Abstract
This study was aimed to establish the possibility of using an eco-friendly plant-based pomelo peel with different concentrations to formulate a novel corrosion green inhibitor for steel applications in 3.5% NaCl and 0.1 M HCl solutions at 30 °C. The corrosion rate and inhibition efficiency were evaluated using weight loss, potentiodynamic polarization (PDP), and electrochemical impedance spectroscopy (EIS) techniques corresponded with surface morphology characterization of the corroded steel samples without and with inhibitors using a scanning electron microscope (SEM) and energy-dispersive x-ray analysis (EDS). Based on the Fourier-transform infrared spectroscopy (FTIR) results, it was found that the pomelo peels extract is a mixture of the chemical compounds of naringin (C27H32O14), auraptene (C19H22O3) and naringenin-4′-O-glucoside (C21H22O10). The results were also indicated that there is a remarkable improvement in the inhibition efficiency and corrosion rate of low-carbon steel after the addition of inhibitor, and as well as the immersion time was profound to have a significant effect on the corrosion behavior of the different concentrations of inhibitor. The pomelo peels extract of 8000 ppm concentration gave the best inhibition efficiency of 74.64 and 71.15% in 0.1 M HCl and 3.5% NaCl, respectively. The adsorption isotherm of pomelo peels within different concentrations on the steel surface conforms Langmuir’s isotherm, and the thermodynamic parameters of Kads and ΔG have also been calculated and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mild steel or low-carbon steel is widely used in various applications such as the oil and gas industries, machinery, and nuclear power (Ref 1). Low-carbon steel is extensively used due to its high strength, good malleability, low cost and easy availability. However, when mild steel is used in an aggressive environment, it is susceptible to corrosion and its properties degrade. Low-carbon steel is commonly used in building construction, acid storage, oil and gas industrial, food industrial as well as water desalination system applications(Ref 1). Compared with other types of metal such as stainless steel, high-carbon steel or aluminum, mild steel is the primary option for most applications because of its availability, low cost and good mechanical workability. Corrosion can have a large economic impact on the industry because of parts of the machine not in function when it corroded, thus having a social impact through affecting the safety and health of the workers. For instance, if there is corrosion that causes leakage of hydrogen sulfide gas (H2S) to the environment, it is extremely dangerous to all the surrounding people, possibly even causing human death. Therefore, the use of corrosion inhibitor is the most economical way to reduce the rate of corrosion to protect the metal surface from corrosion. Organic inhibitors are widely used in the petroleum refining process (acid medium); whereby the corrosion inhibitors can produce a protective film at the surface in the acid media. There are some chemical families that exhibit excellent performance as corrosion inhibitors, for instance, primary amines and diamines (Ref 2), pyridines (Ref 3) and polymers. The incorporation of corrosion inhibitors in a small amount to the corrosive medium results in slowing down the reaction between metal surface compared with the corrosive element in the electrolyte (Ref 4). The corrosion inhibitors decrease the corrosion rate through blocking either the anodic reaction or cathodic reaction at the reaction. Recently, organic inhibitors are reported as one of the most environmentally friendly corrosion inhibitors which can protect copper (Ref 5), aluminum alloys and steel (Ref 6). These organic inhibitors normally contain heteroatoms (N, O, S and P), heterocyclic rings, polar functional groups or conjugated double bonds (Ref 7,8,9). The corrosion inhibition of these organic compounds is the result of the adsorption of organic molecules or ions at the metal surface and the formation of a protective layer, thus preventing and slowing the process of metal corrosion.
According to Ref 10 and 11, the possibility of forming a passive film on the metal is highly affected by the environment and the presence of corrosive ions such as chloride and sulfate ions. Hence, inhibitors are used to reduce the anodic or cathodic reaction (Ref 12). Organic, inorganic and polymeric corrosion inhibitors show good performance for various types of metal or alloys; however, these compounds are toxic and are difficult to filter out during disposal. To replace the toxic compound, a new generation of green inhibitors was developed. Green inhibitors are mainly from plant extracts which are either from their peels, leaves or seeds (Ref 13). The investment cost of corrosion inhibitors is low, and green inhibitors are widely investigated nowadays. In addition, the compounds are non-toxic, environmentally friendly, save to use and do not require extra cost for waste treatment (Ref 13, 14). The aim of this study is to investigate the corrosion of inhibition properties of pomelo peels in 3.5% NaCl and 0.1 M HCl solutions using different corrosion techniques to help provide excellent, green, and biodegradable replacements for toxic inhibitors that are currently used in the industry. There is limited literature on the use of natural products such as pomelo peels from plants as corrosion inhibitors, as presented in this work. Therefore, this paper deliberates the use of the pomelo peels, which have the desired chemical characteristics, as corrosion inhibitors for low-carbon steel applications.
Experimental Procedure
Composition Analysis
To measure the elemental composition of the low-carbon steel sample, a glow discharge spectrometer (GDS) LECO GDS850A was used. In the GDS, the cathodic sputtering removed atoms of the low-carbon steel sample layer by layer. Then, the removed atoms from the metal surface are converted into a plasma that was excited by collisions with electrons or inert gas atoms. Lastly, the elemental concentration of the steel was determined based on the light emission.
Microstructural Analysis
An inverted metallurgical optical microscope, Olympus GX 41, was used to observe the morphology of the material surface. The prepared low-carbon steel sample was etched in a 2% NITAL solution (Ref 15, 16) for a few seconds to reveal the microstructure. The compounds or functional groups (chemical bonds) of pomelo peels extract were determined by FTIR (Perkin Elmer Universal ATR Sampling Accessory).
Preparation of the Inhibitor (Pomelo Peels)
The pomelo fruits, each having a mass of about 2 kg, were obtained from Parit Bunga, Muar. The skin or peels (both green and white peel) were removed from the fruit and left to dry at room temperature for 14 days. The dried pomelo peels were cut into small pieces for the extraction process, because the pomelo peels were used as an inhibitor in the form of a liquid extract.
