Abstract
The “degree of wetting,” which is related to the contact angle (θ) between the molten solder and the substrate, is a useful parameter on the solderability process control. The contact angle, however, is strongly dependent on the type of substrate surface finish and used atmosphere (inert or non-inert). Furthermore, the surface tension, being an important parameter on the solderability process and performance, can also be achieved if the contact angle is known. In this study, the SAC405 [Sn4.0Ag0.5Cu (in wt.%)] solder paste contact angle was measured, by the “sessile drop” method, as a function of the temperature, surface pad finish and used atmosphere. The results are discussed, and the contact angles obtained for the different conditions are compared and discussed. Then, the surface tension (experimental) was obtained from the measured contact angle and compared with the obtained by using computation models (theoretical). The experiments performed in high vacuum conditions, i.e., low oxygen content, over a temperature range, allowed the evaluation and understanding of the surface oxides layers role on the solder wettability. The present study shows that in the soldering process, even in an inert atmosphere, usually used in industry, occurs the formation of superficial oxides, over the liquid solder and/or at the pad surfaces, that strongly affects the solder paste wettability, specially with Sn and OSP (organic solderability preservative) finishing. Differences in contact angle of ≥ 10° were determined between the two types of used atmospheres. The experimental surface tension and theoretical surface tension obtained, for the NiAu substrate type, present good correlation. The lower contact angle values were obtained for the NiAu and OSP finish types, independently of the atmosphere type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The soldering process consists of the formation of a joint between the molten solder and the metal surface (from now on designated as substrate). Thus, the ability of the molten solder to flow or spread on the substrate is important for the formation of a proper metallic bond (Ref 1,2,3,4). To achieve a reliable solder joint to sustain the performance and overall product reliability, the study of the wetting behavior is a crucial step in the characterization of the solder alloys (Ref 2, 5,6,7,8,9,10), especially for the lead-free solders.
The wettability depends on the ability of the molten solder to react with a substrate, forming intermetallic compounds (IMCs). These IMCs will be the adhesion layer between the solder/weld and the substrate (Ref 7, 11,12,13). However, the solder spreading over a metallic surface is a complex problem, involving several physical and chemical processes (Ref 14), namely the diffusion between the liquid and the solid, the nucleation conditions followed by the formation and growth of the intermetallic compound layer(s) at the interface, which together will determine the behavior of the metal–metal system (Ref 2, 15).
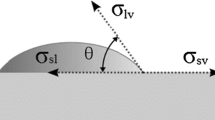
The extent of the solder wetting depends on degree and rate of wetting and also on the surface tension. The degree of wetting is generally indicated by the contact angle (θ) between the surface and the liquid (Ref 1, 16,17,18,19,20,21,22). The surface tension is a complex issue even for the simplest case of a liquid drop on a solid substrate. For a complete surface study, three surface tensions must be considered (Ref 16): solid–liquid (σsl), liquid–gas (σlg) and solid–gas (σsg). Assuming the simplest situation which corresponds to the spread of a non-reactive liquid on an ideal solid surface (physically and chemically inert, smooth, homogeneous and rigid) (Ref 7), the surface tension (balance of surface tensions at the junction (Ref 17)) and the equilibrium contact angle (θ) are related by following Young’s Eq 1:
Although these ideal conditions are rarely achieved in practical situations, this relationship is of higher importance since the Young’s equation is a fundamental starting point for understanding the complex process of wetting (Ref 7).
To get more information concerning the surface tension, several methods, based on the Laplace equation, have been developed in order to express the surface tension (σ) as a function of the droplet shape and size (Ref 23,24,25,26,27). The well-known Bashforth and Adams (Ref 27) tables (ADSA—axisymmetric drop shape analysis) can be used to determine the interfacial tension and the contact angle based on the experimental drop profile. (The use of these tables is limited to drops of a certain size and θ > 90°.) These tables were generated from sessile drop profiles for different values of surface tension and curvature radius at the apex of the drop. Malcolm and Elliott (Ref 26) implemented a semiempirical method to estimate the surface tension based on the total height (H) and maximum diameter (D) of a sessile drop, designated as axisymmetric drop shape analysis-height and diameter (ADSA-HD). The advantage of this method is the possibility of application to sessile drops of any contact angle. Moreover, Rio and Neumann (Ref 28) presented a software, to overcome the deficiencies of the numerical approaches, called axisymmetric liquid–fluid interface (ALFI) which uses more efficient algorithms. In this ALFI software, the theoretical Young–Laplace curves are obtained by integrating the Laplace equation for recognized values of surface tension and drop curvature at the apex. In this way, the procedure proposed by Bashforth and Adams becomes extended and automatic.
