Abstract
Corrosion tests were conducted in a high-temperature high-pressure (HTHP) autoclave to simulate the conditions of CO2 auxiliary steam drive in gas injection wells. Weight loss tests were performed with the sheets of N80 and 9Cr steels under the testing conditions. The morphology and composition of corrosion products were explored by SEM, EDS, XRD and XPS. The corrosion resistance of 9Cr steel was better than that of N80 steel under the testing conditions. The corrosion rates of N80 and 9Cr met the application requirements in CO2 auxiliary steam drive. The results broke the constraint in ISO-15156 standards. The corrosion process of N80 steel was mainly affected by the flow velocity. However, the corrosion process of 9Cr steel was mainly affected by temperature. The corrosion resistance of 9Cr steel depended on the FeCO3 content of Cr-rich layer, which was closely related to temperature. The low flow velocity influenced the diffusion process of N80 steel corrosive ions, whereas the high flow velocity influenced the integrity of corrosion scales. Considering the influence of flow velocity on the corrosion of tubing and casing, in the gas injection well, 9Cr steel and N80 steel were, respectively, selected as the materials of tubing and casing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CO2 auxiliary steam drive is a new technology to improve the recovery performance of heavy oil reservoir in recent years and shows a good application prospect in oil recovery (Ref 1, 2). Heavy oil viscosity can be reduced through injecting steam and a small amount of CO2 into heavy oil reservoir. However, during steam injection, the wellbore temperature (160-20 °C) of gas injection wells is high and injected CO2 will cool the wellbore to generate the water film on the inner wall of wellbore. When steam injection stops, the temperature of wellbore gradually decreases and the water film is formed on the inner wall of wellbore. When CO2 is dissolved in water, it will form carbonic acid. Carbonic acid will significantly increase the corrosion rate of tubing and casing steel and cause serious corrosion (Ref 3,4,5,6). Therefore, a reasonable choice of tubing and casing steels is the key to ensure the safe implementation of CO2 auxiliary steam drive.
According to the study results of the Kawasaki Heavy Industries and Sumitomo Metal Industries, when the temperature was higher than 180 °C, stainless steel (22Cr and 25Cr steel) containing the higher level of Cr should be adopted (Ref 7). However, the expensive 22Cr and 25Cr steel significantly increased the exploitation cost of heavy oil reservoir. The commonly used tube and case steels are much cheaper than recommended steel (22Cr and 25Cr steel), but the applicability of the common tube and case steels in the injection well should be further explored.
The tube and case often serve in complex environments involving multiple corrosion factors, such as temperature, CO2 partial pressure, and flow velocity (Ref 8, 9). The failure probability of tube and case was higher in the complex environment of corrosion factors. Zhu et al. reported that the deposition rate of FeCO3 increased with the increase in temperature (Ref 10). Gao et al. found that as the temperature increased, the corrosion scales of N80 steel were changed from a membrane structure into a two-layer structure (Ref 11). Zhang et al. observed that the increase in CO2 partial pressure resulted in the decreased pH value of the solution and the serious corrosion of steels (Ref 12). Guo et al. studied that the corrosion rate of 3Cr steel under the flowing conditions was higher than that under static conditions (Ref 13). Xu et al. reported that the CO2 partial pressure affected the corrosion morphology of Cr-containing steel through changing the solution pH (Ref 14). Temperature, CO2 partial pressure, and flow rate had the significant influences on the structure and composition of corrosion products (Ref 15,16,17). Previous studies focused on the influences of individual factors on the corrosion mechanisms. However, the corrosion failure of tubes and cases is the consequence of multiple factors and it is difficult to determine the main factors affecting the corrosion of tube and case steels based on previous studies. Therefore, it is necessary to determine the main factors affecting the corrosion for developing proper protective measures in gas injection wells.
Moreover, previous studies on the simulated environment of CO2 corrosion mainly involved the environment of liquid, gas or gas–liquid, which was significantly different from the corrosion environment of high-temperature steam containing CO2 (Ref 18,19,20). In CO2 auxiliary steam drive, gas injection temperature should be controlled within the range of 160-220 °C and CO2 pressure should be controlled within the range of 1-5 MPa. The main corrosion media in the gas injection wells involve high-temperature steam and CO2. However, the corrosion mechanism of steels in the environment involving high-temperature steam and CO2 was seldom reported.
