Abstract
Porous Ti-Nb-xSn shape memory alloys (SMAs) are fabricated by microwave sintering technology. The microstructures exhibit needle-like morphologies, β(N) (normal straight and crossed needles along with needle-like morphology that resembles spaghetti or irregular lines with α-phases in between) as well as plate-like morphologies [normal straight plate-like morphology, α′′ and dendritic plate-like morphology, β(D)]. Increases in Sn addition significantly induce an increase in the density of the α-phase. XRD patterns exhibited three phases, namely the β-main phase with smaller intensities of α′′ and α. Further, the addition of 0.25% Sn led to more effective improvement in the intensity of the α′′-phase compared with 0.5% and 1.5% Sn addition. Additions of Sn also enhanced the fracture strength and its corresponding strain along with the shape memory effect (SME), where the best enhancement was achieved at 0.25% Sn. The corrosion rate (Ri) was reduced by rising Sn content, while both corrosion resistance and antibacterial zones were increased. The lower elastic modulus, as well as the robust mechanical properties and bioactivity, made these SMAs rather suitable for biomedical application purposes, where the low elastic modulus had value in terms of avoiding the problem of “stress shielding.”
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pure Ti and Ti alloys have been widely used as implanting materials and for clinical repair based on their excellent mechanical properties, superb corrosion resistance and biocompatibility (Ref 1,2,3,4,5,6). At present, the elastic modulus of Co-Cr and Ti-6Al-4V is leveraged for orthopedic implant applications, with greater than 100 GPa (Ref 7), while the elastic modulus of the bone is between 4 and 30 GPa depending on measurement direction and bone type (Ref 8, 9). This large difference between bone tissues and implant devices causes stress-shielding problems because of bone resorption (Ref 10), and as such, total hip arthroplasty is necessary based on the loosening of devices. To overcome the critical issue of stress shielding, Ti-Nb-based alloys for biomedical applications have recently attracted much attention, and many alloys with low elastic modulus as well as robust biocompatibility and high strength have been developed, e.g., Ti-33.6Nb-4Sn (Ref 11), Ti-29Nb-13Ta-4.6Zr (Ref 12), Ti-35Nb-5Ta-7Zr (Ref 13), and Ti-13Nb-13Zr (Ref 14). The existence of pores in titanium alloys reduces the elastic modulus (Ref 15,16,17) as well as allows for the implant cells to grow into the pores and integrate with the host tissue (Ref 17,18,19). It was reported by Yang et al. (Ref 16) that porous Ti-(10-35) wt.% Nb alloys were produced by powder metallurgy (PM) technology and featured low elastic modulus (Young’s modulus) of 6-15 GPa. Matsumoto et al. (Ref 20) reported that adding Sn to Ti-Nb alloys can enhance the strength of these alloys. It is evident the corrosion rate was reduced after adding Sn, Khalifa et al. (Ref 21) described the presence of Sn with the oxide layer of TiO2 that improved the effect of this layer in an aggressive environment, enhanced corrosion resistance and diminished the Ri, while Ghoranneviss et al. (Ref 22) noted that pure Sn exhibits antibacterial properties. Ti-Nb-based alloys can be fabricated by PM through several methods, including conventional sintering (Ref 23, 24), metal-injection molding (Ref 25,26,27), self-propagating high-temperature synthesis (Ref 28), hot-isostatic pressing (Ref 29), spark-plasma sintering (Ref 23, 30, 31) and microwave sintering (MWS). The MWS technique is a relatively new method for preparing Ti-Nb alloys, and it is considered a novel sintering method for metals, composites, ceramics and semiconductors (Ref 32,33,34). Overall, MWS has several advantages, such as an enhanced diffusion process, reduced energy and sintering-process time, rapid heating rates and improved mechanical and physical properties (Ref 32, 33). Therefore, the purpose of this study was to evaluate the impact of the amount of Sn on the microstructure characteristics, martensitic transformation, mechanical properties, bio-corrosion and antibacterial effects of Ti-Nb-xSn SMAs. The results are beneficial for the development of Ti-Nb-xSn SMAs for biomedical applications.
Experimental Procedure
Sample Preparation
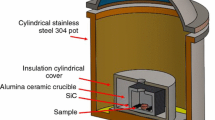
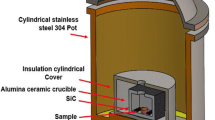
Ti of particle sizes of 45-150 µm and a purity of 99.5% mixed with Nb of a particle size of 74 µm and a purity of 99.85% and a particle size of 45 µm for Sn were utilized. Figure 1 depicts the morphologies of the Ti, Nb and Sn powders. The powders for this research were supplied by Stanford Advanced Materials Company. These powders were prepared for 1 h in a planetary ball mill (PM100) at 300 rpm until becoming well-mixed powder with a weight ratio of ball to powder of 4:1. The Ti(77−x)-Nb23-xSn (x = 0, 0.25, 0.5, and 1.5) were expressed in atomic percentage (at.%); powders were cold-pressed to green samples of Φ25 × 10 mm under a uniaxial pressure of 230 kg/cm2 for 5 min followed by MWS (HAMiLab-V3, Synotherm Corp.). These samples underwent MWS at a temperature of 900 °C for 30 min at a heating rate of 30 °C/min and furnace cooling to 250-300 °C, followed by water cooling (during furnace cooling, the cylindrical stainless steel 304 was under water cooling). The cooling rate at all parameters shows a small range of 8-9 °C/min. Next, these samples were coupled with microwaves, where electromagnetic energy was absorbed and transformed into heat to adjust the sintering temperature. An insulation barrel was set up into a continuous 2.45 GHz and 4.5 kV. The sintering was under Ar gas, which fills the sintering chamber with a gas flow purity of 99.999%. The compacts were placed in an alumina crucible surrounded by SiC particles (at the corners of the alumina crucible). SiC was used as an auxiliary heat material. An infrared pyrometer was employed to measure the temperature of the samples during the MWS process.
