Abstract
In this work, a NiCr-Cr3C2 coating was prepared on the AISI 1045 steel substrate by plasma spraying, then subjected to tungsten inert gas arc (TIG) remelting treatment. The microstructure, hardness and the wear test under different temperatures (25, 200, 400, and 600 °C) of the NiCr-Cr3C2 coatings before and after remelting were studied comparatively. The results show that after TIG remelting, the internal structure of the coating was dense, and the defects were greatly reduced. The hardness of the remelted coating surface became uniform and increased from 1007 HV0.1 to 1141 HV0.1, with an increase of 13.3%. At different temperatures, the weight loss of the remelted coating was always less than that of the sprayed coating, with a significant reduction of about 75%. The friction coefficients of the remelted coating and sprayed coating at 25 and 200 °C were basically the same, while the friction coefficients of the remelted coating at 400 and 600 °C were smaller than that of the sprayed coating. The wear mechanism of the remelted coating under different temperature were abrasive wear and adhesive wear, while that of the sprayed coating were fatigue wear, abrasive wear, and adhesive wear. Therefore, the TIG remelting treatment can optimize the microstructure of the NiCr-Cr3C2 coating and remarkably improve the wear resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromium carbide is a commonly used thermal spraying material in engineering. There are three common Cr-C compounds, namely, Cr3C2, Cr7C3, and Cr23C6, whose hardness are quite high even at high temperatures (Ref 1,2,3). NiCr is a kind of commonly used alloy with excellent heat resistance and corrosion resistance (Ref 4). Thus, the coating prepared by NiCr-Cr3C2 has wonderful oxidation resistance and abrasion resistance even under severe conditions such as high temperature and heavy load. Its typical applications are boiler tube, metallurgical furnace roll, engine cylinder, etc., (Ref 5, 6).
A common process for preparing NiCr-Cr3C2 coating is plasma spraying (Ref 7). However, the coating prepared by this method is mechanically bonded to the substrate, and has many defects such as cracks, inclusions, and pores. This makes the coating easy to fail in practical applications, leading to the excellent properties of NiCr-Cr3C2 cannot be fully utilized (Ref 8, 9). Studies have shown that remelting the sprayed coating can optimize the microstructure and improve properties of the coating, and further extend the service life of the components (Ref 10,11,12). Tungsten inert gas arc (TIG) remelting has high processing temperature, concentrated energy, and its equipment cost is low which is beneficial for popularization (Ref 13,14,15). However, there are few reports on the research of using TIG remelting method to improve the performance of plasma-sprayed NiCr-Cr3C2 coating up to now. In particular, the effect of TIG remelting on the wear resistance of plasma-sprayed NiCr-Cr3C2 coating under different temperatures is unclear.
In this study, TIG process was used to remelt plasma-sprayed NiCr-Cr3C2 coating, aiming to study the wear resistance and wear mechanism of the remelted NiCr-Cr3C2 coating under different temperatures. This study has a reference value for improving the performance of NiCr-Cr3C2 coating and expanding its application.
Experimental Methods
Experimental Materials
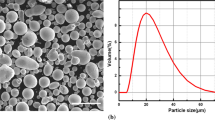
In this work, AISI 1045 steel was selected as the substrate with a size of 60 mm × 30 mm × 10 mm. Coated NiCr-Cr3C2 composite powder was used, in which Cr3C2 was coated by NiCr. This structure can reduce the possibility of carbon loss during spraying (Ref 16, 17). The ratio of NiCr to Cr3C2 was 3:7, and composition of the powder was 19.38 C, 75.87 Cr, and 4.74 Ni (at.%). Particle size of the powder was 25-30 μm, and its morphology is shown in Fig. 1. The powder was spherical and porous, which was benefical to be heated evenly and had good fluidity in the spraying process.
Preparation of Coatings
The surface of the substrate was cleaned with acetone to remove oil, and sandblasted to improve the surface roughness before spraying. The BT-G3 plasma spraying system was used to prepare coating with a thickness of 300 μm. The sample was preheated before remelting, and it was held in a X-G01123 box-type furnace at 300 °C for 1 h. TIG remelting treatment was carried out by YC-300WX welding machine, and then the sample was placed in a furnace for heat preservation with 2 h at 300 °C. The parameters of plasma spraying and TIG remelting are shown in Table 1.
