EABF are obtained from GP-fitting. Figs. (15 and 16) symbolized the (EBF) and EABF of glasses against the gamma energy. The values of EBF and EABF are affected by the energy and composition of the glass samples (Ref 38,39,40,41,42,43,44). EBF and EABF values are small at the lower energy because the energy photons will be absorbed by the glasses and then expanded with the energy increase because of Compton scattering. After that, EBF and EABF decrease with increasing energy because of pair production. In addition, enhanced protective properties such as G 6 glass should be achieved with the Y2O3 content of studied glasses (Tables 7 and 8).
Fast Neutron Removal Cross Section (FNRCS) (1/cm)
Effective removal cross section (ΣR),projected:\(\left(\frac{{\Sigma }_{\mathrm{R}}}{\uprho }\right)= \sum_{\mathrm{i}}{{\mathrm{w}}_{\mathrm{i}}\left(\frac{{\Sigma }_{\mathrm{R}}}{\uprho }\right)}_{\mathrm{i}}\) and \(\mathrm{R }= \sum_{\mathrm{i}}{ {\uprho }_{\mathrm{i}} \left(\frac{\mathrm{R}}{\uprho }\right)}_{\mathrm{i}} .\) Fig. 17 (ΣR) of glass samples against gamma energy was illustrated. At small energy, it is noticed that the (ΣR) increased. Small variations of these glasses with a decrease in the value of (ΣR) are obtained at higher energy because of increased Y2O3 at the expense of Na2O. In our sample (Y2O3: 225.81) increased in prepared glasses. The increase in the Y2O3 content may lead to an improvement in the shielding of neutrons. The increase in Y2O3 enhances ΣR values; therefore, we can say that the addition of Y2O3 to glasses improves the γ -radiation attenuation (Ref 38,39,40,41,42,43,44).
Figure 18 shows the FNRCS of the prepared glasses. It is observed that the FNRCS values are increased as the Y2O3 content increased. The rise in FNRCS is influenced by the composition and density of glass. So, we can say that the addition of Y2O3 to prepare glasses increase the FNRCS.\(\mathrm{Ti}=\left(\frac{6.023\times {10}^{23}\mathrm{x mol fraction of cation}\times \mathrm{valency of cation}}{\mathrm{Vm}}\right)\) \(\mathrm{Ti}=\left(\frac{6.023\times {10}^{23}\mathrm{x mol fraction of cation}\times \mathrm{valency of cation}}{\mathrm{Vm}}\right)\)
Conclusions
In the present investigation, six glasses of titanium lanthanum sodium silicate glasses containing different amount of yttrium with the chemical composition 50SiO2-25TiO2-5La2O3- (20-x) Na2O-xY2O3 where \(x\) = \((0\le x\ge 10\) \(\mathrm{0,5},\mathrm{10,15,20,25}\)) have been fabricated by conventional melt-quenching method. The structure, mechanical, and shielding parameters for these glasses were investigated. The results reveal the following items:
-
1.
The amorphous nature of glasses was confirmed by XRD measurements.
-
2.
The structural changes of the studied glass have been estimated via FTIR spectroscopy.
-
3.
FT-IR spectral pointed out with the increase in the concentration of yttrium, there is a high increase in the strength of the bond associated with increasing the settled glass arrangement to provide more stable structures in cooperation with yttrium in the structural network.
-
4.
The density of the samples was increased with increasing Y2O3 concentration while the molar volume decreased.
-
5.
The ultrasonic velocities these glasses were increased with increasing Y2O3 concentration
-
6.
Elastic modulus of these glasses was experimentally and theoretically calculated based on the Makishima-Mackenzie model is increased.
-
7.
The gamma shielding features of the proposed glasses were estimated using Phy-X / PSD program between 0.015 and 15 MeV. The effect of the addition of Y2O3 on the shielding ability of the glasses was discussed and we found that: (i) The mass attenuation coefficient increased with the increase in the concentration of Y2O3 from 0 mol. % to 10 mol. %, (ii) The addition of Y2O3 can improve the shielding effectiveness the glasses, and (iii) The sample coded as G 6 possesses the lowest HVL while highest Zeff.