The sintered samples were cut to dimensions of 7 mm × 7 mm × 14 mm using electrical discharge machining (EDM) wire cut for the compression test and 10 mm × 10 mm × 20 mm for the shape memory test according to the ASTM E9-09 standards. Corrosion testing was performed with samples of dimensions Φ13.5 × 2 mm while the sample dimension for the antibacterial testing was Φ5 × 1.5 mm.
Microstructural Characterization
Several techniques were used to analyze and characterize the Ti-Nb-xSn SMAs, such as scanning electron microscopy (SEM), x-ray diffraction (XRD), differential scanning calorimetry (DSC), and energy-dispersive x-ray spectroscopy. After cutting, the samples were ground and polished, and this was followed by etching in 10%HF-40%HNO3-50% distilled water solution for 15-40 s. SEM (Hitachi Model S-3400N) was employed to evaluate the microstructure. The Archimedes drainage method was utilized to determine the porosity of the samples. The x-ray diffractometer (XRD; D5000 Siemens) was employed to characterize the phase composition of the Ti-Nb-xSn SMAs; the diffraction patterns were identified by Jade software, version 9. The XRD used a CuKα x-ray source, and the scanning mode was locked couple with a scan rate of 0.05°/s and a 2θ range between 20 and 90 °C. DSC (DSC; Q200, TA Instrument) was used to determine the phase transformation temperatures of these alloys via the TA Instrument software under heating/cooling rates of 10 °C/min. For the DSC test, the temperature ranges were from − 80 to 500 °C in two both heating and cooling cycles and the transformation temperatures after each cycle nearly the same. The weight of the samples for DSC test was about 10 mg.
Mechanical Tests
An Instron 600 DX-type universal testing machine was used to perform the compression testing at a displacement speed of 0.5 mm/min at 25 °C. Shape memory test was also performed with an Instron 600 DX-type universal testing machine, which was operated by special program parameters according to the shape memory effect (SME) testing. The loading–unloading cycle compressive test was conducted at a strain of 4%, and moreover, the tests were carried out below the martensite start temperature (Ms) to establish shape recovery followed by heating at 200 °C for 30 min [above austenite finish temperature (Af)] with subsequent water quenching. The SME and total strain recovery (εT) were calculated by the following formals: \({\text{SME}} = (L_{\text{f}} - L_{\text{o}} )/L_{\text{f}}\) and \(\varepsilon_{\text{T}} = (\varepsilon_{\text{p}} - \varepsilon_{\text{R}} )/\varepsilon_{\text{p}} \times 100,\) where L0 is sample length after compression testing, Lf is sample length following heating at 200 °C with water quenching thereafter, (\(\varepsilon_{\text{p}}\)) is loading–unloading cycle strain, and εR is residual strain.
Electrochemical Testing/Bio-corrosion
To evaluate the corrosion behavior of Ti-Nb-xSn SMAs, a potentiodynamic polarization (PDP) in a simulated body fluid (SBF) was used. The specimen was connected to an open-air glass cell with a surface area of 0.2 cm2, the open-air glass cell contained approximately 300 mL of SBF at a pH of 7.4, and the experiment was accomplished at human body temperature (37 °C) with a scan rate of 2 mV/s begun at − 250 mV. The VersaSTAT 3 machine (Princeton Applied Research) was used to record the measured data. Each electrochemical test was repeated three times for each sample to ensure the corresponding results. Hereafter, the Ri and polarization resistance (Rp) of these samples was calculated according to (Ref 35, 36) the equations; \(R_{\text{i}} = 22:85_{{i{\text{corr}}}}\) and \(R_{\text{p}} = \beta a\beta c/\left( {2.3\left( {\beta a + \beta c} \right)i_{\text{corr}} } \right),\) where icorr is the corrosion current density, βc is the cathodic Tafel slope and βa is the anodic Tafel slope. Eventually, the calculations of the output results were based on the polarization curves for the Ti-Nb-xSn SMAs.
Antibacterial Test
The antibacterial activities of metal before and after adding the third alloying element (Sn) were determined by the ager-disk diffusion technique making use of the bacterial species, Escherichia coli (gram-negative bacteria) (Ref 37). These bacteria were gotten from the Institute of Medical Research (IMR)/Kuala Lumpur/Malaysia. The glassware was sterilised for 20 min in an autoclave at 120 °C preceding to the experiment. The stock solution for each organism was produced via mixing 9 mL of Luria–Bertani (LB) broth with 1 mL of each bacterial solution followed by incubation at 37 °C for 24 h with shaking at 250 rpm. For disk diffusion, solidified nutrient agar media was swabbed with the respective organisms (n = 2) (1 × 108 CFU/mL). The disks on each plate were fixed at an equalized distance and incubated at 37 °C for 24 h. The optimized image was captured with a digital camera. ImageJ software (v 1.47) was used to measure the inhibition zone (mm) around the film.