Coating Characterization and Performance Test
The Hitachi S4800 field emission scanning electron microscopy (SEM) was used to observe the microscopic morphology of the coatings, and the energy-dispersive spectroscopy (EDS) attached to the SEM was used for energy spectrum analysis. X-ray diffraction (XRD) model D/max/2500PC was used for phase analysis of powders and coatings. Tecnai F20 field emission transmission electron microscopy (TEM) was used to observe the crystal structure and high-resolution image of the coatings. HMV-2000 Vickers hardness tester was used to measure the hardness of the coatings. MG-2000 pin-disk wear tester was used to test the wear resistance of the coatings under different temperatures. The schematic diagram of the wear test is shown in Fig. 2. The material of the disk was AISI E52100 steel and its hardness is 58HRC. The test time was 20 min with a 400 N load and 200 r/min rotation. The test temperatures were 25 °C, 200 °C, 400 °C, and 600 °C, respectively. Before the test, the samples were kept in the furnace for 5 h at the corresponding temperature to make them completely oxidized, so as to avoid the influence of oxidation weight gain on the data of weight loss in the experiment.
Results and Discussion
Phase Analysis of the Coatings
The XRD patterns of the coatings are shown in Fig. 3. The main phases in the sprayed coating were Cr3C2, Cr7C3, Cr23C6 and Cr3Ni2. Cr3C2, Cr7C3 and Cr23C6 are high-quality ceramic phases (Ref 18, 19), whose existence will greatly improve the hardness of the coating. Diffraction peaks of Fe3C and FeNi appeared in the diffraction spectrum of the remelted coating. The appearance of these two phases proved the occurrence of elements diffusion and the formation of metallurgical reactions between the coating and substrate. In addition, the diffraction peaks of Cr3C2 and Cr7C3 in the remelted coating decreased, while those of Cr23C6 increased, which was due to the carbon loss of Cr3C2 and Cr7C3 transforming into Cr23C6.
Morphology and Microstructure of the Coatings
The surface morphology of the sprayed coating is shown in Fig. 4(a). It can be seen that the surface of the sprayed coating was rough, with many defects such as cracks and pores. The section morphology of the sprayed coating is shown in Fig. 4(b), illustrating the layered structure inside the sprayed coating. The interface between the sprayed coating and the substrate was serrated with poor bonding, which was a typical mechanical one (Ref 20). In addition, light gray phase (A) and dark gray phase (B) can be observed in the sprayed coating. Figure 4(c) and (d) were EDS analysis results, which showed that region A was rich in Ni and region B was rich in Cr. Combined with the results of XRD analysis, it can be determined that the main phase in region A was Cr3Ni2, and the main phases in region B were chromium carbide, including Cr3C2, Cr7C3 and Cr23C6.
The surface morphology of the remelted coating is shown in Fig. 5(a). Compared with the sprayed coating, the surface of the remelted coating was relatively flat with fewer defects. The section morphology of the remelted coating in Fig. 5(b) showed that the interface between the coating and the substrate was smooth and neat, the width was about 10 μm, which was the characteristic of metallurgical bonding. So the bonding strength of the interface will be significantly improved (Ref 21,22,23). Besides, the internal structure of the remelted coating was dense, with few defects and obvious dendritic structure. EDS analysis of region C and region D are shown in Fig. 5(c) and (d), respectively. Combined with the results of XRD analysis, the main phase at the dendrite gaps was chromium carbide, while those in the dendrite were Fe3C and FeNi.
The TEM morphology of the sprayed coating is shown in Fig. 6(a). Its microstructure can be divided into four regions: A, B, C,1 and C1′. Combining the EDS analysis of the region A with the XRD analysis of the sprayed coating, the main phase was determined to be Cr3Ni2. The electron diffraction patterns of region C1 and C1′ are shown in Fig. 6(c) and (d). By measuring and calculating the crystal plane spacing, and comparing with the PDF card (38-0804, 36-1482), the main phases were determined to be Cr3C2 and Cr7C3, respectively. Region B belonged to the mixed crystal part of Cr3Ni2 and Cr3C2.