Extraction Process of Inhibitor
Extraction is a process to remove the active component from the plants that can be from branches, leaves, seeds, peels, shells or stems. In this study, pomelo peels were used for extraction. The pomelo peels were extracted through the following procedure. The pomelo peels were first dried and cut into small pieces. Then, all the peels are soaked in an ethanol solution for 48 h. Afterwards, the specimens were cooled and filtered. The filtrate was placed into an evaporator at 352 K to remove ethanol from the specimen. After obtaining the pomelo peels extract, it was mixed with 5 L of 0.1 M of HCl and 3.5% of NaCl within different concentrations. The concentrations of pomelo peels extracted that were mixed with two immersion environments are calculated and tabulated in Table 1.
Antioxidation Test on Inhibitor
The antioxidant efficiency of plant extracts was determined by using the 2,2-diphenyl-1-picryhydrazyl (DPPH) test, in which this method was developed by Brand-Williams. DPPH method makes use of stable free radicals (purple in color), and this was spectrophotometrically measured. The DPPH liquid changing from purple to discolored means that the compound can transfer an electron or donate hydrogen. Changes in DPPH absorbance is usually used as an index of antioxidant efficiency. The DPPH test for this research was conducted as described in Fig. 1. First, extraction was performed to extract antioxidant material from the pomelo peel. Then, only the antioxidant test was conducted from a range of 100 ppm to 10,000 ppm.
Chemical Characterization of Pomelo Peels Extract
The pomelo peels extract was analyzed by liquid chromatography–high-resolution mass spectrometry under positive and negative electrospray ionization modes. A data-dependent acquisition (DDA) technique was conducted to fragment the five most intense peaks. Molecular network were carried out for the calculations and database matching using 0.02 Da as precursor ion mass tolerance and 0.05 Da as fragment ion mass tolerance, 0.7 as minimum cosine score and 3 as minimum matched fragment ions for edge linkage.
Corrosion Test
Corrosion tests performed were the immersion test and electrochemical tests. The tests were conducted in simulated seawater (3.5% NaCl) and an industrially used acidic environment (0.1 M HCl).
Electrochemical Test
The electrochemical test or known as potential polarization method has been conducted in a standard three-electrode cylindrical glass. Three electrodes include a working electrode (steel sample), counter electrode (carbon electrode) and reference electrode (saturated calomel electrode) in 250 ml of electrolyte (immersed medium) to complete the circuit. Polarization was performed in accordance with the standard ASTM G5-94 by using computer-controlled VersaSTAT 3 software. The Tafel polarization scan performed was at the potential of ± 250 mV, with a potential scan rate of 1 mV/s, to achieve a current steady state. The working electrode, which was the steel sample, was immersed in the immersion medium (e.g., 0.1 M of HCl with inhibitor) at an open circuit potential for 30 min before performing the electrochemical test. The polarization resistance (Rs) was determined at an overvoltage lower than ± 20 mV. The electrochemical measurements were determined in the frequency range between 100 kHz and 10 mHz and with a peak-to-peak amplitude (AC signal at open circuit) of 5 mV. The inhibitor efficiency (%IE) was calculated (Ref 17, 18) as \({\text{\% IE = }}\frac{{R_{{{\text{ct}}\left( {\text{inh}} \right)}} - R_{\text{ct}} }}{{R_{{{\text{ct}}\left( {\text{inh}} \right)}} }} \times 100;\) where Rct represents the charge transfer resistance without the inhibitor and Rct(inh) represents the charge transfer resistance with the inhibitor.
Immersion Test
The immersion test is commonly used to determine the corrosion rate of the metal by weight loss. This method is relatively easy and low cost, because it does not require any equipment during the immersion process. This immersion test was conducted in accordance with ASTM G31-72 (laboratory immersion corrosion testing of metal) for a maximum duration of eight weeks. The low-carbon steel samples were prepared before the immersion test. They were cut and then ground and cleaned to remove any oxides and contaminants on the surface. The prepared samples were tied with a plastic string so they could be hung in the immersion tank. The areas at the edge of the samples were covered with silicone so they would not be exposed to the test solution, and the exposed area calculated would only be the top and bottom surfaces of the samples. The steel samples were immersed in 5-L glass containers, where all four containers were represented as a medium solution of 0.1 M HCl or 3.5% NaCl solution. Pomelo peels extract which acted as an inhibitor was added into the containers at different concentrations of 3000, 8000 and 10,000 ppm. The low-carbon steel samples were weighed before the immersion test. They were immersed in the corrosion media where each separate media represented the first week, second week, fourth week, sixth week and eighth week immersion durations. Each low-carbon steel sample was removed from the immersion tank in accordance with the duration period. The samples were then cleaned according to ASTM-G1 and reweighed. The immersion test was repeated for three times and the standard deviation was recorded. Photographs were also taken before and after the immersion test. A schematic of the immersion setup is shown in Fig. 2(a) and (b). The immersed area was observed using a scanning electron microscope (Hitachi S-3400 N VP) with energy-dispersive x-ray spectroscopy (EDX).
The corrosion rate after the immersion test was determined using (Ref 19)\({\text{Corrosion rate (mm/year) = }}\frac{{\left( {K \times w} \right)}}{{\left( {A \times t \times D} \right) }};\) where K is a constant, w is the weight loss in grams, A is the exposed area, t is the immersion time in hours and D is the density in g/cm3.Corrosion rates without and with an inhibitor can determine the % IE (Ref 19) by using the \({\text{\% IE = }}\frac{{W_{o} - W_{\text{corr}} }}{{W_{o} }} \times 100;\) where Wo is the weight loss in the absence of the inhibitor and Wcorr is the weight loss in the presence of the inhibitor in grams.