The conventional “sessile drop” method is probably the most used technique in the study of solid–liquid interfaces (Ref 1, 11, 23, 25, 29), due to its simplicity and ease of use. Arenas et al. (Ref 1) measured the contact angles of four Sn-based alloys, with and without the use of fluxes, on Cu substrates using the “sessile drop” method. Contact angles between 10° and 30° were obtained using rosin-based fluxes (RMA and RA). Higher values (θ = 30°-40°) were obtained for wetting under vacuum without fluxes. Amore et al. (Ref 2) studying the behavior of the Sn-Cu system on two different metal substrates (Cu and Ni), under high vacuum conditions and for temperatures up to 300 °C, showed a better wettability on the Cu substrate than on Ni substrate, with θ = 40° and θ = 90°, respectively. Zang et al. (Ref 18) investigated the wetting behavior of Sn-3.0Ag-0.5Cu solder, on Cu and Ni substrates under an Ar atmosphere at 250 °C, obtaining a contact angle θ = 29° on Cu substrate and θ = 37° on Ni substrate.
The conventional “sessile drop” method is based on the analysis of drop profile over a substrate (Ref 26), as schematically shown in Fig. 1. Although it is experimentally a simple method, it is necessary to keep in mind that both the working temperature and atmosphere as, also, the composition of the substrate will influence the wettability, the scattering characteristics and the interfacial morphology (Ref 24, 27). In this way, the measurement of the contact angle, for the purpose of this work, involves the control of a number of parameters that affect the wetting behavior of the solder.
Good mechanical adhesion of a solder joint is achieved by the intermetallic layers obtained from the reaction between the solder and the PCB (printed circuit board) substrate. The presence of a superficial oxide layer, at the substrate pad or the liquid solder ball, has a great influence on the wettability and soldering reliability. In industrial production, formation of oxide layers is usually prevented by the use of a flux. A better knowledge of the physical mechanisms of soldering in the presence of oxide layers may help to improve the reflow soldering quality (Ref 30, 31).
Since the type of the surface finish play a crucial role (Ref 2, 30,31,32,33,34,35) in the wetting properties, in this work we studied the surface finish generally used in commercial PCB, namely OSP, NiAu and Sn (Ref 36). The aim of this work is the study and characterization of the wetting behavior of the SAC405 solder paste by measuring the solder contact angle (by the “sessile drop” method) on different surface finish types and to obtain the corresponding surface tension. The effect of superficial oxides on the solder wetting was, also, evaluated. The experimental alloy surface tension, achieved using the experimental contact angle values, will be compared and validated with analytical surface tension obtained by computational methods based on the Butler’s model.
Experimental Procedures
Due to the excellent performance of the SAC405 solder paste (Sn4.0Ag0.5Cu, in wt.%) it is widely used in electronics manufacturing and so was the selected solder for this study. The wetting behavior was studied on three different commercial PCB surface pad finish types: Sn, OSP and NiAu, cut into pieces of 20 × 20 mm2, and used as substrates in the experiments. Table 1 shows the composition and layer structure of the three different FR4 (glass-reinforced epoxy laminate material) substrates used in the wettability tests.
From the obtained solder paste bubble over the PCB images, the contact angle, at different temperatures (Fig. 1), was obtained. For each substrate/PCB finishing type, the wettability tests were performed as a function of temperature, for two different atmospheric conditions: vacuum and inert atmosphere. The used experimental apparatus was presented in a previous work (Ref 10). For the wetting measurements, a primary heating step, up to 170 °C, was used to reach the internal pressure of 1 × 10−5 mbar (~ 1-2 °C/min). In this first step, the solder flux is activated, cleaning the solder paste and pad surfaces. Then, the temperature is increased in the selected atmosphere (vacuum or inert). The flux evaporation during the heating cycle was studied by simultaneous differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) with the experimental conditions presented in Table 2. This study (simultaneous DSC/TGA equipment, SDT2960 from TA Instruments) allows to follow and quantify the solder weight changes as a function of the imposed heating rate.