Results and Discussion
Microstructure Characteristics
Figure 2 portrays the SEM micrographs of Ti-Nb-xSn. The microstructures exhibit two forms of morphologies; needle-like morphologies, β(N) (normal straight and crossed needles along with needle-like morphology that resembled spaghetti or irregular lines with α-phases in between, present with β phase) as well as plate-like morphologies (normal straight plate-like morphology, α′′ and dendritic plate-like morphology, β(D)). Adding Sn potentially induced an increase in the density of the α-phase because Sn acts as a α-Ti stabilizer. Sn hampers the formation of the ω-phase and the associated stabilization of the parent β-phase (Ref 38,39,40). It has been reported that Ti-Nb alloys of 15-35 at.%Nb exhibit superelastic and shape memory properties associated with the β to α′′ martensitic transformation (Ref 41), while 20-24 at.%Nb exhibit self-accommodation morphologies of the α′′ (Ref 42). It was also described that during the cooling process with a Nb content of 11 < Nb < 27 at.%, the α′′ structure forms from the retained β-phase (Ref 43, 44). The titanium structure is HCP (α), while niobium structure is BCC (β). Therefore, Nb-rich region is β phase and the diffusion of Nb to Ti-rich region affects as β-Ti stabilizer, then Ti-rich region is (α + α′′) + β. The darkest parts in Ti-rich region contained more α phase while the less darkness regions or contain needles and plates are β phase except the spaghetti (irregular) shape needles contains α phase companies with β phase. Furthermore, the SME decreases with increasing precipitates of the equilibrium α-phase (Ref 45). As well, the density of these alloys was increased gradually with rising amounts of Sn, and these densities were in the range between 77 and 86%. Terayama et al. (Ref 30) reported that elemental additions with lower melting points effectively rose the density of the sintered alloy. Based on EDS, the morphology of the surface exhibited two regions—a bright area related to the Nb-rich region and a dark area referred to as the Ti-rich region (Ref 46, 47). Figure 2(b) portrays the EDS data of Ti-Nb-0.25%Sn, where 1 and 2 indicate the EDS points (spot scanning) on the micrograph in Fig. 2(a). Point 1 refers to Ti with 55.97 at.%Ti and 30.05 at.%Nb associated with an existence of O. Point 2 reflects Ti with 63.20 at.%Ti and 20.35 at.%Nb, with the rest being O. The bright region without the presence of any needle-like or plate-like morphologies refer to the Nb-rich region, while the presence of these morphologies were close to the Ti-rich region and populated the area between the two rich regions. Sn was not observed at either point. Figure 3 shows the elemental mapping of the Ti-Nb-0.25%Sn SMA with evident diffusion between the elements, and it can be observed there is a Ti-rich region (dark area) and Nb-rich region (bright area). Sn diffused finely in both the Ti and Nb regions.
Figure 4(a) portrays the XRD data of the sintered Ti-23Nb-xSn samples. The influence of Sn on the XRD patterns of Ti-Nb was investigated, and the β, α′′ and α phases were observed. The main phase was the β-phase that was indicated on planes (110), (200), (211), (112) and (220) at the angles of 38.8°, 55.9°, 70°, 76° and 82.7°, respectively. The α′′-phase appeared at planes (020), (021) and (130) of angles 36.7°, 40.2° and 63°, respectively, while the α-phase appeared at planes (100), (101), (102), (110) and (201) at angles 35.17°, 40.2°, 52.7°, 63° and 77.12°, respectively. Observation revealed that at 0.25% Sn, there was a slight increase in the intensity of the α′′-phase peak at an angle of 36.7° of plane (020) as well as the peak at an angle of 40.2° containing the largest intensity of the α′′-phase of the plane (021). With a further increase in Sn additions to 0.5% and 1.5%, what resulted was a minor reduction in the intensity of the α′′-phase, which was also reported by Ozaki and Hu (Ref 11, 48), where large amounts of Sn addition in Ti alloys can lead to inadequate occurrence of martensitic transformation. The increase in the α′′-phase may have improved the mechanical and shape memory behaviors. Furthermore, there were also many elemental oxides (these oxidize of small intensities due to using argon gas during the sintering process, the oxidation maybe occurs due to the exposing to the atmosphere after furnace cooling to 250-300 °C), such as TiO2, Ti2O3, Nb2O5 and Sn2O3. TiO2 appeared at planes (022), (112) and (224) of angles 40.2°, 70° and 82.7°, respectively. Ti2O3 appeared at plane (113) of an angle 40.2°, Nb2O5 appeared at planes (-518) and (017) of angles 38.8° and 55.9°, respectively, while Sn2O3 appeared at plane (141) of an angle 55.9°. As well, there were various compounds observed, such as NbTi4 and Ti3Sn. NbTi4 was located at plane (002) of angle 38.8°, Ti3Sn appeared at plane (201) of angle 40.2°. Figure 4(b) portrays the XRD data of the sintered Ti-13.34Nb sample, and the matched phases were the same for the Ti-23Nb alloy (for the β and α phase, the planes and angles were the same as for the Ti-23Nb alloy, but the α′′-phase was not observed, nor for the oxides and compounds that appeared based on the presence additional Sn). The presence of Nb at 13.34 at.% was less than that in Fig. 4(a), causing an increase in the intensity of the α-phase, and this may have been because Nb is β stabilizer element (Ref 49). As well, one could not observe the α′′-phase because of the reduction in Nb, which decreased the stability of the β-phase, hence diminishing the α′′-phase that formed from the retained β-phase during cooling, as mentioned previously. It is reported by Lee et al. (Ref 50) that the results indicate that crystal structure and morphology of the Ti-Nb alloys are sensitive to the Nb content.