Results and Discussion
Chemical Characterization of Pomelo Peels
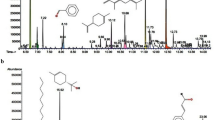
For the identification of the compounds present in the extract of pomelo peels, we acquired mass spectra of high chemical resolution. These results were presented to the calculation of similarities between MS fragment grams obtained and the Marinlit, Nist14 and GNPS databases (Ref 20). It was possible to identify three compounds that are in agreement with what is expected for their chemotaxonomic characteristics acquired with their molecular structures are shown in Fig. 3(a-c). The pomelo peels extract is a mixture of the following chemical compounds, including naringin, auraptene and naringenin-4′-O-glucoside. On the other hands, an FTIR spectrum for the pomelo peels extract is illustrated in Fig. 3(d), shows these functional groups present such as 3350 cm−1 (–OH); 1635 cm−1 (–C = O); 2973 cm−1 (–CH3); 1400-1600 cm−1 (benzene ring); 1000-1200 cm−1 (C–O–C), in which these peaks indicate the possible chemical composition mixture in the extract of pomelo peels, which they are represented complicated compounds with aromatic or heterocyclic rings and majorly functional groups of hydroxyls.
Weight Loss
The weight losses of the low-carbon steel samples immersed in 3.5% NaCl and 0.1 M HCl solutions are shown in Fig. 4(a) and (b), respectively. The sample immersed in HCl acid medium experienced aggressive corrosion because of the chloride attack (Ref 21, 22). The presence of halide ions broke down any passive films on the surface of the low-carbon steel and may prevent a passive film forming on the surface. Figure 4(a) illustrates the weight loss within the sequence of no inhibitor > 3000 ppm > 10,000 ppm > 8000 ppm in 3.5% NaCl medium, and the lowest values were observed with 8000 ppm to imitate the optimum value of inhibitor to form an adsorptive passive layer at the surface of the low-carbon steel. In contrast, as for hydrochloric acid medium, the weight loss was much greater than the same for the 3.5% NaCl medium. In an acid medium, the optimum concentration is 8000 ppm pomelo peel inhibitor, as shown in Fig. 4(b). Because of the presence of chloride ions, the passive layer can break down and the corrosion becomes severe (Ref 22, 23).
The corrosion rate of low-carbon steel samples after immersion test is calculated and tabulated in Table 2. The results show that the 3.5% NaCl medium without inhibitors has the highest corrosion rate associated with a sudden increase in corrosion rate between week 4 and week 6 that may be because of the corrosion formation on the surface of samples, and the samples did not have a protective film to prevent corrosion. As immersion duration increased, the corrosion product was too heavy and unable to withstand its own weight; consequently, it dropped off, increasing the corrosion rate drastically from week 4 to week 6. In comparison with the 3.5% NaCl solution with inhibitors, the corrosion rate decreased with the following sequence of 8000 ppm < 10,000 ppm < 3000 ppm, and this order is mainly because the lowest corrosion rate with an 8000 ppm inhibitor concentration may refer to the formation of adsorptive passive film, therefore reducing the corrosion rate. In addition, the results show that the rate of corrosion in acid medium is much higher than the stimulated seawater medium, because the halide ions (chloride ions) reacted actively to the Fe ions (Ref 24). This reaction prevents the formation of passive layers on the surface of the low-carbon steel, or the passive layer was easily attacked by the chloride ions. From the same perspective, the results revealed that the corrosion rate in the 0.1 M hydrochloride acid medium obtained the highest values of 4.957 mm/yr compared with the stimulated seawater medium of 0.196 mm/yr. The results also showed that as much as the concentration of the pomelo peel inhibitor increased, and the corrosion rate tended to decrease in the acidic medium. Furthermore, the acid medium with inhibitors had a slightly steady rate from week 2 to week 6 possibly because of the passive layer formation at the surface of the low-carbon steel. During week 6 to week 8, there is a decrease in corrosion rate that may be because the chloride ions actively attacked the low-carbon steel sample (Ref 25, 26). The chloride ions were already being consumed; therefore, fewer chloride ions were left in the medium.
Inhibition efficiency represents the performance of the inhibitors in the immersed medium (Ref 27). As the inhibition efficiency percentage increases, the performance of the selected inhibitor increases. After determining the weight loss and corrosion rate with and without having inhibitors, the inhibition efficiency can be calculated, and the data are tabulated in Table 3. The inhibition efficiency in the acid medium is slightly higher than in the stimulated seawater medium at the end of the first week. However, the inhibition efficiency dropped drastically to around 40% at the end of week 8. This indicates that the adsorptive passive film may be broken off, because the low-carbon steel sample was actively attacked by chloride ions. In the 3.5% sodium chloride medium, the overall trend of the inhibition efficiency decreased as time increased. In three different pomelo peel extract concentrations, the 8000 ppm concentration showed the highest inhibition efficiency. During the end of the first week, the inhibitor with a concentration of 8000 ppm was able to achieve the inhibition efficiency of 65.52%. After the eighth week of immersion, the passive film could not protect the surface of the low-carbon steel sample, possibly breaking off; therefore, the inhibition efficiency dropped to 49.90% for 8000 ppm pomelo peel concentration. The IE with 0.1 M HCl almost shows the same trend of decrement as the immersion time increased. This IE achieved the highest efficiency with 8000 ppm concentration of 67.76%, which it mainly is attributed to the organic molecules forming a passive film at the surface of the low-carbon steel to decelerate the contact between the low-carbon steel surface and aggressive ions in the media (Ref 28, 29).