After the extraction of the droplet line (contact angle) for the different studied conditions, the solder surface tension was calculated using the ADSA-HD analysis given by Rio and Neumann (Ref 28).
Surface Tension Calculation
In the Butler’s model, the surface is assumed as an additional thermodynamic phase, in equilibrium with the bulk (Ref 37,38,39). Considering a regular solution model approximation, the surface tension of a binary and ternary liquid alloys can be calculated as (Ref 39):
where R, T, σi and Si are the gas constant, absolute temperature, the surface tension of pure components and the surface area, respectively. X s i and X b i correspond to the composition of the surface and of the bulk phase for the component i, respectively. \(G_{i}^{{{\text{xs}},{\text{s}}}} \left( {T,X_{{j\left( {j = 2,3} \right)}}^{\text{s}} } \right)\) and G xs,b i (T, X b j( j = 2,3) ) are the partial excess Gibbs energies of a component i in the surface phase and in the bulk phase, respectively. Both free energies are given as functions of temperature (T) and composition of the surface (Xs) and bulk phase (Xb).
The surface area of component i (Si) is calculated using the Avogadro’s number (No), the atomic mass (Mi) and the density (ρi) (Ref 40) as follows:
The excess energy term of a component i can be derived from the standard thermodynamic relation, in the form (Ref 28):
where δij is Kronecker’s symbol.
Assuming that the alloy free energy is always proportional to the number of interactive contacts between neighboring atoms, it is possible correlate the respective coordination numbers in the surface layer and in the bulk phase:
where β is the ratio between the two coordination numbers, i.e., a parameter describing the reduced coordination in the liquid phase. In the present work, β was assumed as 0.75 (Ref 41, 42).
The excess Gibbs energy of the Ag-Cu-Sn ternary subsystem is calculated combining the corresponding values of the Ag-Cu, Ag-Sn and the Cu-Sn (Ref 34, 40, 43) binary subsystems, with an additional ternary contribution (xi xj xk L123), in the form:
In order to validate the as-referred surface tension calculations, we also performed the theoretical surface tension calculations based on Butler’s equation, by using the SURDAT program (Ref 41) (which has a library containing data of the surface tensions and densities for an extensive range of temperatures and concentrations/solder compositions).
Then, the surface tensions calculated from Butler’s model and the experimental ones (using the measured contact angles obtained by the sessile drop method inn Eq 1) will be compared for the three different substrates and the two atmospheric conditions used. In this way, this simple and rapid approach for the surface tension determination based on the sessile drop method becomes validated.
Results and Discussion
Effect of the Heating Rate on the Solder Paste Flux Evaporation
The solder paste used in this study (SAC405) consists of a mixture of microscopic solder balls, flux, activator and solvents. Table 2 and Fig. 2 present the weight change, in the solder solid state range, as a function of the heating rate.
From Fig. 2(a), it is seen that the flux evaporation (total solder paste weight change) is more efficient using a low heating rate. According to these results, the influence of the flux on the soldering process is reduced/favored by using a low heating rate. Accordingly, in the present work, the heating rate used on the wettability tests, during the solder melting range, was lower than the 5 °C/min, thereby ensuring the total solder flux evaporation before the solder melting starts. The fluxes activation is time dependent and consequently varies with the heating rate as shown in Fig. 2(b). On the vacuum atmosphere experiments the flux is temperature activated, up to 170 °C, cleaning the solder balls and the pad surface and then evaporates.
Wettability Properties of SAC405 Solder Past
The measured contact angles of the lead-free solder SAC405 for the three different substrates are shown in Fig. 3(a) for the measurements taken in high vacuum atmosphere (~ 1 × 10−5 mbar) and in Fig. 3(b) for the measurements taken in an inert atmosphere (~ 100 mbar). Two tests were done, for each configuration, with a θ scatter lower than ~ 2°. The contact angle (θ) for NiAu substrate was not possible to measure, above 230 °C, because the solder fully spreads on the pad substrate.