The study aims to explore the corrosion behaviors of common tube and case steels in high-temperature steam environment through testing the corrosion behaviors of tube and case steels in simulated CO2 auxiliary steam drive environment in the high-temperature and high-pressure (HTHP) autoclave. This study explored the main factors affecting the corrosion in gas injection wells as well as the corrosion mechanism of steel in high-temperature steam environment. In addition, a new method for selecting the tube and case steels in gas injection wells was established.
Materials and Experiments
Specimen Preparation
The commonly used tube and case steels include N80 and 9Cr steels. The composition of steels is shown in Table 1. According to the metallographic structures of N80 and 9Cr steels (Fig. 1), the N80 and 9Cr steels microscopically belong to the tempered sorbate and martensite, respectively.
Weight Loss Test
Testing Scheme
Based on the statistics data of gas injection wells, the main influencing factors of corrosion were determined. The effects of temperature, CO2 pressure and flow velocity on the corrosion behaviors of steel were studied in the experiment. The experimental scheme is provided in Table 2.
Experimental Methods
According to the ASTM standards, all specimens were machined into a size of 30 × 15 × 3 mm, and each material had 4 parallel specimens in each group. Prior to the test, the specimens were abraded with silicon carbide paper (300-mesh, 600-mesh, 800-mesh, and 1200-mesh) to eliminate scratches, rinsed with distilled water, degreased with acetone, and finally dried with cold air.
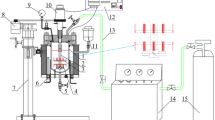
Prior to the test, deionized water was pre-oxidized with pure N2 for 8 h. The each parallel specimen was mounted on specimen holders. The specimen holders were placed in self-designed high-temperature (HT) autoclave (Fig. 2). The volume of high-temperature autoclave was 4 L. Deionized water (60 mL) was poured into the autoclave. The specimen was in the steam environment. Before the autoclave was sealed, the autoclave was deoxygenated with pure N2 gas for 3 h. When the autoclave was sealed, N2 was purged into the autoclave for 30 min to remove air. After the autoclave was heated to the experimental temperature, CO2 gas was injected into the autoclave and the flow velocity was then set for the test. After the test, the four parallel specimens of each group were taken out. After each corrosion test, the specimens were rinsed with deionized water, dehydrated with alcohol and dried in the air. Three specimens were, respectively, descaled in the solution (100 mL of 1.19 g/mL hydrochloric acid +1000 mL of deionized water + 10 g hexamethylene tetramine), rinsed with distilled water, dehydrated with alcohol and then dried in air. The another specimen was used to analyze the morphology and composition of corrosion products.
Before and after the tests, the specimen was weighed in an electronic balance with a precision of 0.1 mg and then stored in a desiccator. The corrosion rate is calculated as (Ref 11, 21):
where \( v \) is corrosion rate (mm/a); \( \Delta m \) is the weight loss (g); \( \rho \) is the density of steel (g/cm3); \( A \) is the surface area (cm2); \( \Delta t \) is the corrosion time (h).
Morphological Observation and Composition Analysis
The corrosion scales were characterized by scanning electron microcopy (JSM-7500F). The corrosion products on the corroded specimens were analyzed by using x-ray diffraction (XPert Pro MPD) and XPS (Phi-Quantera II).
Results and Discussion
Corrosion Rate
Figure 3 shows the average corrosion rate of steels under various testing conditions. The average corrosion rate of the steels was the average of three specimens. As shown in Fig. 3, the corrosion rate of N80 steel is higher than that of 9Cr steel. Compared with N80 steel, 9Cr steel showed the better corrosion resistance under experimental conditions.
Macroscopic Appearance of Corrosion Scales
The macroscopic morphologies of N80 and 9Cr steels under the maximum corrosion rate are shown in Fig. 4. As exhibited in Fig. 4a, corrosion products of N80 steel are black. In Fig. 4b, the corrosion of 9Cr was slight and corrosion products of 9Cr steel are blue.
SEM Observation and EDS Analysis of Corrosion Products
Figure 5 shows the surface morphology and corresponding EDS results of 9Cr steel at various temperatures. The corrosion films of specimen were complete and dense (Fig. 5A). The main elements in the corrosion products of 9Cr steel were Fe, C, Cr and O (Fig. 5B). The Cr content in the 9Cr steel corrosion products was higher than that in the matrix, indicating that Cr enrichment occurred on the surface of the specimen. The crystals of corrosion products on the surface of the specimen were cubic, and the crystal size was 1-1.5 μm (Fig. 5C). The Cr content in the area (marked with a in Fig. 5A) was slightly higher than that in the matrix (Fig. 5E). Round crystals were scattered on the surface of the specimen, and the size of crystals was below 1 μm (Fig. 4f). Scaly corrosion products completely covered the metal matrix (Fig. 5I). Some corrosion products were mixed together. The Cr content in corrosion products was much lower than that in the matrix (Fig. 5J).