Transformation Temperatures
Figure 5 features the DSC curves of the Ti-Nb-xSn samples with and without Sn addition, and the extracted data were determined and tabulated in Table 1. It was found that the peak of martensite transformation temperature (Ms to Mf) was shifted to the left to the lowest temperatures with the addition of 0.25% Sn, and this shifting was reduced gradually with rising additional Sn. The range of the austenite transformation temperatures [austenite start temperature (As to Af)] became smaller with increasing Sn additions, moderately shifting from As without any changes in the Af values. Based on previous research (Ref 11, 48), with a large amount of Sn addition, martensitic transformation has difficulty taking place in Ti alloys potentially because the Ms and Mf temperatures (see Table 1) were reduced after adding small amounts of Sn (0.25%) to the Ti-23Nb SMA, while adding greater amounts of Sn (i.e., 0.5% and 1.5%) had smaller effects on Ms and Mf. Furthermore, Sn addition may affect the stabilization of Ti-based alloys, thus increasing the stability of the β-phase. This effect of Sn on martensitic transformation was compatible with the fluctuations in the intensities of the α′′-phase in the XRD patterns. In other words, at this temperature, the martensitic transformation will be achieved while applying stress, and hence this martensitic transformation from the β-phase to the martensite α′′-phase will enhance shape memory behavior (Ref 51, 52). There are appearance of peaks not belong to the martensitic transformation during cooling process (see Fig. 5); the reason for the presence of this peak (or peaks) is due to the concentration of different stress regions close to and around the precipitates (α-precipitates/intermetallic compounds) that are formed during the addition of tin (Ref 53, 54). Based on the Clausius–Clapeyron equation, the presence of stresses in the alloy consistently improves the martensitic transformation temperature. Once martensite forms (Ref 55), the stress field is relaxed near the precipitates and additional martensite transformation inhibited temporarily. Upon further cooling, as the temperature decreases to stress-free martensite start temperature, the martensitic transformation again starts. In this type of transformation, the initial martensite peak increases with greater transformation heat release when compared with the final martensite peak (Ref 56).
Mechanical Properties
Stress–Strain Curves
The impact of Sn content on deformation behavior based on compression testing is found in Fig. 6, where all the engineering stress-engineering strain curves of Ti-Nb-Sn exhibit three stages: (a) elastic deformation of the β-austenite; (b) elastic deformation of stress-induced martensite; and (c) plastic deformation of the stress-induced martensite. However, the β-phase can transform into the martensitic α′′-phase by adding stress during loading, while during unloading, the unstable martensitic α′′-phase mostly transforms back into the β-phase (Ref 51). In general, the compression stress of the material decreased with further increases in Sn content as formed via the fracture strain. The sample order of the compression stress and strain was in the sequence of: Ti-Nb-0.25Sn > Ti-Nb-0.5Sn > Ti-Nb-1.5Sn > Ti-Nb. The fraction strength and strain of these SMAs with 0, 0.25, 0.5 and 1.5 at.% Sn were 515 MPa at 26.3%, 643 MPa at 34%, 626 MPa at 30.48% and 589 MPa at 30%, respectively. The highest fracture strength and strain for the Ti-Nb-0.25%Sn alloy were possibly because of the effect of the amount of Sn on α-Ti or β-Ti stabilization, where as previously mentioned, Sn acts as α-Ti stabilizer (Ref 38,39,40). However, Gutiérrez-Moreno et al. (Ref 57) reported for Ti-Nb alloys that the β-phase was stable at low Sn substitutions, though increasing Sn concentrations induce instability. Therefore, the highest fracture strength and strain were at 0.25% Sn, while greater Sn content caused a slight decrease in fracture strength and its strain. Table 2 presents the effect of Sn addition on maximum stress and its strain, elastic modulus and Vickers hardness. The elastic modulus increased slightly after adding 0.5% Sn content and was reduced slightly at 1.5% Sn, while hardness diminished. The low elastic modulus is utterly important to solving the problem of stress shielding, which occurs based on the significant difference in the elastic modulus of implants and human body tissues (Ref 9, 58, 59). This low elastic modulus was based on the MWS process and its effect on the pores, as well as the low hardness (Ref 15,16,17).
Shape Memory Test
The influence of Sn addition on the shape memory behavior of Ti-Nb-xSn is depicted in Fig. 7. The total strain recovery (εT) was improved with the addition of Sn compared to the Ti-Nb SMA. The maximum improvement of the εT was observed after adding 0.25 at.% Sn to Ti-Nb SMA sintered at 900 °C for 30 min. The εT was reduced with increasing Sn content more than 0.25 at.% Sn. Table 3 features the residual strain (εR) and εT of the Ti-Nb-xSn SMAs. The εT indicates the percentage of strain recovery by the superelastic recovery and SME (see Fig. 7b). The order of the εT with respect to the samples was: Ti-Nb-0.25Sn > Ti-Nb-0.5Sn > Ti-Nb-1.5Sn > Ti-Nb. The εT of the Ti-Nb-xSn SMAs was determined by utilizing a specially designed compression test at T < Ms (human body temperature of 37 °C). The εT varied with the changes in the amount of Sn at the strain value of 4%. The changes in the εT of these SMAs were exhibited after the preheating process at T > Af. It was found that the εT rose from 40.537 to 46.95% after adding 0.25 at.%Sn. The increase in Sn addition to more than 0.25 at.% led to a decrease of the εT. Further addition of 0.5 at.% Sn obtained a εT of roughly 42.95%, while 1.5 at.% Sn attained approximately 42.15%. This reduction in εT may be based on Sn precipitates that formed with the addition of Sn. It was proposed that the increase in the amount of Sn addition can result in enhancing α-phase stability, also may be due to the influence of Sn addition on the transformation temperatures as mentioned earlier (Ref 11, 48), where small amounts of Sn made the martensite transformation temperatures closer to that of human body temperature of (37 °C). The εR of these SMAs at x = 0, 0.25, 0.5 and 1.5 at.% Sn were 2.3785, 2.122, 2.282 and 2.314, respectively.