Achieved results revealed that the increase in the concentration of Y2O3 in SiO2-La2O3-TiO2-Na2O-Y2O3 glass system can lead to a significant improvement in the structural, mechanical, and gamma photons attenuation. In addition, theses glass can be used as in aircraft bodies, a shield from radiation in the x-ray centers, facades of houses photoelectric, optoelectronic, nonlinear optical devices and optical instruments like solar cells and wave guide-based optical circuits.
References
D.R. Messier, Preparation and Properties of Y-Si-Al-O-N Glasses, Int. J. High Technol. Ceram., 1987, 3(1), p 33–41. https://doi.org/10.1016/0267-3762(87)90061-0
K.S. Shaaban, E.A.A. Wahab, E.R. Shaaban et al., Electronic Polarizability, Optical Basicity, Thermal, Mechanical and Optical Investigations of (65B2O3–30Li2O–5Al2O3) Glasses Doped with Titanate, J. Elec. Mater., 2020, 49, p 2040–2049. https://doi.org/10.1007/s11664-019-07889-x
K.S. Shaaban, S.M. Abo-Naf and M.E.M. Hassouna, Physical and Structural Properties of Lithium Borate Glasses Containing MoO3, Silicon, 2019, 11, p 2421–2428. https://doi.org/10.1007/s12633-016-9519-4
E.A. Abdel Wahab, K.S. Shaaban, R. Elsaman et al., Radiation Shielding, and Physical Properties of Lead Borate Glass Doped ZrO2 Nanoparticles, Appl. Phys. A, 2019, 125, p 869. https://doi.org/10.1007/s00339-019-3166-8
W.M. Abd-Allah, H.A. Saudi, K.S. Shaaban et al., Investigation of Structural and Radiation Shielding Properties of 40B2O3–30PbO–(30–x) BaO-x ZnO Glass System, Appl. Phys. A, 2019, 125, p 275. https://doi.org/10.1007/s00339-019-2574-0
K.S. Shaaban, S.M. Abo-naf, A.M. Abd Elnaeim and M.E.M. Hassouna, Studying Effect of MoO3 on Elastic and Crystallization Behavior of Lithium Diborate Glasses on Elastic and Crystallization Behavior of Lithium Diborate Glasses, Phys. A, Appl, 2017. https://doi.org/10.1007/s00339-017-1052-9
I. Hussain, E.K. Barimah, Y. Iqbal, G. Jose and R. Muhammad, Thermal, Mechanical and Optical Properties of TiO2-doped Sodium Silicate Glass-Ceramics, Trans. Indian Ceram. Soc., 2019 https://doi.org/10.1080/0371750x.2019.1626287
E.A.A. Wahab and K.S. Shaaban, Effects of SnO2 on Spectroscopic Properties of Borosilicate Glasses Before and After Plasma Treatment and its Mechanical Properties, Mater. Res. Express, 2018, 5(2), p 025207. https://doi.org/10.1088/2053-1591/aaaee8
M. Rezvani, B. Eftekhari-Yekta, M. Solati-Hashjin and V.K. Marghussian, Effect of Cr2O3, Fe2O3 and TiO2 Nucleants on the Crystallization Behaviour of SiO2–Al2O3–CaO–MgO(R2O) Glass-Ceramics, Ceram. Int., 2005, 31(1), p 75–80. https://doi.org/10.1016/j.ceramint.2004.03.037
S. Banijamali, B. Eftekhari Yekta, H.R. Rezaie and V.K. Marghussian, Crystallization and Sintering Characteristics of CaO–Al2O3–SiO2 Glasses in the Presence of TiO2, CaF2 and ZrO2, Thermochim. Acta, 2009, 488(1–2), p 60–65. https://doi.org/10.1016/j.tca.2008.12.031