Figure 6 shows the surface morphology and corresponding EDS results of N80 steel under various flow velocities. Cubic corrosion crystals were deposited on the surface of the specimen to form uneven corrosion scales. Corrosion scales peeled off, and a circular hole was formed in a local area (Fig. 6B). There were many holes on the surface of corrosion products (Fig. 6C). The main elements in the N80 steel corrosion products were Fe, C, Cr and O (Fig. 6D–F).
XRD and XPS Analysis of Corrosion Scales
The XRD results of N80 and 9Cr steels under the maximum corrosion rate are shown in the Fig. 7. Fe2O3 and Fe could be detected (Fig. 7a). The result was consistent with the XPS results. Only 3 peaks were observed and determined as Fe peak through the jade analysis (Fig. 7b).
The corrosion product film of 9Cr was thin, so it was difficult to analyze the composition of corrosion products with XRD. To further study the corrosion products, the XPS was used to analyze to the elemental composition. Figure 8 shows the XPS results of 9Cr steel under static conditions (160 °C and 2 MPa CO2). The C 1s peaks at 283.6 eV and 284.6 eV corresponded to adventitious carbon (Fig. 8a). The Cr 2p3/2 peaks at 577.1 eV was ascribed to Cr(OH)3 and Cr2O3 (Fig. 8b). The Fe2p1/2 peaks at 710.7 eV and Fe2p3/2 peaks at 724.4 eV could be ascribed to FeCO3 or Fe2O3 (Fig. 8c). The O1s peaks at 530.8 eV and 531.4 eV corresponded to FeCO3, Fe2O3, Cr(OH)3 and Cr2O3 (Fig. 8d). The XPS analysis results indicated that the corrosion products were FeCO3, Fe2O3, Cr(OH)3 and Cr2O3 (Ref 22).
In the XPS results of 9Cr steel, a small quantity of Fe2O3 and Cr2O3 appeared on the surface of 9Cr steel (Fig. 8). FeCO3 was resolved at the high temperature (Eq 2). The specimens were taken out from the autoclave and FeO of corrosion scales was oxidized in air to form Fe2O3 (Eq 3) (Ref 22). Cr(OH)3 was dehydrated in air to form Cr2O3 (Eq 4) (Ref 23, 24).
Figure 9 shows XPS results of N80 steel after corrosion at 160 °C under 2 MPa CO2 at 6 m/s. The C1s peak only appears at 284.6 eV, corresponding to adventitious carbon (Fig. 9a). The Fe 2p3/2 peaks at 724.4 eV corresponded to FeCO3 (Fig. 9b). As for Fe 2p1/2, the peaks at 710.2 eV could be assigned to FeCO3 (Fig. 9b). The peaks of O1s at 530.6 and 531.9 eV were associated with oxygen in FeCO3 and Fe2O3 (Fig. 9c). The XPS analysis results indicated that the corrosion products were FeCO3 and Fe2O3 (Ref 22).
Discussion
Analysis of Corrosion Factors
During the process of CO2 auxiliary steam drive, the corrosion of oil tube and casing tube was mainly affected by temperature, CO2 pressure, and flow velocity. To quantitatively analyze the influences of temperature, CO2 pressure, and flow velocity on the corrosion rate, the influencing rate of a corrosion factor is defined as follows:
where \( \varphi_{\text{i}} \) is the influencing rate of a corrosion factor; \( v_{i,\hbox{max} } \) is the maximum corrosion rate of a material under a certain testing condition; \( v_{i,\hbox{max} } \) is the maximum corrosion rate of a material at different temperatures; \( v_{p,\hbox{max} } \) is the maximum corrosion rate of a material under different CO2 pressures; \( v_{f,\hbox{max} } \) is the maximum corrosion rate of a material under different flow velocities.
Figure 10 shows the corrosion factor index of the steels under different testing conditions. The main control factor in the corrosion of N80 steel is flow velocity (Fig. 10a). The main control factor in the corrosion of 9Cr steel is temperature (Fig. 10b). The influences of corrosion factors on oil casing tube should be considered in the selection of materials.