As expected, these results reveal that the contact angle value depends on the temperature and on the surface pad finishing. Besides this dependence, the present study shows that the atmosphere used also affects the contact angle, for the same solder/substrate combination. Moreover, the obtained results show that, regardless of the atmosphere used in the tests, among the three types of substrates studied, the contact angle values obtained for the Sn pad are always much higher than for the other substrates. Indeed, a significantly higher contact angle was obtained for the tests performed in an inert atmosphere, specially at the initial melting temperature (217 °C). Moreover, the differences in the θ values, between Sn pad and the others surfaces, are much higher for the tests performed in an inert atmosphere. This effect is probably related to surface oxidation, due to the high tendency of Sn to oxidize (Ref 44). Although the experimental conditions used in the first heating step are favorable to the almost total evaporation of the solder flux, the observed differences in the contact angles, for the two atmospheres used, as also the presence of some fluctuations (with temperature), especially in the tests made in inert atmosphere, indicate some level of oxidation, i.e., the presence of oxide layers. Indeed, among the pad finishes under study, the Sn is the most sensitive to oxidation and is the one that show higher θ angle at the initial melting temperature. Moreover, as the temperature increases, the differences in the θ values, between the different surface types, start to decrease. This study shows the importance of the contact angle determination and the effect of the surface condition on the solder wetting behavior and consequently on the final soldering quality.
The results shown in Fig. 3(a) and (b) present some variations of the contact angle value evolution with the temperature. The contact angle decrease, observer at 222 and 245 °C (seen in Fig. 3a and b), might be related to the presence of oxide layers at the pad and/or at the solder liquid surfaces. Indeed, the observed decrease on the θ value is probably related to the oxide layer break (at the solder liquid or at the pad surface), as also have been observed by other researchers (Ref 45, 46).
In Fig. 4 is shown the difference in the contact angle (Δθ = θi − θv) obtained for the experiments performed in inert (θi) and vacuum atmosphere (θv) for the three tested surface finishes, at 220 and 230 °C. It is clear that these differences in the contact angle strongly decrease as the temperature increases, as also the contact angle value, independently of the pad finish. Moreover this behavior, differences in the contact angle between the pads type finish are observed in the range of tested temperatures. All these behaviors, namely contact angle versus temperature, contact angle versus used atmosphere and, independently of the atmosphere and temperature, the different contact angle values for the three studied pads can be attributed to the presence of oxide barriers at the solder liquid bubble (that will break at lower T) and/or pad surface (that will break at higher T).
In fact, Protsenko et al. (Ref 45) showed that, at low temperatures, the oxide films that form in the liquid (solder) or solid (substrate) are the main factors affecting the process of wetting. For this type of lead-free solder, with a high Sn content in its composition, it is expected that a protective oxide layer will be formed when heated to a liquid phase (Ref 30, 47), due essentially to the chemical nature of Sn. Indeed, our results indicate that if the temperature is further increased, this protective layer breaks and the solder spreads (as seen in Fig. 3a and b), probably due to the break of the oxide protective layer. Since the contact angle behavior with temperature observed for the NiAu pad finish tested in an inert atmosphere (Fig. 3b), does not show the second transition (higher temperature- related to the break of the oxide at the pad surface) it indicates that this type of substrate is less sensitive to surface oxidation and, so, only the first transition, corresponding to the liquid solder surface oxide breaking, was detected. Indeed, this effect of oxidation in an inert atmosphere also explains the higher temperatures that were observed for the solder start spreading, mainly for NiAu and OSP substrates, as also reported by Görlich et al. (Ref 30). Then, we can conclude that the higher initial contact angle observed in inert atmosphere conditions is due to a thicker superficial oxidation film. Furthermore, this oxide layer, present at liquid solder and/or substrate surfaces, acts as a barrier to the wetting process, preventing the solder from spreading and properly reacting with the substrate, corresponding to higher contact angle values. This observation allows us to conclude that the initial surface pad condition plays an important role in the solder wettability behavior.
Surface Tension: Comparison Between Experiment and Modeling/Theoretical
The theoretical surface tension, obtained for temperatures between the solder melting point (Tm) and 650 °C, was determined for different SAC type alloys and also for pure Sn, for comparison, and the results are shown in Fig. 5. Also in Fig. 5 are present the obtained values of the surface tension of SAC405 on NiAu substrate (using the ADSA-HD analysis) together with the experimental values. These results were obtained using the same experimental device and procedure used for contact angle determination (Fig. 3).
The results show that (1) the surface tension decreases as the Sn content increases; (2) the change in the surface tension due to the increase in Ag, for the same Cu content, is very small; (3) the experimental data, for the surface tension, are in good agreement with the calculations. Taking into account the experimental systematic errors (measurement procedure and the accuracy and repeatability of the profile acquisitions), the error associated with the surface tension values was estimated to be less than 5%.