K. Shaaban, E.A. Abdel Wahab, A.A. El-Maaref et al., Judd-Ofelt Analysis and Physical Properties of Erbium Modified Cadmium Lithium Gadolinium Silicate Glasses, J. Mater. Sci. Mater. Electron, 2020, 31, p 4986–4996. https://doi.org/10.1007/s10854-020-03065-8
K.S. Shaaban, E.S. Yousef, E.A. Abdel Wahab et al., Investigation of Crystallization and Mechanical Characteristics of Glass and Glass-Ceramic with the Compositions xFe2O3-35SiO2-35B2O3-10Al2O3-(20–x) Na2O, J. Mater. Eng. Perform., 2020 https://doi.org/10.1007/s11665-020-04969-6
A.A. El-Maaref, S. Badr, K.S. Shaaban, E.A.A. Wahab and M.M. ElOkr, Optical Properties and Radiative Rates of Nd3+ Doped Zinc-Sodium Phosphate Glasses, J. Rare Earths, 2019, 37(3), p 253–259. https://doi.org/10.1016/j.jre.2018.06.006
K.S. Shaaban, E.A.A. Wahab, E.R. Shaaban et al., Electronic Polarizability, Optical Basicity and Mechanical Properties of Aluminum Lead Phosphate Glasses, Opt. Quant. Electron, 2020, 52, p 125. https://doi.org/10.1007/s11082-020-2191-3
A.A. El-Rehim, H. Zahran, I. Yahia et al., Radiation, Crystallization, and Physical Properties of Cadmium Borate Glasses, Silicon, 2020 https://doi.org/10.1007/s12633-020-00798-3
H.A. Saudi, W.M. Abd-Allah and K.S. Shaaban, Investigation of Gamma and Neutron Shielding Parameters for Borosilicate Glasses Doped Europium Oxide for the Immobilization of Radioactive Waste, J Mater Sci: Mater Electron, 2020, 31(9), p 6963–6976. https://doi.org/10.1007/s10854-020-03261-6
E.A. Abdel Wahab, A.A. El-Maaref, Kh.S. Shaaban, J. Börcsök and M. Abdelawwad, Lithium cadmium phosphate glasses doped Sm3+ as a host material for near-IR laser applications, Opt. Mater., 2020 https://doi.org/10.1016/j.optmat.2020.110638
K.S. Shaaban, M.S.I. Koubisy, H.Y. Zahran et al., Spectroscopic Properties, Electronic Polarizability, and Optical Basicity of Titanium-Cadmium Tellurite Glasses Doped with Different Amounts of Lanthanum, J. Inorg. Organomet. Polym., 2020 https://doi.org/10.1007/s10904-020-01640-4
A. Makehima, Y. Tamura and T. Sakaino, Elastic Moduli and Refractive Indices of Aluminosilicate Glasses Containing Y2O3, La2O3, and TiO2, J. Am. Ceram. Soc., 1978, 61(5–6), p 247–249. https://doi.org/10.1111/j.1151-2916.1978.tb09291.x
K.S. Shaaban, E.S. Yousef, S.A. Mahmoud et al., Mechanical, Structural and Crystallization Properties in Titanate Doped Phosphate Glasses, J. Inorg. Organomet. Polym, 2020 https://doi.org/10.1007/s10904-020-01574-x
K.S. Shaaban and E.S. Yousef, Optical Properties of Bi2O3 Doped Boro Tellurite Glasses and Glass Ceramics, Optik Int. J. Light Electron Opt., 2020, 203, p 163976. https://doi.org/10.1016/j.ijleo.2019.163976
A.F.A. El-Rehim, K.S. Shaaban, H.Y. Zahran et al., Structural and Mechanical Properties of Lithium Bismuth Borate Glasses Containing Molybdenum (LBBM) Together with their Glass-Ceramics, J. Inorg. Organomet. Polym., 2020 https://doi.org/10.1007/s10904-020-01708-1