Corrosion Mechanism
Effects of Temperature on Water Film
During the corrosion process of steel in high-temperature steam containing CO2, H2O molecules are firstly absorbed on the surface of specimens to form a thin water film (Fig. 11a). The amount of water-film adsorption is closely related to temperature. Generally, the adsorption of H2O molecules on steel surface corresponds to a heat release process. The temperature rise is harmful to the adsorption process of H2O molecules on steel surface. Therefore, the elevated temperature is also not conducive to the formation of water film on steel surface.
Effects of Temperature on the Structure of Cr-Rich Films
In a CO2 environment, Cr-rich films are formed on the surface of Cr-containing steel. Cr-rich films are mainly composed of a small amount of amorphous FeCO3 and Cr(OH)3 generated in the hydrolysis of Cr3+ (Fig. 11c). The hydrolysis reaction of Cr3+ is an endothermic reaction, so the temperature rise can accelerate the hydrolysis of Cr3+ (Ref 25, 26). The hydrolysis reaction of Cr3+ also produces more H+ ions, thus causing the rapid decrease in the pH value of the water film (Ref 27, 28). The pH value of the water film determines the growth and nucleation processes of FeCO3 crystals (Ref 29, 30). More FeCO3 crystals are generated at low pH. Therefore, a large number of FeCO3 crystals also accumulate on the Cr-rich layer in the high-temperature environment (Fig. 11d).
Effects of Fluid Flow on the Corrosion Scales
The flow velocity of a fluid determines corrosion scales. At a low flow velocity, the fluid promotes the mass transfer process of corrosive ions (Ref 31, 32). Corrosive ions (H+, HCO3− and CO32−) rapidly diffuse to the electrode surface and Fe2+ quickly leaves from the metal surface. Fe2+ ions react with corrosive ions to form the dense FeCO3 layer (Fig. 12b). At a high flow velocity, the wall shearing stress of fluid increases. The combination between the FeCO3 corrosion scales and the metal matrix is weak, so corrosion scales on the surface easily peel off (Fig. 12c).
Selection of the Materials of Oil Casing Tube
To further study the safe service conditions of gas injection well, the corrosion safety factor of the materials is defined as:
where \( \varPhi \) is corrosion safety factor; 0.076 is the corrosion safety control value; \( v_{i,\hbox{max} } \) is the maximum corrosion rate of a material under a certain testing condition. The corrosion safety factor of materials was the ratio of corrosion control index to material corrosion rate. The corrosion safety factor value of a material was higher than 1, indicating that the material was in the safe service state. If the value of the corrosion safety factor of a material was less than 1, the material was in a dangerous service state.
Figure 12 shows the corrosion safety factors of steels under different testing conditions. Under different testing conditions, 9Cr steel was in safe service state, whereas N80 steel was in a dangerous service state under different current conditions. During the CO2 auxiliary steam drive, the steam and CO2 were injected into the heavy oil reservoir through the tubing. In the tubing material selection, the gas injection pressure, temperature, and flow velocity should be considered; in the casing material selection, the gas injection pressure and temperature should be considered. According to the corrosion safety factor of the material, 9Cr steel was selected as the tubing material, whereas N80 steel was selected as the casing material. Based on the corrosion impact index, the corrosion conditions of 9Cr steel were closely monitored in the high-temperature steam environment.
Conclusions
-
1.
The corrosion resistances of 9Cr and N80 steel were compared under testing conditions. The corrosion rates of N80 and 9Cr met the application requirements in CO2 auxiliary steam drive. The results broke the constraint of ISO-15156 standards. The flow velocity had a significant influence on the corrosion of N80 steel, and the temperature had a significant influence on the corrosion of 9Cr steel.
-
2.
The corrosion resistance of 9Cr steel depended on the Cr-rich layer, whereas the temperature affected the amount of FeCO3 on the surface and interior part of the Cr-rich layer. At the low temperature, the FeCO3 content of the Cr-rich layer was relatively low. At the high temperature, the FeCO3 content of the Cr-rich layer was relatively high.
-
3.
The flow velocity affected the mass transfer process of N80 steel and the integrity of the corrosion scales. At the low flow velocity, the corrosion process was dominated by the ion diffusion process. At the high flow velocity, the corrosion process mainly depended on the integrity of the corrosion scales.