Conclusions
The contact angle of lead-free SAC405 solder paste as a function of temperature was measured using three different surface finishes (NiAu, OSP and Sn), by the “sessile drop” method, in two atmospheric conditions (vacuum and inert atmosphere). The lowest contact angle was obtained for NiAu and OSP substrates, indicating a solder better wettability, for both types of tested atmospheres.
The variation of the solder surface tension with temperature was calculated, based on the ADSA-HD method, using the solder contact angle experimentally obtained. A good correlation between experimental and calculated solder surface tension was obtained.
Experiments performed in vacuum and inert atmosphere showed that the presence of superficial oxide films strongly affect the solder wettability. Differences in the contact angle higher than 10° were found, between experiments performed in vacuum and inert atmosphere, for the same pad finish. These differences strongly decrease as the temperature increases. From the behavior of the contact angle with temperature, for the different pads and the two used atmospheres the breaking temperatures of the surface oxide layer at the solder bubble and at the pad were determined and explained. Firstly, the oxide layer breaks on liquid solder surface and later, i.e., at higher temperatures, on the substrate surface.
References
M.F. Arenas and V.L. Acoff, Contact Angle Measurements of Sn-Ag and Sn-Cu Lead-Free Solders on Copper Substrates, J. Electron. Mater., 2004, 33(12), p 1452–1458
S. Amore, E. Ricci, G. Borzone, and R. Novakovic, Wetting Behaviour of Lead-Free Sn-Based Alloys on Cu and Ni Substrates, Mater. Sci. Eng., A, 2008, 495(1–2), p 108–112
V. Vuorinen, T. Laurila, H. Yu, and J.K. Kivilahti, Phase Formation Between Lead-Free Sn-Ag-Cu Solder and Ni(P)/Au Finishes, J. Appl. Phys., 2006, 99(2), p 023530
C. Leinenbach, F. Valenza, D. Giuranno, H.R. Elsener, S. Jin, and R. Novakovic, Wetting and Soldering Behavior of Eutectic Au-Ge Alloy on Cu and Ni Substrates, J. Electron. Mater., 2011, 40(7), p 1533–1541
T. Chellaih, G. Kumar, and K.N. Prabhu, Effect of Thermal Contact Heat Transfer on Solidification of Pb-Sn and Pb-Free Solders, Mater. Des., 2007, 28(3), p 1006–1011
C.-T. Lin and K.-L. Lin, Contact Angle of 63Sn-37Pb and Pb-Free Solder on Cu Plating, Appl. Surf. Sci., 2003, 214(1-4), p 243–258
L. Boinovich and A. Emelyanenko, Wetting and Surface Forces, J. Colloid Interface Sci., 2011, 165(2), p 60–69
F. Guo, S. Choi, J.P. Lucas, and K.N. Subramanian, Effects of Reflow on Wettability, Microstructure and Mechanical Properties in Lead-Free Solders, J. Electron. Mater., 2000, 29(10), p 1241–1248
J. Lee, S. Chen, H. Chang, and C. Chen, Reactive Wetting Between Molten Sn-Bi and Ni Substrate, J. Electron. Mater., 2003, 32(3), p 13–17
C. Gonçalves, H. Leitão, C.S. Lau, J.C. Teixeira, L. Ribas, S. Teixeira, M.F. Cerqueira, F. Macedo, and D. Soares, Wetting Behaviour of SAC305 Solder on Different Substrates in High Vacuum and Inert Atmosphere, J. Mater. Sci.: Mater. Electron., 2015, 26(7), p 5106–5112
V.H. López and A.R. Kennedy, Flux-Assisted Wetting and Spreading of Al on TiC, J. Colloid Interface Sci., 2006, 298(1), p 356–362
K.N. Prabhu, Reactive Wetting, Evolution of Interfacial and Bulk IMCs and Their Effect on Mechanical Properties of Eutectic Sn-Cu Solder Alloy, Adv. Colloid Interface Sci., 2011, 166(1–2), p 87–118