The TEM morphology of the remelted coating is shown in Fig. 7(a), which comprised three regions, i.e., D, C,2 and C2′. Based on EDS analysis of region D and XRD analysis of the remelted coating, the main phase was determined to be FeNi. The electron diffraction patterns of region C2 and C2 ‘ are shown in Fig. 7(c) and (d). By measuring and calculating the crystal plane spacing, and comparing with the PDF card (36-1482, 35-0783), the main phases were determined to be Cr7C3 and Cr23C6, respectively. This was because Cr3C2 and Cr7C3undergone a carbon loss reaction during remelting to produce Cr23C6.
In Fig. 7(a), according to rough measurement, the area of region C2 was about 4 μm2, and the area of region C2′ was about 0.4 μm2. It can be found that the region C2 was relatively thick, while the region C2′ was relatively tiny and evenly distributed. As a hard phase, tiny Cr23C6 was evenly dispersed in the coating by the form of particles. Such a tiny structure could hinder the movement of dislocations, thereby, achieving the effect of dispersion strengthening. This was very beneficial to the homogenization of the hardness distribution and the improvement in the hardness and wear resistance of the coating.
Hardness of the Coatings
Figure 8 shows the comparison diagram of the surface hardness of the sprayed coating and the remelted coating. The average hardness of them were 1007 HV0.1 and 1141 HV0.1, respectively, and the original data are shown in the Table 2. It indicates the remelted coating was slightly harder than the sprayed coating. Moreover, the hardness error value of the remelted coating(51 HV0.1) was obviously lower than that of the sprayed coating(388 HV0.1). This was due to the existence of defects such as pores and cracks in the sprayed coating led to the looseness of the structure and weak adhesion between the phases. While, the microstructure of the remelted coating was dense, moreover, tiny Cr23C6 distributed dispersively in the coating to play a strengthening role. Therefore, the hardness increased and its distribution became uniform.
Wear Resistance of the Coatings at Different Temperatures
Wear Resistance
The friction coefficients(FC) and weight loss(WL) of the coatings at different temperatures are shown in Table 3. The FC refers to the average value after the coefficient reached a stable value until the end of the test. It can be seen that at four temperatures, the wear loss of the remelted coating is significantly less than that of the sprayed coating, and the former is only 25% of the latter. The friction coefficients of the remelted coating at 25 °C and 200 °C are basically the same as that of the sprayed coating, while the friction coefficients of the remelted coating at 400 °C and 600 °C are smaller than that of the sprayed coating.
Wear Mechanism
-
(1)
Wear Mechanism at 25 °C
The wear scar morphologies of the coatings at 25 °C are shown in Fig. 9. There were pits and furrows on the wear scars of the sprayed coating. Pit was the characteristic of fatigue wear. While, there were furrows and oxide films on the wear scars of the remelted coating. EDS analysis was conducted on the oxide film (A) in Fig. 9(b). As shown in Fig. 9(c), the oxide film was rich in iron and oxygen, indicating the main component was iron oxide. Therefore, the wear mechanism of the sprayed coating was fatigue wear and abrasive wear, while that of the remelted coating was abrasive wear.
The formation mechanism of the pits on the sprayed coating can be illustrated in Fig. 10. There were many cracks inside the layer structure of the sprayed coating. Under the extrusion pressure and shear force of the grinding pair, the cracks expanded, and the surface material peeled off the coating, leaving pits. The remelted coating was dense with few defects, and its internal structure was not layered, so no pits formed, which means that no fatigue wear occurred. In the wear test of remelted coating, wear debris on the grinding ball fell after oxidation and stuck to the surface of the coating, thus forming an oxide film on the coating surface. Oxide film played a role of lubrication in the process of friction (Ref 24), so wear is reduced.
the oxide film in the wear scar of the remelted coating played a role of lubrication in the process of friction, so, wear is reduced.
-
(2)
Wear Mechanism at 200 °C
The wear scar morphologies of the coatings at 200 °C are shown in Fig. 11. There were delamination and adhesion marks in the wear scars of the sprayed coating. Delamination was also the characteristic of fatigue wear. While, furrows and oxide films can be found in the wear scar of the remelted coating. Therefore, the wear mechanism of the sprayed coating were fatigue wear and adhesive wear, while that of the remelted coating was abrasive wear.