A comparison between inhibition efficiency in acid medium and stimulated seawater media is shown in Fig. 5. In an acid medium, the corrosion inhibitors have better inhibition efficiency, although the acid corroded the low-carbon steel severely at the end of the experiment. This relationship is because during the first week of the immersion time, the low-carbon steel with corrosion inhibitors in acid medium has a thin passive layer that protects it from severely corroding. After the protective layer is attacked by the halide ions, it corroded faster and more severely than when the sample is immersed in the stimulated seawater medium.
Tafel Polarization Measurement
Figure 6(a) and (b) illustrate the Tafel polarization curves obtained on low-carbon steel in 3.5% NaCl and 0.1 M HCl containing different pomelo peel extract concentrations, respectively, and the specific corrosion parameters such as the equilibrium corrosion potential (Ecorr), corrosion current density (icorr), slope of anodic (βa), slope of cathodic (βc), inhibition efficiency in percentage (IE%) and corrosion rate are tabulated in Table 4. Both the cathodic and anodic reactions were suppressed in the presence of the inhibitor. Adding the pomelo peel extract inhibited both the anodic dissolution and the cathodic hydrogen evolution reactions (Ref 30). In addition, the corrosion current density (icorr) decreases as different pomelo peels extract concentrations are added. This indicates that the corrosion rate of low-carbon steel in 0.1 M HCl was inhibited by the pomelo peels extract. Pomelo peels have aromatic or heterocyclic rings which contain functional groups of oxygen, whereas the low-carbon steel surface has vacancies at their d-orbitals. It is expected that the inhibitor adsorbs on the low-carbon steel surface by the interaction between the donor and acceptor. The Tafel polarization curve shows that the equilibrium corrosion potential (Ecorr) values and corrosion current density (icorr) decrease as the pomelo peel extract is added. In contrast, the Tafel polarization curve of the samples without adding any extract has the highest icorr value and the lowest Ecorr value.
According to the Tafel polarization results, as the pomelo peels extract (inhibitors) concentration increases, the corrosion current density is lower than that of the medium without any inhibitor added. This result indicates that the inhibitor molecules react dissolution effectively at the interface. As given in Table 4, the equilibrium corrosion potential value (Ecorr) shifted between 32 and 80 mV as the inhibitor is added in both media. When using anodic type or cathodic type inhibitors, they usually provide a high displacement Ecorr value that is greater than 85 mV (Ref 31). Therefore, it is expected that the present inhibitor has mixed type behaviors. With the addition of inhibitor, the inhibition efficiency (IE%) showed a positive result; thereby, at 8000 ppm, the 3.5% NaCl has an IE % of 71.15%, whereas the 0.1 M HCl solution only has an IE% of 74.64%. These results suggest that the organic molecules form a passive film at the surface of the low-carbon steel to decelerate the contact between the low-carbon steel surface and aggressive ions in the medium (Ref 32). The corrosion rate is lower with the presence of pomelo peels extract in both media. As compared with the corrosion rate of acid medium and stimulated seawater medium, the acid medium has a higher corrosion rate because of the acid medium containing very aggressive ions that promote corrosion. The corrosion rate becomes very low when the concentration of extract is 8000 ppm, indicating that this amount is the best concentration for corrosion inhibition.
The inhibition efficiency can be determined by using the following equation of \({\text{IE}} \left( \% \right) = \frac{{i_{0} - i_{\text{inh}} }}{{i_{0} }} \times 100 \% ;\) where \(i_{\text{inh}}\) represents the corrosion current density with inhibitors and \(i_{0}\) represents the corrosion current density without any inhibitor (Ref 33).
Figure 7 shows that the pomelo peel extract can effectively inhibit the corrosion rate of low-carbon steel in both immersion media. By increasing addition of pomelo peel inhibitor, the corrosion efficiency increased from 30.27 to 74.64% and then slightly dropped at a pomelo peel concentration of 10,000 ppm, in which the inhibition efficiency achieved the highest value at a pomelo peel extract concentration of 8000 ppm. Further increasing the pomelo peel extract concentration reduced the corrosion inhibition possibly, because the chemical reaction may have occurred between the adsorption layer and medium (Ref 34).
Microstructure Analysis
Figure 8 shows the immersion surface area of the low-carbon steel samples in 3.5% NaCl and 0.1 M HCl solutions without and with the addition of inhibitor for different periods of time. The immersed samples in 3.5% NaCl medium without any inhibitor showed severe corrosion conditions on the surface compared with the immersed samples with 10,000 ppm of pomelo peels extract. In the first week, the low-carbon steel showed dark and black corrosion products on the surface, and as the time increased to the eighth week, the sample surface becomes rough as the corrosive layer been removed, and some black spots are left on the surface of the low-carbon steel sample associated with some circular pumps that form after the washing process. In contrast, the immersion medium becomes more yellowish in color along with precipitation of yellowish-brown particles at the bottom of the container, in which these corrosive particles were pulled out from the sample surface because of the effect of gravity. More circular pits were shown on the surface of sample after being immersed in a modified medium with 3000 , 8000 and 10,000 ppm of pomelo peel within the periods of 4, 6 and 8 weeks. There were no significant changes shown on the surface sample throughout the immersion with 3.5% NaCl modified with 8000 ppm and 10,000 ppm. As the immersion time increased, the low-carbon steel sample formed dark-blacked corrosion products and the surface of the sample was rougher after being washed.
From different perspectives, the results of the low-carbon steel surface show uniformly formed corrosion after 1 and 8 weeks of immersion in 0.1 M hydrochloric acid. At the end of week one, the sample surface had a grey and shiny appearance, and the medium solution became a light-green color, and as the immersion time increased to the second week, few grey-like particles participated at the bottom of the container. In the fourth week, the color of 0.1 M HCl solution changed to be somewhat yellow and then had a dark brown-like appearance associated with the formation of a brown product at the end of the container. This formation may be attributed to the oxide film being too thick and breaking off and the corrosion thus becoming more severe (Ref 35). As the samples been removed from the immersion medium, a high roughness with an irregular shapes pits formed at the surface of samples, and the density of these pits increased as the immersion time increased.