G. Humpston and D.M. Jacobson, Principles of Soldering, ASM International, Ohio, 2004
N. Eustathopoulos, M.G. Nicholas, and B. Drevet, Wettability at High Temperatures, Pergamon, Oxford, 1999
J.A. Warren, W.J. Boettinger, and A.R. Roosen, Modelling Reative Wetting, Acta Mater., 1998, 46(9), p 3247–3264
D. Bonn, J. Eggers, J. Indekeu, J. Meunier, and E. Rolley, Wetting and Spreading, Rev. Mod. Phys., 2009, 81(2), p 739–805
E.E.M. Noor, N.M. Sharif, C.K. Yew, T. Ariga, A.B. Ismail, and Z. Hussain, Wettability and Strength of In-Bi-Sn Lead-Free Solder Alloy on Copper Substrate, J. Alloy. Compd., 2010, 507(1), p 290–296
L. Zang, Z. Yuan, H. Xu, and B. Xu, Wetting Process and Interfacial Characteristic of Sn-3.0Ag-0.5Cu on Different Substrates at Temperatures Ranging From 503 K to 673 K, Appl. Surf. Sci., 2011, 257(11), p 4877–4884
F. Gnecco, E. Ricci, S. Amore, D. Giuranno, G. Borzone, G. Zanicchi, and R. Novakovic, Wetting Behaviour and Reactivity of Lead Free Au-In-Sn and Bi-In-Sn Alloys on Copper Substrates, Int. J. Adhes. Adhes., 2007, 27(5), p 409–416
R. Voitovitch, A. Mortensen, F. Hodaj, and N. Eustathopoulos, Diffusion-Limited Reactive Wetting: Study of Spreading Kinetics of Cu-Cr Alloys on Carbon, Acta Mater., 1999, 47(4), p 1117–1128
G.W. Liu, F. Valenza, M.L. Muolo, G.J. Qiao, and A. Passerone, Wetting and Interfacial Behavior of Ni-Si Alloy on Different Substrates, J. Mater. Sci., 2009, 44(22), p 5990–5997
L. Zang, Z. Yuan, H. Zhao, and X. Zhang, Wettability of Molten Sn-Bi-Cu Solder on Cu Substrate, Mater. Lett., 2009, 63(23), p 2067–2069
J. Drelich, C. Fang, and C.L. White, Measurement of Interfacial Tension in Fluid–Fluid Systems, Encycl. Surf. Colloid Sci., 2002, 3, p 3158–3163
L. Zang, Z. Yuan, H. Xu, and B. Xu, Wetting Process and Interfacial Characteristics of Sn–3.0Ag–0.5Cu on Different Substrates at Temperatures Ranging From 503 K to 673 K, Appl. Surf. Sci., 2011, 257, p 4877–4884
N. Sobczak, A. Kudyba, R. Nowak, W. Radziwill, and K. Pietrzak, Factors Affecting Wettability and Bond Strength of Solder Joint Couples, Pure Appl. Chem., 2007, 79(10), p 1755–1769
J.D. Malcolm and D.D. Elliot, Interfacial Tension from Height and Diameter of a Single Sessile Drop or Captive Bubble, J. Chem. Eng., 1980, 58, p 151
J.C. Bashforth and F. Adams, An Attempt to Test the Theory of Capillary Action, Cambridge University Press, Cambridge, 1892
O. Río and A. Neumann, Axisymmetric Drop Shape Analysis: Computational Methods for the Measurement of Interfacial Properties from the Shape and Dimensions of Pendant and Sessile Drops, J. Colloid Interface Sci., 1997, 196(2), p 136–147
J.J. Sundelin, S.T. Nurmi, T.K. Lepistö, and E.O. Ristolainen, Mechanical and Microstructural Properties of SnAgCu Solder Joints, Mater. Sci. Eng., A, 2006, 420(1–2), p 55–62
J. Görlich, C. Oberdorfer, D. Baither, G. Schmitza, C. Reinke, and U. Wilke, The Role of Oxide Layers in Solder Joints, J. Alloy. Compd., 2010, 490, p 336–341
M. Ramireza, L. Henneken, and S. Virtanen, Oxidation Kinetics of Thin Copper Films and Wetting Behaviour of Copper and Organic Solderability Preservatives (OSP) with Lead-Free Solder, Appl. Surf. Sci., 2011, 257, p 6481–6488
J. Li, J. Karppinen, T. Laurila, and J.K. Kivilahti, Reliability of Lead-Free Solder Interconnections in Thermal and Power Cycling Tests, IEEE Trans. Compon. Pack Technol., 2009, 32(2), p 302–308
J. Han, H. Chen, and M. Li, Role of Grain Orientation in the Failure of Sn-Based Solder Joints Under Thermomechanical Fatigue, Acta Met. Sin., 2012, 25(3), p 214–224