The formation mechanism of delamination on the sprayed coating at 200 °C can be illustrated in Fig. 12. At 200 °C, the ductility of the the sprayed coating was increased. Hence, the material of surface-layer plastically was pressed into a flaky shape by the grinding pair. As the wear progressed, the material of surface layer was squeezed and cracked under the combined force of friction shear force and extrusion force. The crack then propagated, causing the surface material to detached from the sprayed coating. The remelted coating was dense, and its internal structure was not layered, so no delamination formed, and the area of the oxide film reduced the wear.
-
(3)
Wear Mechanism at 400 °C
The wear scar morphologies of the coatings at 400 °C are shown in Fig. 13. The surface of the wear scars were wavy, and there were traces of adhesion. The phenomenon of adhesion was serious in the sprayed coating, while that of remelted coating was relatively slight. EDS analysis of the wavy front end is shown in Fig. 13(c) and (d), indicating its main component was iron oxide. At 400 °C, plastic deformation and adhesive wear occurred in both sprayed coating and remelted coating.
The wear mechanism of the coatings at 400 °C is shown in Fig. 14. Although the surface of the coating was carefully polished, it was still uneven at the micro-level. The plastic deformation capacity of the coating was improved at 400 °C. During the wear process, the surface of the coating presented wavy plastic deformation along the sliding direction due to the squeezing and shearing forces of the grinding pair. The front end of the wave after plastic deformation was tightly attached to the grinding pair. When it was hardly to withstand the shearing force, it would detach from the coating, causing adhesive wear. The front end of some waves was not easily detached from the coating. But, the surface material of the grinding pair in contact with the wave was sheared off and transferred to the coating surface, thereby, forming iron oxide film on the surface of the coating. Compared with the sprayed coating, the remelted coating had a dense internal structure, and the bonding force between the phases would also increase, so it was not easy to cause adhesive wear. While there were more materials transferred from the friction pair, forming a large oxide film area, so the wear was slight.
-
(4)
Wear Mechanism at 600 °C
The wear scar morphologies of the coatings at 600 °C are shown in Fig. 15. Furrows and abrasive grains were found in the wear scars of the sprayed coating and the remelted coating. In addition, iron oxide was also present, and EDS analysis shows that its main component was iron oxide. Therefore, the wear mechanisms of sprayed coating and remelted coating were both abrasive wear.
The wear mechanism of the coatings at 600 °C is shown in Fig. 16. At this temperature, the plastic deformation ability of the alloy phase was enhanced. While the ceramic phases have a stable structure. Even at 600 °C, they were still hard and the plastic deformation ability was still weak, which would cause a large hardness difference between the alloy phases and the carbide phases. The ceramic phases protruded from the surface of the coatings during the test and detached from the coatings under shearing force, and then they were used as abrasive grains between the coatings and the grinding pairs, resulting in abrasive wear. The ceramic phase particles in the sprayed coating were relatively large, and the furrows caused by abrasive grains were deep, resulting in serious wear. For the remelted coating, the main ceramic phase in the coating was Cr23C6, which was fine and dispersed, so the furrows caused by the abrasive grains were shallow. More importantly, the structure of Cr3C2 and Cr7C3 is orthogonal, and that of Cr23C6 is cubic. In comparison, the capacity of plastic deformation for Cr23C6 was relatively strong, so it was not easy to protrude from the coating. With the formation of FeNi alloy in the remelted coating, the bonding of the alloy phases to ceramic phases were enhanced. The ceramic phases did not easily detached from the coating, so the wear of the remelted coating was reduced.
According to the above wear test results, the wear resistance of the remelted coating is better than that of the sprayed coating at different temperatures. The reasons can be summarized as follows:
-
(1)
In the process of wear, the loss of coating material was accompanied by the generation of cracks (Ref 25). The original pores or layered structure in the coating would accelerate the crack propagation, making the material easily detach from the coating. Compared with the sprayed coating, the remelted coating had less pores and the layered structure disappeared, so the wear resistance was improved.
-
(2)
The ceramic phase in the remelted coating was finer. When the crack extended to the grain edge of the ceramic phase, the distance along the grain boundary of the ceramic phase was short. After entering the alloy phase, the crack propagation work was absorbed by the alloy phase through the plastic deformation capacity, the crack propagation was blocked and the material was not easy to fall off, so the wear resistance was improved.
-
(3)
The wear resistance of the material was proportional to the hardness (Ref 26). The remelted coating was harder than the sprayed coating, and the hardness distribution was even, so the remelted coating had excellent wear resistance.