The microstructures of the low-carbon steel were observed under a scanning electron microscope with and without inhibitors immersed in 3.5% NaCl and 0.1 M HCl media for different periods of times shown in Fig. 9 and 10, respectively. Without the presence of the inhibitor and within the first week, the low-carbon steel surface appeared rough; then, after four weeks, few circular pits were observed on the surface; and after eight weeks, the whole surface was covered with irregular pits and had a rough surface, as shown in Fig. 9(a-c). In 3.5% NaCl medium, the solution which contains inhibitors, fewer pits were observed on the surface. Based on the immersion area in Fig. 9(e), (f), for low-carbon steel samples that were immersed after eight weeks, the corrosion occurred less severe than without adding any inhibitor. However, the surface shows smaller irregular pits after eight weeks of immersion. Moreover, the low-carbon steel samples immersed in the acid medium were attacked by halide ions; therefore, the surface of the low-carbon steel appeared rough and contained many irregular pits, particularly, in the medium without adding any inhibitors, as shown in Fig. 10(a-c). After subsequent immersion of one week, the surface was covered with irregular tiny pits, and as immersion time increased, the size of the pits also increased. The size of the pits became even larger after eight weeks of immersion. The samples appeared rough, and some of the low-carbon steel was worn out. As the low-carbon steel was immersed in the acid medium with inhibitors added with a concentration of 8000 ppm (see in Fig. 10(d-f)), the surface appearance has lesser irregular pits. After eight weeks of immersion, the low-carbon steel surface became rough and covered with numerous circular tiny pits as shown in Fig. 10(f). These results indicate that inhibitors form a passive film at the surface of the low-carbon steel at the beginning of immersion weeks. The halide ions were aggressive ions, and they broke the adsorptive passive film and caused subsequent corrosion to occur on the surface of the low-carbon steel. According to the EDX analysis at the surface of the corroded surface of low-carbon steel, the presence of oxygen indicates that the pomelo peels extract has acceptor and donor interaction with the low-carbon steel, as presented in Figs. 9(g) and 10(g) for 3.5 NaCl and 0.1 M HCl media, respectively. The pomelo peel extract has aromatic or heterocyclic rings with a functional group of oxygen that acts as a donor (Ref 36); low-carbon steel has a d-orbital vacancy that acts as an acceptor (Ref 37). Because of this interaction, the corrosion rate decreases. At the end of the first week, the low-carbon steel surface has 2.74% of oxygen, the percentage of oxygen increases to 15.64% at the eighth week of immersion.
Electrochemical Impedance Spectroscopy (EIS)
The most common method for determining the corrosion behavior is by using EIS. The results show the resistivity and capacitive process of the metal and inhibitor interface. According to Bentiss et al.(Ref 38), an equivalent circuit model is shown in Fig. 11. In the equivalent circuit, Rs represents the resistance in the solution, Rp is the polarization resistance and Qdl is the constant phase element (CPE). The impedance spectra were analyzed based on this type of circuit. Normally, to fit the double layer accurately, a pure capacitor is substituted by a CPE, and this CPE was calculated by equation as follows (Ref 39):
where Q is the magnitude of the CPE, \(j\) = −1 is the imaginary number, \(\omega\) is the angular frequency in rad/s, and η is an exponent of CPE which can be used as gauge roughness or heterogeneity of the metal surface. The CPE value is considered as resistance when η= 0, and it represents an ideal capacitor when η = 1. The electrochemical impedance parameters of Rs (resistance of the solution), Rct (resistance of charge transfer), Q (constant phase element), η (exponent of CPE), Cdl (capacitance value) and IE % (inhibition efficiency in term of percentage) are given in Table 5.
According to Table 5, the Rct value increased and the Cdl value decreased as the pomelo peel extract concentration increased. These results indicate that the inhibitor molecules adsorbed between the metal and solution interface. As the concentration of pomelo peel inhibitor increased, the η value also increased because of the adsorption of inhibitor molecules and decreased the inhomogeneity of low-carbon steel surface. In addition, the inhibition efficiency (IE%) achieved the highest value at 8000 ppm in both 0.1 M HCl and 3.5% NaCl media. The IE % with a pomelo peel extracts concentration of 8000 ppm added to the acid medium was 84.07%, whereas the IE % of the 3.5% NaCl medium was 69.21%. These results show that the addition of pomelo peel extracts can decelerate the corrosion reaction. Figure 11a and b illustrate the Nyquist plot for the low-carbon steel with different pomelo peel extract concentrations in 3.5% NaCl and 0.1 M HCl media, respectively. The electrochemical impedance diagram contains the semicircular-shaped patterns, and the sample without adding inhibitors has the smallest semicircle; with adding pomelo peels extract, the diameter of semicircle increased. When the concentration reaches 8000 ppm, it has the largest semicircle diameter, and by further increasing the concentration of inhibitors to 10,000 ppm, the semicircle diameter decreased. This semicircle shape indicates the surface roughness of the low-carbon steel surface and inhomogeneity is affected by the corrosion reaction (Ref 40). In the presence of the inhibitors, the semicircle becoming larger is because the corrosion mechanism remained the same for the three inhibitor concentrations. The addition of inhibitors increases the charge transfer resistance and decelerates the corrosion process at the interface between the metal and the solution. The Nyquist plot with depressed semicircles consists of a large capacitive loop at high frequency and an inductive loop at low frequency. The large capacitive loop is related to the charge transfer resistance process between the low-carbon steel surface and the solution, whereas the inductive loop is about the relaxation process by the adsorption species \(H_{\text{ads}}^{ + }\) or the corrosion inhibitor species that is present at the metal surface (Ref 41). From the Bode plot in Fig. 12a and b, the magnitude of impedance increased as the pomelo peels extract was added to both media. This phenomenon is because the surface roughness and homogeneity decreased as the inhibitors were added to the 0.1 M HCl and 3.5% NaCl media (Ref 42). According to Table 6, the results of the current study in both corrosion mediums have shown significant improvements compared with other studies.