K. Kanlayasiri, M. Mongkolwongrojn, and T. Ariga, Influence of Indium Addition on Characteristics on Sn-03-Ag-0.7Cu Solder Alloy, J. Alloys Compd., 2009, 485(1/2), p 225–230
J.-W. Yoon, B.-I. Noh, B.-K. Kim, C.-C. Shur, and S.-B. Jung, Wettability and Interfacial Reaction of Sn-Ag-Cu/Cu and Sn-Ag-Ni/Cu Solder Joints, J. Alloys Compd., 2009, 486(1/2), p 142–147
L. Zhang, S.B. Xue, G. Zeng, L.L. Gao, and H. Ye, Interface Reaction Between SnAgCu/SnAgCuCe Solders and Cu Substrate Subjected to Thermal Cycling and Isothermal Aging, J. Alloy. Compd., 2012, 510(1), p 38–45
T. Gancarz, Density, Surface Tension and Viscosity of Ga-Sn Alloys, J. Mol. Liq., 2017, 241, p 231–236
A. Zdziennicka, K. Szymczyk, J. Krawczyk, and B. Janczuk, Some Remarks on the Solid Surface Tension Determination From Contact Angle Measurements, Appl. Surf. Sci., 2017, 405, p 88–101
H. Tavana and A.W. Neumann, Recent Progress in the Determination of Solid Surface Tensions from Contact Angles, Adv. Coll. Interface. Sci., 2007, 132, p 1–32
V. Sklyarchuka, Y. Plevachuka, I. Kabanb, and R. Novakovićc, Surface Properties and Wetting Behaviour of Liquid Ag-Sb-Sn Alloys, J. Min. Metall. Sect. B Metall., 2012, 48(3), p 443–448
Z. Moser, W. Gasior, A. Debski, and J. Pstrus, SURDAT—Database of Physical Properties of Lead-Free Solders, J. Min. Metall. Sect. B Metall., 2007, 43(2), p 125–130
T. Tanaka, K. Hack, T. Lida, and S. Hara, Application of Thermodynamic Databases to the Evaluation of Surface Tensions of Molten Alloys, Salt Mixtures and Oxide Mixtures, Z. Metall., 1996, 875, p 380–389
J. Lee, W. Shimoda, and T. Tanaka, Surface Tension and its Temperature Coefficient of Liquid Sn_X (X = Ag, Cu) Alloys, Mater. Trans., 2004, 45(9), p 2864–2870
T. Hetschel, K. Wolter, F. Phillipp, R.B. Gmbh, Wettability Effects of Immersion Tin Final Finishes with Lead Free Solder, in Electronics System-Integration Technology Conference, 2008. ESTC 2008, 2nd (2008)
P. Protsenko, A. Terlain, V. Traskine, and N. Eustathopoulos, The Role of Intermetallics in Wetting in Metallic Systems, Scr. Mater., 2001, 45, p 1439–1445
O. Dezellus and N. Eustathopoulos, Fundamental Issues of Reactive Wetting by Liquid Metals, J. Mater. Sci., 2010, 45(16), p 4256–4264
K. Suganuma, Advances in Lead-Free Electronics Soldering, Curr. Opin. Solid State Mater. Sci., 2001, 5, p 55–64
Acknowledgments
This work is supported by FCT with the reference Project UID/EEA/04436/2013, Compete 2020 with the Code POCI-01-0145-FEDER-006941 and project in co-promotion No. 002814/2015 (iFACTORY 2015-2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is an invited submission to JMEP selected from presentations at the Symposium “Interface Design and Modelling, Wetting and High-Temperature Capillarity,” belonging to the topic “Processing” at the European Congress and Exhibition on Advanced Materials and Processes (EUROMAT 2017), held September 17-22, 2017, in Thessaloniki, Greece, and has been expanded from the original presentation.
Rights and permissions
About this article
Cite this article
Soares, D., Leitão, H., Lau, C.S. et al. Effect of the Soldering Atmosphere on the Wettability Between Sn4.0Ag0.5Cu (in wt.%) Lead-Free Solder Paste and Various Substrates. J. of Materi Eng and Perform 27, 5011–5017 (2018). https://doi.org/10.1007/s11665-018-3419-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-018-3419-2