Conclusion
In this paper, a NiCr-Cr3C2 coating was prepared on AISI 1045 steel substrate by plasma spraying, and was remelted by TIG remelting process. On this basis, the effect of TIG remelting on the microstructure, mechanical properties, and wear resistance of the coatings at different temperatures was studied. The conclusions are as follows:
-
1.
The sprayed NiCr-Cr3C2 coating had a layered structure with many defects, and the coating was mechanically bonded to the substrate. After TIG remelting treatment, the interior of the coating was dendrite structure with few defects, and there was a metallurgical bond between remelted coating and substrate. Hence, TIG remelting optimized the structure of the sprayed coating.
-
2.
After TIG remelting, the carbon loss of Cr3C2 and Cr7C3 produced tiny and dispersed Cr23C6, whose dispersion strengthening effect increased the coating hardness from 1007HV0.1 to 1141HV0.1. In addition, the hardness distribution became uniform.
-
3.
The wear resistance of the coatings at 25 °C, 200 °C, 400 °C, and 600 °C were studied, respectively. The weight loss of remelted coating was always less than that of sprayed coating, and the magnitude of reduction was about 75%. Therefore, TIG remelting significantly improved the wear resistance of sprayed NiCr-Cr3C2 coating.
References
L. Venkatesh, B. Venkataraman, M. Tak, G. Sivakumar, R.C. Gundakaram, and S.V. Joshi, Room Temperature and 600 °C Erosion Behaviour of Various Chromium Carbide Composite Coatings, Wear, 2019, 422–423, p 44–53
T. Min, Y.M. Gao, Y.F. Li, Y. Ying, R.T. Li, and X.J. Xie, First-principles Study of the Tlectronic Structure, Hardness and Debye Temperature of Chromium Carbide, Rare Metal Mat. Eng., 2012, 41(02), p 87–91 (in Chinese)
R. Pileggi, M. Tului, D. Stocchi, and S. Lionetti, Tribo-corrosion Behaviour of Chromium Carbide Based Coatings Deposited by HVOF, Surf. Coat. Technol., 2015, 268, p 247–251
E.A. Lazareva, Heat-Resistant Sitall Coatings for High-Temperature Corrosion Protection of Nichrome Alloys, Mater. Sci. Forum, 2020, 992, p 633–639
M. Mathapati, M. Doddamani, and M.R. Ramesh, High-Temperature Erosive Behavior of Plasma Sprayed Cr3C2-NiCr/Cenosphere Coating, J. Mater. Eng. Perform., 2018, 27(3), p 1–9
Q. Liu, T. He, W.Y. Guo, Y. Bai, Y.S. Ma, and Z.D. Chang, Tribological Behavior of SAPS Sprayed Al2O3-TiO2 and NiCr-Cr3C2 Coatings under Severe Load Conditions, Surf. Coat. Technol., 2019, 370, p 362–373
S. Matthews, Development of High Carbide Dissolution/Low Carbon Loss Cr3C2-NiCr Coatings by Shrouded Plasma Spraying, Surf. Coat. Technol., 2014, 258, p 886–900
S. Xuanyu and Y. Suyuan, Performance in Resistance to Surface Fatigue for Cr3C2-25%NiCr Coatings by Plasma Spray and CDS Spray, Tribol. Lett., 2004, 16(3), p 173–180
E. Qin, B. Wang, W. Li, W. Ma, H. Lu, and S. Wu, Optimized Microstructure and Properties of Cr3C2-NiCr Cermet Coating by HVOF/Laser Hybrid Processing, J. Therm. Spray Technol., 2019, 28, p 1072–1080
K.A. Habib, D.L. Cano, C.T. Caudet, M.S. Damra, I. Cervera, and J. Bellés, Influence of Al2O3 Particle Size on Microstructure, Mechanical Properties and Abrasive Wear Behavior of Flame-Sprayed and Remelted NiCrBSi Coatings, J. Mater. Eng. Perform., 2017, 26(4), p 1647–1656
R. Shoja, Reza, Laser Surface Treatment of Stellite 6 Coating Deposited by HVOF on 316L Alloy, J. Mater. Eng. Perform., 2016, 25(7), p 2583–2595