Bio-corrosion and Antibacterial Tests
Figure 8 portrays the PDP curves of the Ti-23Nb-xSn SMAs in SBF solution. Table 4 lists the electrochemical parameters of the Ti-Nb-Sn samples in SBF solution obtained from polarization testing. The current density (icorr) values were reduced with increasing Sn addition. The corrosion potential shifted in the noble direction after rising the proportion of Sn from 0.25 to 0.5 at.%, but an elevation to 1.5 at.% Sn diminished the corrosion potential to -54 mV. However, the order of corrosion potential was: Ti-Nb-0.5Sn > Ti-Nb-1.5Sn > Ti-Nb > Ti-Nb-0.25Sn. The order of corrosion rate (Ri) based on Sn addition was: Ti-Nb-1.5Sn < Ti-Nb-0.5Sn < Ti-Nb-0.25Sn < Ti-Nb. Ti-Nb-1.5Sn had the highest polarization resistance (Rp) compared to the rest of the alloys. However, increasing Sn additions of 0-1.5 at.% rose the Rp value from 1.172 to 111.949 kΩ. Therefore, the Rp of ternary Ti-Nb-xSn SMAs was observed in the following sequence: Ti-Nb < Ti-Nb-0.25Sn < Ti-Nb-0.5Sn < Ti-Nb-1.5Sn. It was reported by Wu et al. (Ref 60) that adding (0-8) wt.%Sn to Ti60Zr10Ta15Si15 alloy reduced the corrosion rate and increased the corrosion resistance gradually with increasing Sn content. The antibacterial activity results with the Ti-Nb-Sn samples against E. coli are presented in Fig. 9. The average of the inhibition zones for Ti-Nb-xSn was found to be 1 mm, 1.3 mm, 1.7 mm and 1.9 mm against E. coli for 0, 0.25, 0.5 and 1.5 at.%Sn, respectively. The order of the average of the inhibition zones was: Ti-Nb < Ti-Nb-0.25Sn < Ti-Nb-0.5Sn < Ti-Nb-1.5Sn. The average inhibition zone with these SMAs increased with elevated Sn addition; it is reported that tin and its oxides have strong antibacterial effect (Ref 22, 61, 62). The presence of TiO2 had good antibacterial effects on Ti and its alloys (Ref 63,64,65,66), while Nb and Nb oxides moderately enhanced antibacterial properties (Ref 67).
Conclusions
Based on the data presented, the following conclusions can be drawn:
-
1.
The microstructures exhibited man β(N) and β(D) morphologies. The β(N) morphology contained normal straight and crossed needles along with needle-like morphology that resembled spaghetti or irregular lines with α-phases in between. The plate-like morphology featured normal straight plate-like morphology, α′′ and dendritic, β(D). The XRD patterns of the Ti-23Nb-xSn SMAs exhibited β, α′′ and α phases, with β-phase being the main phase with lower intensities of α′′ and α phases. Ti-13.34Nb possessed β and α phases, with the α-phase being the primary phase with a lower intensity of the β-phase without any presence of the α′′-phase, potentially because of the effect of Nb as a β-stabilizer.
-
2.
Adding Sn in small amounts (0.25%) improved the martensitic transformation while larger amounts (0.5% and 1.5%) hindered martensitic transformation.
-
3.
Adding Sn improved fracture strength and its strain, with the highest fracture strength and strain being for the Ti-Nb-0.25%Sn SMA. The low elastic modulus and Vickers hardness was based on the MWS process. The low elastic modulus diminished the effect of stress shielding for these SMAs. Further, the SME was enhanced for all Sn additions, but the highest SME was for the Ti-Nb-0.25%Sn SMA.
-
4.
Ri was reduced gradually with increasing Sn content while corrosion resistance was elevated. The corrosion potential shifted in the noble direction after increasing the proportion of Sn to 0.5 wt.% and 1.5 at.% compared to the Ti-23Nb alloy with less nobility at 0.25%Sn.
-
5.
The average of the inhibition zones of the Ti-Nb-xSn samples against E. coli increased gradually with greater Sn additions. The robust effect of Sn additions on Ti-23Nb SMA made it able to replace the Ti-Ni alloy for biomedical application purposes.