R.S. Gedam and D.D. Ramteke, Synthesis and Characterization of Lithium Borate Glasses Containing La2O3, Trans. Indian Inst. Met., 2012, 65, p 31–35. https://doi.org/10.1007/s12666-011-0107-4
S. Kaewjaeng, S. Kothan, W. Chaiphaksa, N. Chanthima, R. Raja Ramakrishna, H.J. Kim and J. Kaewkhao, High Transparency La2O3-CaO-B2O3-SiO2 Glass for Diagnosis X-Rays Shielding Material Application, Radiat. Phys. Chem., 2019, 160, p 41–47. https://doi.org/10.1016/j.radphyschem.2019.03.018
R. Rajaramakrishna, S. Karuthedath, R.V. Anavekar and H. Jain, Nonlinear Optical Studies of Lead Lanthanum Borate Glass Doped with Au Nanoparticles, J. Non-Cryst. Solids, 2012, 358(14), p 1667–1672. https://doi.org/10.1016/j.jnoncrysol.2012.04.031
Y. Yamamoto, K. Kita, K. Kyuno and A. Toriumi, Structural and electrical properties of HfLaOx films for an amorphous high-k gate insulator, Appl. Phys. Lett., 2006, 89(3), p 032903. https://doi.org/10.1063/1.2227630
A.M. Fayad, W.M. Abd-Allah and F.A. Moustafa, Effect of Gamma Irradiation on Structural and Optical Investigations of Borosilicate Glass Doped Yttrium Oxide, Silicon, 2018, 10, p 799–809. https://doi.org/10.1007/s12633-016-9533-6
K. Singh, N. Gupta and O.P. Pandey, Effect of Y2O3 on the crystallization behavior of SiO2–MgO–B2O3–Al2O3 glasses, J Mater Sci, 2007, 42, p 6426–6432. https://doi.org/10.1007/s10853-006-1188-z
V. Kumar, O.P. Pandey and K. Singh, Effect of A2O3 (A = La, Y, Cr, Al) on Thermal and Crystallization Kinetics of Borosilicate Glass Sealants for Solid Oxide Fuel Cells Glasses, Ceram Int, 2010, 36(5), p 1621–1626. https://doi.org/10.1016/j.ceramint.2010.02.040
S. Singh, G. Kalia and K. Singh, Effect of Intermediate Oxide (Y2O3) on Thermal, Structural and Optical Properties of Lithium Borosilicate Glasses, Mol. Struct., 2015, 1086, p 239–245. https://doi.org/10.1016/j.molstruc.2015.01.031
E.A. AbdelWahab, M.S.I. Koubisy, M.I. Sayyed, K.A. Mahmoud, A.F. Zatsepin, S.A. Makhlouf and K.S. Shaaban, Novel Borosilicate Glass System: Synthesis Average Electronics Polarizability Optical Basicity and Gamma-Ray Shielding Features, J. Non-Cryst. Solids, 2020 https://doi.org/10.1016/j.jnoncrysol.2020.120509
E. Şakar and Özpolat, Öü.Fı., Alım, Bü., Sayyed, M.I., Kurudirek, M., , PhyX / PSD: Development of a User Friendly Online Software for Calculation of Parameters Relevant To Radiation Shielding and Dosimetry, Radiat. Phys. Chem., 2020, 166, p 108496. https://doi.org/10.1016/j.radphyschem.2019.108496
E.A. Abdel Wahab, K.S. Shaaban and E.S. Yousef, Enhancement of Optical and Mechanical Properties of Sodium Silicate Glasses Using Zirconia, Opt. Quant. Electron, 2020, 52, p 458. https://doi.org/10.1007/s11082-020-02575-3
A.A. El-Maaref, E.A.A. Wahab, K.S. Shaaban, M. Abdelawwad, M.S.I. Koubisy, J. Börcsök and E.S. Yousef, Visible and mid-infrared spectral emissions and radiative rates calculations of Tm3+ doped BBLC glass, Spectrochim. Acta Part A Mol. Biomol. Spectrosc., 2020 https://doi.org/10.1016/j.saa.2020.118774