Adsorption Isotherm
The surface adsorption process can be approached by isotherm models using the magnitude of the surface coverage (\(\theta\); \(\theta = IE_{\text{EIS}} /100\)) for a particular range of inhibitor concentration (Ref 52, 53). The results revealed that the best linear fit was matched with Langmuir adsorption isotherm and the values of regression parameter (R2) were obtained in the range of 0.915 and 0.993 for the 0.1 M HCl and 3.5% NaCl solutions, respectively, as given in Table 6. The Langmuir isotherm (Ref 54) is represented by using \(\frac{{C_{\text{inh}} }}{\theta } = \frac{1}{{K_{\text{ads}} }} + C_{\text{inh}}\); where Cinh is the inhibition concentration in ppm; θ is the par of metal surface covered by inhibitor and Kads is the constant equilibrium of adsorption. Figure 13 displays the linear fitted graphs of the ratio of Cinh/θ versus Cinh that resulted from the adsorption of different pomelo peel inhibitor concentrations on the surface of low carbon steel as substrate. The calculated parameters of adsorption that been derived from the implemented model are listed in Table 6. On the other hands, the Gibbs free energy (ΔGads) of the pomelo peel inhibitor adsorption is empirically related to the value of Kads, which can be calculated using equation as follows (Ref 54):
where the R is the gas constant (8.314 J/mol.K) and T is the absolute test temperature in kelvin. The calculated values are tabulated in Table 7, hence, the range of ΔGads is in between − 16.35 kJ/mol and − 13.36 kJ/mol, and then the electrostatic metal–inhibitor interactions are consistent with a physical adsorption mechanism (physisorption) (Ref 54, 55). The thermodynamic spontaneity was presented by the negative magnitude of ΔGads in which it corresponded to the adsorption process of the pomelo peel inhibitor onto the low-carbon steel surface.
Conclusions
According to the overall experimental results, the following conclusions are determined:
- 1.
The pomelo peels extract provides a good inhibition of corrosion on low-carbon steel surface either in 3.5% NaCl or 0.1 M HCl media.
- 2.
The inhibition efficiency increases with the addition of pomelo peels extract to both media and reaches a maximum value of 84.07% inhibition efficiency at 8000 ppm concentration. The IE % dropped slightly to 70.25% at 10,000 ppm concentration, and this result may be because the peel off occurred on the passive layer formed by the inhibitor.
- 3.
Based on the immersion test, pomelo peel inhibitor has higher inhibition efficiency in 3.5% NaCl (49.90%) compared with 0.1 M HCl (40.21%). However, based on the polarization test, the % IE is slightly higher in 0.1 M HCl (74.64%) than in 3.5% NaCl (71.15%), while % IE in EIS test shown the best record of 84.07% in 0.1 M HCl.
- 4.
This research was conducted for eight weeks testing; thus, the author(s) would recommend further testing time to be considered for the future work.
References
F. Bentiss, M. Lagrenée, and M. Traisnel, 2,5-bis(n-pyridyl)-1,3,4-oxadiazoles as corrosion inhibitors for mild steel in acidic media, Corrosion, 2000, 56(7), p 733–742
O. Olivares-Xometl, N.V. Likhanova, M.A. Domínguez-Aguilar, E. Arce, H. Dorantes, and P. Arellanes-Lozada, Synthesis and corrosion inhibition of α-amino acids alkylamides for mild steel in acidic environment, Mater. Chem. Phys., 2008, 110(2), p 344–351
S.A.A. El-Maksoud and A.S. Fouda, Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium, Mater. Chem. Phys., 2005, 93(1), p 84–90
P. Muthukrishnan, B. Jeyaprabha, and P. Prakash, Corrosion inhibition and adsorption behavior of Setaria verticillata leaf extract in 1 M sulphuric acid, J. Mater. Eng. Perform., 2013, 22(12), p 3792–3800
E.-S.M. Sherif, Corrosion mitigation of copper in acidic chloride pickling solutions by 2-amino-5-ethyl-1, 3, 4-thiadiazole, J. Mater. Eng. Perform., 2010, 19(6), p 873–879
D.T. Nguyen, H.T.X. To, J. Gervasi, Y. Paint, M. Gonon, and M.-G. Olivier, Corrosion inhibition of carbon steel by hydrotalcites modified with different organic carboxylic acids for organic coatings, Prog. Org. Coat. , 2018, 124, p 256–266
K. Hu, J. Zhuang, J. Ding, Z. Ma, F. Wang, and X. Zeng, Influence of biomacromolecule DNA corrosion inhibitor on carbon steel, Corros. Sci., 2017, 125, p 68–76
P.B. Raja and M. Sethuraman, Studies on the inhibition of mild steel corrosion by Rauvolfia serpentina in acid media, J. Mater. Eng. Perform., 2010, 19(5), p 761–766
Ü. Ergun and K.C. Emregül, Azole compounds as corrosion inhibitors: part I, J. Mater. Eng. Perform., 2014, 23(1), p 213–221
A. Fateh, M. Aliofkhazraei, and A.R. Rezvanian, Review of corrosive environments for copper and its corrosion inhibitors, Arab. J. Chem. , 2017, 13(1), p 481–544