-
4.
Considering the influence of flow velocity on the corrosion of the oil casing, 9Cr steel and N80 steel were, respectively, selected as the materials of tubing and casing in the gas injection well.
References
D.W. Zhao, J. Wang, and I.D. Gates, Thermal Recovery Strategies for Thin Heavy Oil Reservoirs, Fuel, 2014, 117(1), p 431–441
D.W. Zhao and I.D. Gates, On Hot Water Flooding Strategies for Thin Heavy Oil Reservoirs, Fuel, 2015, 153, p 559–568
L. Xu, B. Wang, J. Zhu, W. Li, and Z. Zheng, Effect of Cr Content on the Corrosion Performance of Low-Cr Alloy Steel in a CO2 Environment, Appl. Surf. Sci., 2016, 379, p 39–46
X. Lin, W. Liu, F. Wu, C. Xu, J. Dou, and M. Lu, Effect of O2 on Corrosion of 3Cr Steel in High Temperature and High Pressure CO2-O2 Environment, Appl. Surf. Sci., 2015, 329, p 104–115
Y.S. Choi, S. Nesic, and D. Young, Effect of Impurities on the Corrosion Behavior of CO2 Transmission Pipeline Steel in Supercritical CO2-Water Environments, Environ. Sci. Technol., 2010, 44(23), p 9233–9238
L.N. Xu, S.Q. Guo, C.L. Gao, W. Chang, T.H. Chen, and M.X. Lu, Influence of Microstructure on Mechanical Properties and Corrosion Behavior of 3%Cr Steel in CO2 Environment, Mater. Corros., 2015, 63(11), p 997–1003
L.M. Tavares, E.M.D. Costa, J.J.D.O. Andrade, R. Hubler, and B. Huet, Effect of Calcium Carbonate on Low Carbon Steel Corrosion Behavior in Saline CO2 High Pressure Environments, Appl. Surf. Sci., 2015, 359, p 143–152
Y. Hua, R. Barker, and A. Neville, Effect of Temperature on the Critical Water Content for General and Localised Corrosion of X65 Carbon Steel in the Transport of Supercritical CO2, Int. J. Greenhouse Gas Control, 2014, 31(2), p 48–60
R. Elgaddafi, R. Ahmed, S. Hassani, S. Shah, and S.O. Osisanya, Corrosion of C110 Carbon Steel in High-Pressure Aqueous Environment with Mixed Hydrocarbon and CO2 Gas, J. Petrol. Sci. Eng., 2016, 146, p 777–787
S.D. Zhu, A.Q. Fu, J. Miao, Z.F. Yin, G.S. Zhou, and J.F. Wei, Corrosion of N80 carbon steel in oil field formation water containing CO2 in the absence and presence of acetic acid, Corros. Sci., 2011, 53(10), p 3156–3165
M. Gao, X. Pang, and K. Gao, The Growth Mechanism of CO2 Corrosion Product Films, Corros. Sci., 2011, 53(2), p 557–568
Y. Zhang, X. Pang, S. Qu, X. Li, and K. Gao, Discussion of the CO2 Corrosion Mechanism Between Low Partial Pressure and Supercritical Condition, Corros. Sci., 2012, 59(3), p 186–197
S. Guo, L. Xu, L. Zhang, W. Chang, and M. Lu, Corrosion of Alloy Steels Containing 2% Chromium in CO2 Environments, Corros. Sci., 2012, 63, p 246–258
L. Xu, S. Guo, W. Chang, T. Chen, L. Hu, and M. Lu, Corrosion of Cr Bearing Low Alloy Pipeline Steel in CO2 Environment at Static and Flowing Conditions, Appl. Surf. Sci., 2013, 270(4), p 395–404
J. Sun, C. Sun, G. Zhang, X. Li, W. Zhao, T. Jiang, H. Liu, X. Cheng, and Y. Wang, Effect of O2 and H2S Impurities on the Corrosion Behavior of X65 Steel in Water-Saturated Supercritical CO2 System, Corros. Sci., 2016, 107, p 31–40
W. Li, L. Xu, L. Qiao, and J. Li, Effect of Free Cr Content on Corrosion Behavior of 3Cr Steels in a CO2 Environment, Appl. Surf. Sci., 2017, 425, p 32–45
L. Wei, X. Pang, and K. Gao, Effect of Small Amount of H2S on the Corrosion Behavior of Carbon Steel in the Dynamic Supercritical CO2 Environments, Corros. Sci., 2016, 103, p 132–144