A.F.A. El-Rehim, H.Y. Zahran, I.S. Yahia et al., Physical, Radiation Shielding and Crystallization Properties of Na2O-Bi2O3- MoO3-B2O3- SiO2-Fe2O3 Glasses, Silicon, 2020 https://doi.org/10.1007/s12633-020-00827-1
K.S. Shaaban and Y.B. Saddeek, Effect of MoO3 Content on Structural, Thermal, Mechanical and Optical Properties of (B2O3-SiO2-Bi2O3-Na2O-Fe2O3) Glass System, Silicon, 2017, 9(5), p 785–793. https://doi.org/10.1007/s12633-017-9558-5
A.K. Varshneya, Fundamentals of Inorganic Glasses, Academic Prese Limited, Cambridge, 1994, p 33
K.M. Mahmoud and Y.S. Rammah, Investigation of gamma-ray shielding capability of glasses doped with Y, Gd, Nd, Pr and Dy rare earth using MCNP-5 code, Phys. B, 2019 https://doi.org/10.1016/j.physb.2019.411756
K.S. Shaaban, H.Y. Zahran, I.S. Yahia et al., Mechanical and Radiation-Shielding Properties of B2O3–P2O5–Li2O–MoO3 Glasses, Appl. Phys. A, 2020, 126(10), p 804. https://doi.org/10.1007/s00339-020-03982-9
R.M. El-Sharkawy, K.S. Shaaban, R. Elsaman, E.A. Allam, A. El-Taher and M.E. Mahmoud, Investigation of Mechanical and Radiation Shielding Characteristics of Novel Glass Systems with the Composition xNiO-20ZnO-60B2O3-(20–x) CdO Based on Nano Metal Oxides, J. Non-Cryst. Solids, 2019, 528, p 119754. https://doi.org/10.1016/j.jnoncrysol.2019.119754
S. Kaewjaeng, S. Kothan, W. Chaiphaksa, N. Chanthima, R. Rajaramakrishna, H.J. Kim and J. Kaewkhao, High Transparency La2O3-CaO-B2O3-SiO2 Glass for Diagnosis X-rays Shielding Material Application, Radiat. Phys. Chem., 2019 https://doi.org/10.1016/j.radphyschem.2019.03.018
E. Kavaz and N.Y. Yorgun, Gamma Ray Buildup Factors of Lithium Borate Glasses Doped With Minerals, J. Alloy. Compd., 2018, 752, p 61–67. https://doi.org/10.1016/j.ceramint.2019.05.028
P. Kaur, D. Singh and T. Singh, Heavy Metal Oxide Glasses as Gamma Rays Shielding Material, Nucl. Eng. Des., 2016, 307, p 364–376. https://doi.org/10.1016/j.nucengdes.2016.07.029
H.O. Tekin, E. Kavaz, A. Papachristodoulou, M. Kamislioglu, O. Agar, E.E. Altunsoy Guclu, O. Kilicoglu and M.I. Sayyed, Characterization of SiO2–PbO–CdO–Ga2O3 Glasses for Comprehensive Nuclear Shielding Performance: Alpha, Proton, Gamma, Neutron Radiation, Ceram. Int., 2019, 45(15), p 19206–19222. https://doi.org/10.1016/j.ceramint.2019.06.168
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through research groups program under grant number R.G.P. 2/93/41
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed Consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the Supplementary Information.
Rights and permissions
About this article
Cite this article
El-Rehim, A.F.A., Zahran, H.Y., Yahia, I.S. et al. Structural, Elastic Moduli, and Radiation Shielding of SiO2-TiO2-La2O3-Na2O Glasses Containing Y2O3. J. of Materi Eng and Perform 30, 1872–1884 (2021). https://doi.org/10.1007/s11665-021-05513-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-021-05513-w