P. Hengst, R. Zenker, T. Süß, and K. Hoffmann, Improvement of the Load-bearing Capacity of Thermal Spray Coatings in Combination with Electron Beam Profiling and Electron Beam Remelt-bonding, J. Heat Treat. Mater., 2016, 71(6), p 265–271
T. Dong, X. Zheng, G. Li, H. Wang, M. Liu, and X. Zhou, Effect of Tungsten Inert Gas Remelting on Microstructure, Interface, and Wear Resistance of Fe-Based Coating, J. Eng. Mater. Tech., 2018, 140(4), p 0410071–0410078
K. Sudhir and P.K. Ghosh, TIG Arc Processing Improves Tensile and Fatigue Properties of Surface Modified of AISI, 4340 Steel, Int. J. Fatigue, 2018, 116, p 306–316
S.C. Juang and Y.S. Tarng, Process Parameter Selection for Optimizing the Weld Pool Geometry in the Tungsten Inert Gas Welding of Stainless Steel, J. Mater. Eng. Perform., 2002, 122(1), p 33–37
V. Matikainen, H. Koivuluoto, P. Vuoristo, J. Schubert, and Š. Houdková, Effect of Nozzle Geometry on the Microstructure and Properties of HVAF-Sprayed WC-10Co4Cr and Cr3C2-25NiCr Coatings, J. Therm. Spray Technol., 2018, 27, p 680–694
Y. Li, X. Meng, R. Li, F. Zeng, and Y. Gu, Effect of Ni Content on Microstructure and Performance of Ni/Ceramic Composite Coating, J. Mater. Eng. Perform., 2020, 29(5), p 2853–2864
J.Y. Du, F.Y. Li, Y.L. Li, and L. Wang, Influences of Plasma Arc Remelting on Microstructure and Service Performance of Cr3C2-NiCr/NiCrAl Composite Coating, Surf. Coat. Technol., 2019, 369, p 16–30
F. Ye, Y. Xu, M. Hojamberdiev, Y. Lai, C. Wang, and X. Wang, Effect of Carbide Ceramic Zone on Wear Resistance of the (Fe, Cr)7C3/Fe Surface Gradient Composite, J. Mater. Eng. Perform., 2015, 24(8), p 2898–2907
G. Xie, Y. Lu, Z. He, B. Hu, and P. Lin, Microstructure and Corrosion Properties of Plasma-Sprayed NiCr–Cr3C2 Coatings Comparison with Different Post Treatment, Surf. Coat. Technol., 2008, 202(13), p 2885–2890
M. Arai, H. Ochiai, and T. Suidzu, A Novel Low-thermal-conductivity Plasma-sprayed Thermal Barrier Coating Controlled by Large Pores, Surf. Coat. Technol., 2016, 285, p 120–127
Z.Y. Piao, B.S. Xu, H.D. Wang, and D.H. Wang, Influence of Surface Nitriding Treatment on Rolling Contact Behavior of Fe-based Plasma Sprayed Coating, Appl. Surf. Sci., 2013, 266(1), p 420–425
J.B. Yu, Y. Wang, F.F. Zhou, and L. Wang, ZY Pan (2018) Laser Remelting of Plasma-sprayed Nanostructured Al2O3-20 wt.% ZrO2 Coatings onto 316L Stainless Steel, Appl. Surf. Sci., 2018, 431(15), p 112–121
Z.X. Zhu, B.S. Xu, S.N. Ma, W. Zhang, and W.M. Liu, Friction Oxidation Behavior of High Velocity Arc Sprayed Fe-Al Coating in High Temperature Wear, Chin. J. Mech. Eng., 2004, 40(11), p 163–168 (in Chinese)
W. Wu, J. Liu, T. Hua, Z. Chen, J. Jiang, H. Wang, L. Liu, and X. Liu, Microstructure and Friction-Wear Behavior of Multi-arc Ion Plating TiAlNC Ceramic Coating on WC-6%Co Substrate, J. Mater. Eng. Perform., 2018, 27, p 4665–4671
D. Shu, Mechanical Properties of Engineering Materials, Mechanical Industry Press., 2016, p 88–127, (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo-lu, L., Jing-min, Y., Tian-shun, D. et al. Wear Resistance under Different Temperatures of NiCr-Cr3C2 Coating Remelted by Tungsten Inert Gas Arc. J. of Materi Eng and Perform 29, 8013–8024 (2020). https://doi.org/10.1007/s11665-020-05254-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-020-05254-2