C.G. Dariva and A.F. Galio, Corrosion inhibitors—principles mechanisms and applications, Developments in Corrosion Protection, M. Aliofkhazraei, Ed., IntechOpen, London, 2014,
I.B. Obot, S. Kaya, and C. Kaya, Conceptual density functional theory and its application to corrosion inhibition studies, Conceptual Density Functional Theory and Its Application in the Chemical Domain, N. Islam and S. Kaya, Ed., Apple Academic Press, Palm Bay, 2018, p 195–216
M. Sohail, A.D. Chandio, and M. Sheikh, High temperature effectiveness of ginger extract as green inhibitor for corrosion in mild Steel, NUST J. Eng Sci, 2019, 11(1), p 26–32
I. Obot, S. Umoren, and N. Ankah, Pyrazine derivatives as green oil field corrosion inhibitors for steel, J. Mol. Liq., 2018, 277, p 749–761
A. Chiba, M. Koyama, E. Akiyama, and T. Nishimura, interstitial carbon enhanced corrosion resistance of Fe-33Mn-xC austenitic steels: inhibition of anodic dissolution, J. Electrochem. Soc., 2018, 165(2), p C19–C26
J.P. Srivastava, P.K. Sarkar, A. Gautam, R. Yadav, H. Kumar, Micromechanical characterisation of Indian rail steel, IOP Conference Series: Materials Science and Engineering, 2018, IOP Publishing, p 012104
M. Belghiti, A. Dafali, Y. Karzazi, M. Bakasse, H. Elalaoui-Elabdallaoui, L. Olasunkanmi, E. Ebenso, Computational simulation and statistical analysis on the relationship between corrosion inhibition efficiency and molecular structure of some hydrazine derivatives in phosphoric acid on mild steel surface, Applied Surface Science, (2019)
A.A. Al-Amiery, M.H.O. Ahmed, T.A. Abdullah, T.S. Gaaz, and A.A.H. Kadhum, Electrochemical studies of novel corrosion inhibitor for mild steel in 1 M hydrochloric acid, Res. Phys., 2018, 9, p 978–981
P.E. Alvarez, M.V. Fiori-Bimbi, A. Neske, S.A. Brandán, and C.A. Gervasi, Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution, J. Ind. Eng. Chem., 2018, 58, p 92–99
M. Wang, J.J. Carver, V.V. Phelan, L.M. Sanchez, N. Garg, Y. Peng, D.D. Nguyen, J. Watrous, C.A. Kapono, and T. Luzzatto-Knaan, Sharing and community curation of mass spectrometry data with global natural products social molecular networking, Nat. Biotechnol., 2016, 34(8), p 828
Y.A. Albrimi, A.A. Addi, J. Douch, R. Souto, and M. Hamdani, Inhibition of the pitting corrosion of 304 stainless steel in 0.5 M hydrochloric acid solution by heptamolybdate ions, Corros. Sci., 2015, 90, p 522–528
P. Morales-Gil, M. Walczak, R. Cottis, J. Romero, and R. Lindsay, Corrosion inhibitor binding in an acidic medium: interaction of 2-mercaptobenizmidazole with carbon-steel in hydrochloric acid, Corros. Sci., 2014, 85, p 109–114
R.T. Loto, Pitting corrosion evaluation of austenitic stainless steel type 304 in acid chloride media, J. Mater. Environ. Sci., 2013, 4(4), p 448–459
H. Song and E.R. Carraway, Reduction of chlorinated ethanes by nanosized zero-valent iron: kinetics, pathways, and effects of reaction conditions, Environ. Sci. Technol., 2005, 39(16), p 6237–6245
C.M. Hansson, Comments on electrochemical measurements of the rate of corrosion of steel in concrete, Cem. Concr. Res., 1984, 14(4), p 574–584
C.G. Berrocal, K. Lundgren, and I. Löfgren, Corrosion of steel bars embedded in fibre reinforced concrete under chloride attack: state of the art, Cem. Concr. Res., 2016, 80, p 69–85
N.J. Nnaji, O.T. Ujam, N.E. Ibisi, J.U. Ani, T.O. Onuegbu, L.O. Olasunkanmi, and E.E. Ebenso, Morpholine and piperazine based carboxamide derivatives as corrosion inhibitors of mild steel in HCl medium, J. Mol. Liq., 2017, 230, p 652–661
D.E. Talbot and J.D. Talbot, Corrosion Science and Technology, CRC Press, Boca Raton, 2018
S.A. Umoren and M.M. Solomon, Synergistic corrosion inhibition effect of metal cations and mixtures of organic compounds: a review, J. Environ. Chem. Eng., 2017, 5(1), p 246–273
P.B. Cranwell, L.M. Harwood, and C.J. Moody, Experimental Organic Chemistry, Wiley, Hoboken, 2017
E. Ferreira, C. Giacomelli, F. Giacomelli, and A. Spinelli, Evaluation of the inhibitor effect of L-ascorbic acid on the corrosion of mild steel, Mater. Chem. Phys., 2004, 83(1), p 129–134
A. Dubey and G. Singh, Corrosion inhibition of mild steel in sulphuric acid solution by using polyethylene glycol methyl ether (PEGME), Portugaliae Electrochimica Acta, 2007, 25(2), p 221–235
M. Kaddouri, M. Bouklah, S. Rekkab, R. Touzani, S. Al-Deyab, B. Hammouti, A. Aouniti, and Z. Kabouche, Thermodynamic, chemical and electrochemical investigations of calixarene derivatives as corrosion inhibitor for mild steel in hydrochloric acid solution, Int. J. Electrochem. Sci., 2012, 7, p 9004–9023
A. Hamdy and N.S. El-Gendy, Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium, Egyptian J. Pet. , 2013, 22(1), p 17–25
M. Uusitalo, P. Vuoristo, and T. Mäntylä, High temperature corrosion of coatings and boiler steels below chlorine-containing salt deposits, Corros. Sci., 2004, 46(6), p 1311–1331