References
X. Wang, Y. Li, J. Xiong, P.D. Hodgson, and C.E. Wen, Porous TiNbZr Alloy Scaffolds for Biomedical Applications, Acta Biomater., 2009, 5(9), p 3616–3624
A. Choubey, R. Balasubramaniam, and B. Basu, Effect of Replacement of V by Nb and Fe on the Electrochemical and Corrosion Behavior of Ti-6Al-4V in Simulated Physiological Environment, J. Alloy. Compd., 2004, 381(1), p 288–294
Y. Tong, B. Guo, Y. Zheng, C.Y. Chung, and L.W. Ma, Effects of Sn and Zr on the Microstructure and Mechanical Properties of Ti-Ta-Based Shape Memory Alloys, J. Mater. Eng. Perform., 2011, 20(4–5), p 762–766
S. Lu, F. Ma, P. Liu, W. Li, X. Liu, X. Chen, K. Zhang, Q. Han, and L.-C. Zhang, Recrystallization Behavior and Super-Elasticity of a Metastable β-Type Ti-21Nb-7Mo-4Sn Alloy During Cold Rolling and Annealing, J. Mater. Eng. Perform., 2018, 27(8), p 4100–4106
T. Ogawa, H. Takada, and K. Maruoka, Corrosion and Mechanical Degradation of Ni-Ti Superelastic Alloy in Neutral Fluoride Solution, J. Mater. Eng. Perform., 2018, 27, p 1–6
Y. Xiao, H. Liu, D. Yi, J. Le, H. Zhou, Y. Jiang, X. Zhao, Z. Chen, J. Wang, and Q. Gao, High-Temperature Deformation Behavior of Ti-6Al-2Sn-4Zr-2Mo Alloy with Lamellar Microstructure Under Plane-Strain Compression, J. Mater. Eng. Perform., 2018, 27, p 1–14
M. Long and H. Rack, Titanium Alloys in Total Joint Replacement—A Materials Science Perspective, Biomaterials, 1998, 19(18), p 1621–1639
D.M. Cullinane and T.A. Einhorn, Biomechanics of Bone, Princ. Bone Biol., 2002, 1, p 16–32
M. Geetha, A. Singh, R. Asokamani, and A. Gogia, Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review, Prog. Mater Sci., 2009, 54(3), p 397–425
H. Kröger, P. Venesmaa, J. Jurvelin, H. Miettinen, O. Suomalainen, and E. Alhava, Bone Density at the Proximal Femur After Total Hip Arthroplasty, Clin. Orthop. Relat. Res., 1998, 352, p 66–74
T. Ozaki, H. Matsumoto, S. Watanabe, and S. Hanada, Beta Ti Alloys with Low Young’s Modulus, Mater. Trans., 2004, 45(8), p 2776–2779
D. Kuroda, M. Niinomi, M. Morinaga, Y. Kato, and T. Yashiro, Design and Mechanical Properties of New β Type Titanium Alloys for Implant Materials, Mater. Sci. Eng., A, 1998, 243(1), p 244–249
T. Ahmed, A New Low Modulus, Biocompatible Titanium Alloy, in Titanium’95: Science and Technology (1996), pp. 1760–1767
A.K. Mishra, J.A. Davidson, R.A. Poggie, P. Kovacs, and T.J. FitzGerald, Mechanical and Tribological Properties and Biocompatibility of Diffusion Hardened Ti-13Nb-13Zr—A New Titanium Alloy for Surgical Implants, Medical Applications of Titanium and its Alloys: The Material and Biological Issuesed, ASTM International, West Conshohocken, 1996
J. Xu, L. Bao, A. Liu, X. Jin, Y. Tong, J. Luo, Z. Zhong, and Y. Zheng, Microstructure, Mechanical Properties and Superelasticity of Biomedical Porous NiTi Alloy Prepared by Microwave Sintering, Mater. Sci. Eng., C, 2015, 46, p 387–393
D. Yang, Z. Guo, H. Shao, X. Liu, and Y. Ji, Mechanical Properties of Porous Ti-Mo and Ti-Nb Alloys for Biomedical Application by Gelcasting, Proc. Eng., 2012, 36, p 160–167
M. Mour, D. Das, T. Winkler, E. Hoenig, G. Mielke, M.M. Morlock, and A.F. Schilling, Advances in Porous Biomaterials for Dental and Orthopaedic Applications, Materials, 2010, 3(5), p 2947–2974
A. Bansiddhi, T. Sargeant, S.I. Stupp, and D. Dunand, Porous NiTi for Bone Implants: A Review, Acta Biomater., 2008, 4(4), p 773–782
G. Ryan, A. Pandit, and D.P. Apatsidis, Fabrication Methods of Porous Metals for Use in Orthopaedic Applications, Biomaterials, 2006, 27(13), p 2651–2670
H. Matsumoto, S. Watanabe, and S. Hanada, Beta TiNbSn Alloys with Low Young’s Modulus and High Strength, Mater. Trans., 2005, 46(5), p 1070–1078
O. Khalifa, E. Wahab, and A. Tilp, The Effect of Sn and TiO2 Nano Particles Added in Electroless Ni-P Plating Solution on the Properties of Composite Coatings, Aust. J. Basic Appl. Sci., 2011, 5(6), p 136–144
M. Ghoranneviss and S. Shahidi, Effect of Various Metallic Salts on Antibacterial Activity and Physical Properties of Cotton Fabrics, J. Ind. Text., 2013, 42(3), p 193–203
M. Wen, C. Wen, P. Hodgson, and Y. Li, Fabrication of Ti-Nb-Ag Alloy Via Powder Metallurgy for Biomedical Applications, Mater. Des., 2014, 56, p 629–634
J. Xiong, Y. Li, X. Wang, P. Hodgson, and C.E. Wen, Mechanical Properties and Bioactive Surface Modification via Alkali-Heat Treatment of a Porous Ti-18Nb-4Sn Alloy for Biomedical Applications, Acta Biomater., 2008, 4(6), p 1963–1968