G.A. Zhang, D. Liu, Y.Z. Li, and X.P. Guo, Corrosion Behaviour of N80 Carbon Steel in Formation Water Under Dynamic Supercritical CO2 Condition, Corros. Sci., 2017, 120, p 107–120
D. Zhu, Y. Lin, H. Zhang, Y. Li, D. Zeng, W. Liu, C. Qiang, and K. Deng, Corrosion Evaluation of Packer Rubber Materials in CO2 Injection Wells under Supercritical Conditions, J. Pet. Sci. Eng., 2017, 151, p 311–317
Q.Y. Liu, L.J. Mao, and S.W. Zhou, Effects of Chloride Content on CO2 Corrosion of Carbon Steel in Simulated Oil and Gas Well Environments, Corros. Sci., 2014, 84(84), p 165–171
Z.Y. Liu, X.Z. Wang, and R.K. Liu, Electrochemical and Sulfide Stress Corrosion Cracking Behaviors of Tubing Steels in a H2S/CO2 Annular Environment, J. Mater. Eng. Perform., 2014, 23(4), p 1279–1287
Y. Tang, X.P. Guo, and G.A. Zhang, Corrosion Behaviour of X65 Carbon Steel in Supercritical-CO2 Containing H2O and O2 in Carbon Capture and Storage (CCS) Technology, Corros. Sci., 2017, 118, p 118–128
A. Pfennig and A. Kranzmann, Effect of CO2 and Pressure on the Stability of Steels with Different Amounts of Chromium in Saline Water, Corros. Sci., 2012, 65, p 441–452
Q. Wu, Z. Zhang, X. Dong, and J. Yang, Corrosion Behavior of Low-Alloy Steel Containing 1% Chromium in CO2 Environments, Corros. Sci., 2013, 75(7), p 400–408
G.A. Zhang and Y.F. Cheng, Localized Corrosion of Carbon Steel in a CO2-Saturated Oilfield Formation Water, Electrochim. Acta, 2011, 56(3), p 1676–1685
Z.F. Yin, X.Z. Wang, and L. Liu, Characterization of Corrosion Product Layers from CO2 Corrosion of 13Cr Stainless Steel in Simulated Oilfield Solution, J. Mater. Eng. Perform., 2011, 20(7), p 1330–1335
Y.S. Choi, S. Nesic, and S. Ling, Effect of H2S on the CO2 Corrosion of Carbon Steel in Acidic Solutions, Electrochim. Acta, 2011, 56(4), p 1752–1760
J.S. Sun, C. Sun, and X.Q. Lin, Effect of Chromium on Corrosion Behavior of P110 Steels in CO2-H2S Environment with High Pressure and High Temperature, Materials, 2016, 9, p 200–214
S. Guo, L. Xu, L. Zhang, W. Chang, and M. Lu, Characterization of Corrosion Scale Formed on 3Cr Steel in CO2-Saturated Formation Water, Corros. Sci., 2016, 110, p 123–133
L. Zhang, X.G. Li, and C.W. Du, Corrosion and Stress Corrosion Cracking Behavior of X70 Pipeline Steel in a CO2-Containing Solution, J. Mater. Eng. Perform., 2009, 18(3), p 319–323
G.A. Zhang and Y.F. Cheng, Electrochemical Characterization and Computational Fluid Dynamics Simulation of Flow-Accelerated Corrosion of X65 Steel in a CO2-Saturated Oilfield Formation Water, Corros. Sci., 2010, 52(8), p 2716–2724
G.A. Zhang, L. Zeng, H.L. Huang, and X.P. Guo, A Study of Flow Accelerated Corrosion at Elbow of Carbon Steel Pipeline by Array Electrode and Computational Fluid Dynamics Simulation, Corros. Sci., 2013, 77, p 334–341
Acknowledgments
The authors acknowledge the support from National Science and Technology Major Project of China (No. 2016ZX05012-001) and the National Natural Science Foundation of China (No. 51374177).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, B., Zeng, D., Yu, Z. et al. Corrosion Mechanism and Applicability Assessment of N80 and 9Cr Steels in CO2 Auxiliary Steam Drive. J. of Materi Eng and Perform 28, 1030–1039 (2019). https://doi.org/10.1007/s11665-018-3847-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-018-3847-z