N. Hackerman and A. Makrides, Action of polar organic inhibitors in acid dissolution of metals, Ind. Eng. Chem., 1954, 46(3), p 523–527
A.Y. Musa, A.A.H. Kadhum, A.B. Mohamad, and M.S. Takriff, Experimental and theoretical study on the inhibition performance of triazole compounds for mild steel corrosion, Corros. Sci., 2010, 52(10), p 3331–3340
F. Bentiss, M. Lebrini, N.E. Chihib, M. Abdalah, C. Jama, M. Lagrenée, S. Al-Deyab, and B. Hammouti, Heat treatment effect of polyphosphate derivatives of guanidine and urea copolymer on the corrosion inhibition of armco iron in acid solution and antibacterial properties, Int. J. Electrochem. Sci., 2012, 7, p 3947–3958
R. Mehdaoui, A. Khelifa, A. Khadraoui, O. Aaboubi, A.H. Ziane, F. Bentiss, and A. Zarrouk, Corrosion inhibition of carbon steel in hydrochloric acid solution by some synthesized surfactants from petroleum fractions, Res. Chem. Intermed., 2016, 42(6), p 5509–5526
A. Singh and M. Quraishi, The extract of Jamun (Syzygiumcumini) seed as green corrosion inhibitor for acid media, Res. Chem. Intermed., 2015, 41(5), p 2901–2914
N. ElHamdani, R. Fdil, M. Tourabi, C. Jama, and F. Bentiss, Alkaloids extract of Retama monosperma (L.) Boiss. seeds used as novel eco-friendly inhibitor for carbon steel corrosion in 1 M HCl solution: electrochemical and surface studies, Appl. Surf. Sci., 2015, 357, p 1294–1305
C.B. Verma, M. Quraishi, and A. Singh, 2-Aminobenzene-1, 3-dicarbonitriles as green corrosion inhibitor for mild steel in 1 M HCl: electrochemical, thermodynamic, surface and quantum chemical investigation, J. Taiwan Inst. Chem. Eng., 2015, 49, p 229–239
A. Ehsani, M.G. Mahjani, M. Hosseini, R. Safari, R. Moshrefi, and H.M. Shiri, Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory, J. Colloid Interface Sci. , 2017, 490, p 444–451
M.S. Al-Otaibi, A.M. Al-Mayouf, M. Khan, A.A. Mousa, S.A. Al-Mazroa, and H.Z. Alkhathlan, Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media, Arab. J. Chem., 2014, 7(3), p 340–346
K.M. Hijazi, A. Abdel-Gaber, and G. Younes, Electrochemical corrosion behavior of mild steel in HCl and H2SO4 solutions in presence of loquat leaf extract, Int. J. Electrochem. Sci., 2015, 10, p 4366–4380
B. Abd-El-Naby, O. Abdullatef, H. El-Kshlan, E. Khamis, and M. Abd-El-Fatahc, Effect of alkaline etching on the inhibition of the acidic corrosion of aluminum by Lupine extract, Portugaliae Electrochimica Acta, 2015, 33(1), p 1–11
S. Aejitha, P. Kasthuri, and S. Jyothi, Corrosion inhibitory action of Commiphora caudata extract on the mild steel corrosion in 1 M H2SO4 acid medium, J. Adhes. Sci. Technol., 2016, 30(7), p 784–802
S. Leelavathi and R. Rajalakshmi, Dodonaea viscosa (L. )leaves extract as acid corrosion inhibitor for mild steel–a green approach, J Mater Environ Sci, 2013, 4(5), p 625–638
A. Khadraoui, A. Khelifa, M. Hadjmeliani, R. Mehdaoui, K. Hachama, A. Tidu, Z. Azari, I. Obot, and A. Zarrouk, Extraction, characterization and anti-corrosion activity of Mentha pulegium oil: weight loss, electrochemical, thermodynamic and surface studies, J. Mol. Liq., 2016, 216, p 724–731
M. Prabakaran, S.-H. Kim, A. Sasireka, K. Kalaiselvi, and I.-M. Chung, Polygonatum odaratum extract as an eco-friendly inhibitor for aluminum corrosion in acidic medium, J. Adhes. Sci. Technol., 2018, 32(18), p 2054–2069
A. Fouda, S. Rashwan, M. Kamel, and N. Arman, Adsorption and inhibition behavior of avicennia marina for Zn metal in hydrochloric acid solution, Int. J. Electrochem. Sci., 2017, 12, p 11789–11804
K. Bouhrira, A. Chetouani, D. Zerouali, B. Hammouti, A. Yahyi, A. Et-Touhami, R. Yahyaoui, and R. Touzani, Theoretical investigation of inhibition of the corrosion of A106 steel in NaCl solution by di-n-butyl bis (thiophene-2-carboxylato-O, O′) tin (IV), Res. Chem. Intermed., 2014, 40(2), p 569–586
M. Mobin and M.A. Khan, Adsorption and corrosion inhibition behavior of polyethylene glycol and surfactants additives on mild steel in H2 SO4, J. Mater. Eng. Perform., 2014, 23(1), p 222–229
S.A. Umoren, A.A. AlAhmary, Z.M. Gasem, and M.M. Solomon, Evaluation of chitosan and carboxymethyl cellulose as ecofriendly corrosion inhibitors for steel, Int. J. Biol. Macromol., 2018, 117, p 1017–1028
S. Bilgic and M. Şahin, The corrosion inhibition of austenitic chromium–nickel steel in H2SO4 by 2-butyn-1-ol, Mater. Chem. Phys., 2001, 70(3), p 290–295
Acknowledgment
The authors would like to thank Universiti Teknologi Malaysia (UTM) for the providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yee, Y.P., Saud, S.N. & Hamzah, E. Pomelo Peel Extract as Corrosion Inhibitor for Steel in Simulated Seawater and Acidic Mediums. J. of Materi Eng and Perform 29, 2202–2215 (2020). https://doi.org/10.1007/s11665-020-04774-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-020-04774-1