D. Zhao, K. Chang, T. Ebel, H. Nie, R. Willumeit, and F. Pyczak, Sintering Behavior and Mechanical Properties of a Metal Injection Molded Ti-Nb Binary Alloy as Biomaterial, J. Alloy. Compd., 2015, 640, p 393–400
D. Zhao, K. Chang, T. Ebel, M. Qian, R. Willumeit, M. Yan, and F. Pyczak, Microstructure and Mechanical Behavior of Metal Injection Molded Ti-Nb Binary Alloys as Biomedical Material, J. Mech. Behav. Biomed. Mater., 2013, 28, p 171–182
F. Kafkas and T. Ebel, Metallurgical and Mechanical Properties of Ti-24Nb-4Zr-8Sn Alloy Fabricated by Metal Injection Molding, J. Alloy. Compd., 2014, 617, p 359–366
A. Aleksanyan, S. Dolukhanyan, V.S. Shekhtman, S. Khasanov, O. Ter-Galstyan, and M. Martirosyan, Formation of Alloys in the Ti-Nb System by Hydride Cycle Method and Synthesis of Their Hydrides in Self-Propagating High-Temperature Synthesis, Int. J. Hydrogen Energy, 2012, 37(19), p 14234–14239
L.W. Ma, C.Y. Chung, Y. Tong, and Y. Zheng, Properties of Porous TiNbZr Shape Memory Alloy Fabricated by Mechanical Alloying and Hot Isostatic Pressing, J. Mater. Eng. Perform., 2011, 20(4–5), p 783–786
A. Terayama, N. Fuyama, Y. Yamashita, I. Ishizaki, and H. Kyogoku, Fabrication of Ti-Nb Alloys by Powder Metallurgy Process and their Shape Memory Characteristics, J. Alloy. Compd., 2013, 577, p S408–S412
X. Wang, Y. Chen, L. Xu, Z. Liu, and K.-D. Woo, Effects of Sn Content on the Microstructure, Mechanical Properties and Biocompatibility of Ti-Nb-Sn/Hydroxyapatite Biocomposites Synthesized by Powder Metallurgy, Mater. Des., 2013, 49, p 511–519
M. Oghbaei and O. Mirzaee, Microwave Versus Conventional Sintering: A Review of Fundamentals, Advantages and Applications, J. Alloy. Compd., 2010, 494(1), p 175–189
S. Das, A. Mukhopadhyay, S. Datta, and D. Basu, Prospects of Microwave Processing: An Overview, Bull. Mater. Sci., 2009, 32(1), p 1–13
R. Roy, D. Agrawal, J. Cheng, and S. Gedevanishvili, Full Sintering of Powdered-Metal Bodies in a Microwave Field, Nature, 1999, 399(6737), p 668–670
H. Bakhsheshi-Rad, M. Idris, M. Abdul-Kadir, A. Ourdjini, M. Medraj, M. Daroonparvar, and E. Hamzah, Mechanical and Bio-corrosion Properties of Quaternary Mg-Ca-Mn-Zn Alloys Compared with Binary Mg-Ca Alloys, Mater. Des., 2014, 53, p 283–292
G. Argade, K. Kandasamy, S. Panigrahi, and R. Mishra, Corrosion Behavior of a Friction Stir Processed Rare-Earth Added Magnesium Alloy, Corros. Sci., 2012, 58, p 321–326
N. Iqbal, M.R.A. Kadir, N.H.B. Mahmood, S. Iqbal, D. Almasi, F. Naghizadeh, H. Balaji, and T. Kamarul, Characterization and Biological Evaluation of Silver Containing Fluoroapatite Nanoparticles Prepared Through Microwave Synthesis, Ceram. Int., 2015, 41(5), p 6470–6477
P.J.S. Buenconsejo, H.Y. Kim, and S. Miyazaki, Effect of Ternary Alloying Elements on the Shape Memory Behavior of Ti-Ta Alloys, Acta Mater., 2009, 57(8), p 2509–2515
P.J.S. Buenconsejo, H.Y. Kim, and S. Miyazaki, Novel β-TiTaAl Alloys with Excellent Cold Workability and a Stable High-Temperature Shape Memory Effect, Scripta Mater., 2011, 64(12), p 1114–1117
H.Y. Kim, T. Fukushima, P.J.S. Buenconsejo, T.-H. Nam, and S. Miyazaki, Martensitic Transformation and Shape Memory Properties of Ti-Ta-Sn High Temperature Shape Memory Alloys, Mater. Sci. Eng., A, 2011, 528(24), p 7238–7246
H. Kim, Y. Ikehara, J. Kim, H. Hosoda, and S. Miyazaki, Martensitic Transformation, Shape Memory Effect and Superelasticity of Ti-Nb Binary Alloys, Acta Mater., 2006, 54(9), p 2419–2429
Y. Chai, H. Kim, H. Hosoda, and S. Miyazaki, Self-Accommodation in Ti-Nb Shape Memory Alloys, Acta Mater., 2009, 57(14), p 4054–4064
J.L. Murray, The Nb-Ti (Niobium-Titanium) System, Bull. Alloy Phase Diagr., 1981, 2(1), p 55–61
H.Y. Kim and S. Miyazaki, Martensitic Transformation and Superelastic Properties of Ti-Nb Base Alloys, Mater. Trans., 2015, 56(5), p 625–634
Y. Guo, beta-bcc and Amorphous Ti-Based Biocompatible Alloys for Human Body Implants, Université Grenoble Alpes, 2014
B. Sharma, S.K. Vajpai, and K. Ameyama, Microstructure and Properties of Beta Ti-Nb Alloy Prepared by Powder Metallurgy Route Using Titanium Hydride Powder, J. Alloy. Compd., 2016, 656, p 978–986
A. Nouri, J. Lin, Y. Li, Y. Yamada, P. Hodgson, C. Wen, Microstructure Evolution of Ti-Sn-Nb Alloy Prepared by Mechanical Alloying, in Materials Forum (CD-ROM), 2007, Institute of Materials Engineering Australasia, pp. 64–70
Q.-M. Hu, S.-J. Li, Y.-L. Hao, R. Yang, B. Johansson, and L. Vitos, Phase Stability and Elastic Modulus of Ti Alloys Containing Nb, Zr, and/or Sn from First-Principles Calculations, Appl. Phys. Lett., 2008, 93(12), p 121902
Y. Guo, K. Georgarakis, Y. Yokoyama, and A. Yavari, On the Mechanical Properties of TiNb Based Alloys, J. Alloy. Compd., 2013, 571, p 25–30
C. Lee, C.-P. Ju, and J. Chern Lin, Structure-Property Relationship of Cast Ti-Nb Alloys, J. Oral Rehabil., 2002, 29(4), p 314–322
R.P. Kolli, W.J. Joost, and S. Ankem, Phase Stability and Stress-Induced Transformations in Beta Titanium Alloys, JOM, 2015, 67(6), p 1273–1280
S. Ehtemam-Haghighi, Y. Liu, G. Cao, and L.-C. Zhang, Influence of Nb on the β → α Martensitic Phase Transformation and Properties of the Newly Designed Ti-Fe-Nb Alloys, Mater. Sci. Eng., C, 2016, 60, p 503–510
N. Vellios and P. Tsakiropoulos, The Role of Sn and Ti Additions in the Microstructure of Nb-18Si Base Alloys, Intermetallics, 2007, 15(12), p 1518–1528
I. Gorna, M. Bulanova, K. Valuiska, M. Bega, O.Y. Koval, A. Kotko, Y.I. Evich, and S. Firstov, Alloys of the Ti-Si-Sn System (Titanium Corner): Phase Equilibria, Structure, and Mechanical Properties, Powder Metall. Met. Ceram., 2011, 50(7–8), p 452–461
M. Kato and H.R. Pak, Thermodynamics of Stress-Induced First-Order Phase Transformations in Solids, Phys. Status Solidi B, 1984, 123(2), p 415–424
T.T. Sasaki, B.C. Hornbuckle, R.D. Noebe, G.S. Bigelow, M.L. Weaver, and G.B. Thompson, Effect of Aging on Microstructure and Shape Memory Properties of a Ni-48Ti-25Pd (At. Pct) Alloy, Metall. Mater. Trans. A, 2013, 44(3), p 1388–1400
J. Gutiérrez-Moreno, Y. Guo, K. Georgarakis, A. Yavari, G. Evangelakis, and C.E. Lekka, The Role of Sn Doping in the β-Type Ti-25 at.% Nb Alloys: Experiment and Ab Initio Calculations, J. Alloy. Compd., 2014, 615, p S676–S679
J. Nagels, M. Stokdijk, and P.M. Rozing, Stress Shielding and Bone Resorption in Shoulder Arthroplasty, J. Shoulder Elbow Surg., 2003, 12(1), p 35–39
M. Niinomi, Metallic Biomaterials, J. Artif. Organs, 2008, 11(3), p 105–110
X. Wu, Q. Peng, J. Zhao, and J. Lin, Effect of Sn Content on the Corrosion Behavior of Ti-Based Biomedical Amorphous Alloys, Int. J. Electrochem. Sci., 2015, 10, p 2045–2054
S.M. Amininezhad, A. Rezvani, M. Amouheidari, S.M. Amininejad, and S. Rakhshani, The Antibacterial Activity of SnO2 Nanoparticles Against Escherichia coli and Staphylococcus aureus, Zahedan J. Res. Med. Sci., 2015, 17(9), p e1053
P. Kamaraj, R. Vennila, M. Arthanareeswari, and S. Devikala, Biological Activities of Tin Oxide Nanoparticles Synthesized Using Plant Extract, Pharm. Pharm. Sci., 2014, 3, p 338–382
L.H. Yun Lu, Y. Hirakawa, and H. Sato, Antibacterial Activity of TiO2/Ti Composite Photocatalyst Films Treated by Ultrasonic Cleaning, Adv. Mater. Phys. Chem., 2012, 2, p 9–12
Y.S. Kim, E.S. Park, S. Chin, G.-N. Bae, and J. Jurng, Antibacterial Performance of TiO2 Ultrafine Nanopowder Synthesized by a Chemical Vapor Condensation Method: Effect of Synthesis Temperature and Precursor Vapor Concentration, Powder Technol., 2012, 215, p 195–199
Y.L. Zhou, M. Niinomi, T. Akahori, H. Fukui, and H. Toda, Corrosion Resistance and Biocompatibility of Ti-Ta Alloys for Biomedical Applications, Mater. Sci. Eng., A, 2005, 398(1), p 28–36
R. Ahmad and M. Sardar, TiO2 Nanoparticles as an Antibacterial Agents Against E. coli, Int. J. Innov. Res. Sci. Eng. Technol., 2013, 2(8), p 3569–3574
G. Ramírez, S. Rodil, H. Arzate, S. Muhl, and J. Olaya, Niobium Based Coatings for Dental Implants, Appl. Surf. Sci., 2011, 257(7), p 2555–2559
Acknowledgments
The authors would like to thank the Ministry of Higher Education of Malaysia and Universiti Teknologi Malaysia for providing the financial support under the University Research Grant No. Q.J130000.2524.12H60 and research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibrahim, M.K., Hamzah, E. & Saud, S.N. Microstructure, Phase Transformation, Mechanical Behavior, Bio-corrosion and Antibacterial Properties of Ti-Nb-xSn (x = 0, 0.25, 0.5 and 1.5) SMAs. J. of Materi Eng and Perform 28, 382–393 (2019). https://doi.org/10.1007/s11665-018-3776-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-018-3776-x