Abstract
In this paper, microstructure evolution and failure analysis of the aluminum-copper interface of cathode conductive heads during their use were studied. The interface morphologies, compositions, conductivity and mechanical properties were investigated and analyzed. Obvious corrosion was found on the surface of the contact interface, which was more prevalent on an Al matrix. The crack increased sharply in the local metallurgical bonding areas on the interface, with the compound volume having no significant change. The phase transformation occurred on the interface during use, which was investigated using the elemental composition and x-ray diffraction pattern. The microhardness near the interface increased accordingly. An obvious electrical conductivity decrease appeared on the Al/Cu interface of the cathode conductive head after use over a specific time interval. Therefore, the deterioration of the microstructures and corrosion are the primary factors that affect the electrical conductivity and effective bonding, which will lead to eventual failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Al/Cu bimetallic composite materials not only have the properties of high electrical and thermal conductivity of copper but also have the lightweight and economic advantages of aluminum (Ref 1,2,3,4,5,6). They have very important applications in circuit transmission, refrigeration (air conditioners, refrigerators, compressors, etc.), hydrometallurgical (cathode conductive heads) and other fields (Ref 7,8,9,10,11,12).
Previous studies have suggested that friction stir welding (Ref 13,14,15) and explosive welding (Ref 16) methods can produce an Al/Cu bimetal structures with reliable welding quality (Ref 17,18,19,20,21). Xue et al. (Ref 17, 18) investigated friction stir welding of aluminum and copper plates by offsetting the tool to the aluminum side. It indicated that excellent metallurgical bond was formed on the Al/Cu interface with the formation of a thin, continuous and uniform Al/Cu intermetallic compound (IMC) layer. Tan et al. (Ref 19) found that nanoscale intercalations appeared in the Al/Cu friction stir welded joint and the interface structures were subsequently confirmed as Al4Cu9, Al2Cu3 and Al2Cu. Excellent metallurgical bond between Al and Cu led to preferable tensile and bending strength. Saeid et al. (Ref 20) studied the feasibility of friction stir welding of Al and Cu in a lap configuration and concluded that a complex microstructure with several IMCs, such as Al2Cu, AlCu and Al4Cu9, was observed. Acarer (Ref 21) and Hoseini Athar et al. (Ref 22) investigated the microstructure and bonding strength of the Al/Cu explosive welding joint. They found that the joint has an acceptable bonding strength and the interface is plane and rough, with a partially intermetallic phase.
An Al/Cu conductive head is the core component of the hydrometallurgical cathode plate. An overwhelming majority of cathode conductive heads were produced by the explosive welding method. Under the action of a large deformation and vibrational wave, the explosive welding method can produce an Al/Cu composite structure with reliable welding quality (Ref 23). Honarpisheh et al. (Ref 24) investigated an annealing treatment on the interfacial properties of an explosive-welded Al/Cu/Al multilayer. It indicated that brittle and low-ductility intermetallic compounds would form during heating. Asemabadi et al. (Ref 25) studied the cold rolling influence on the mechanical properties of explosive-welded Al/Cu bimetal. They found that the nucleation and propagation of microcracks were accelerated under tension and plastic deformation due to the brittle nature of the intermetallic compounds on the joining interface. In view of the corrosive atmosphere and repeated hot and cold cycle conditions, the conductive ability and bonding strength of Al/Cu interface would be weakened in the process of using as the conductive devices. The conductivity of the Al/Cu interface directly influences the current consumption, while the bonding strength and corrosion resistance determine the life of the conductive head. Therefore, the failure process of the Al/Cu interface of the cathode conductive head is important to investigate.
The aim of this study was to analyze the failure mechanism and evaluate the influence of the corrosion and hot/cold thermal cycle on the interface of the Al/Cu cathode conductive head. To achieve this objective, Al/Cu cathode conductive head samples that were unused and used for different lengths of time underwent microstructural observation, compositional analysis of their interface organization, shear strength tests and hardness distribution measurements. To determine the rupture mode and types of IMCs, the fracture surfaces were examined and studied by SEM and XRD.
Experimental Samples and Analysis Methods
Experimental Samples
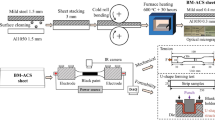
The Al/Cu cathode conductive head materials were commercially pure Cu (99.9 wt.%) and Al (99.6 wt.%). The external dimensions of the specimen were 50 cm in length, 25 cm in width and 25 cm in height, with Al (15 cm) and Cu (10 cm) in this study (Fig. 1). The interface contact area was 50 × 25 cm2.
Three samples with different service time were used to investigate the microstructure evolution and failure analysis. The samples were used for 0, 240 and 480 h, with a current density of 1440 A/3.1-3.4 V and without electricity every 24 h, respectively. Moreover, the cathode conductive head was placed 350-400 mm above the electrolyte rather than being soaked in the electrolyte. The pH of the electrolyte was approximately 0.7-1. The environmental temperature when the samples were working was 313-333 K.
Analysis Methods
The welded joints were cut for section view, in which the structures and compositions were examined by scanning electron microscopy (SEM, JEOL, JSM-6700) with energy-dispersive spectroscopy (EDS). The mechanical properties of the joints were evaluated by tensile shear tests (Instron 5880). The phase composition of the fracture surfaces was investigated using 7000S x-ray diffraction (XRD) instrument with Cu Kα radiation. The size of the specimen samples for the microstructure observations and shear tests is shown in Fig. 1. The hardness was determined on a Brinell hardness tester. The interface conductivity was tested by an eddy current conductivity meter (FD102).
Results and Discussion
Surface and Interface Morphology
Surface Morphology
The comparative macroscopic morphologies of the cathode conductive head before and after use for different periods are presented in Fig. 2. It can be observed that interface corrosion with different degrees occurred on the surfaces of the cathode conductive head. The electrochemical corrosion and chemical corrosion occurred at the same time near the interface during the use of the cathode conductive head. The larger electrode potential difference between Al and Cu caused serious electrochemical corrosion. The corrosion on the Al matrix near the interface was more serious, which can be demonstrated by the microstructure (Fig. 2) and resulted from the Al matrix consumption as a negative electrode due to the lower electrode potential. The cracks in the contact interface increased as the usage time increased. Therefore, the effective bond area was reduced, which would facilitate the cathode conductive head failure.
Interfacial Microstructures
The explosion welding method is a solid process, which uses thermal shock and large plastic deformation to accelerate the interface reaction and diffusion, forming the metallurgical bond between metals. The typical interface morphology of Al/Cu produced by explosive welding is shown in Fig. 3. The entire interface has two distribution patterns, with one as the direct contact region with no obvious color gradient area. The line scan component analysis showed an ~ 5-μm-width transition layer with an Al element drop off and gradual Cu element increase from the Al matrix to the Cu matrix, as shown in Fig. 3(c). The other transition pattern is a spiral structure between the compound and pure metals. The line scan component analysis showed an ~ 110-μm-width transition layer with the element content of Al and Cu relatively fixed, as shown in Fig. 3(b).
The microstructures of the cathode conductive head Al/Cu interface of the failure sample are shown in Fig. 4. It was observed that there are two major changes that appeared on the interface of the failure sample, compared with the sample before use, as shown in Fig. 3(a). One has significantly increased cracks in the interface compounds. The partial compounds were broken into small fragments, as shown in Fig. 4(c). The other is the excessive corrosion that occurred on the edge of the contact interface. The cathode conductive head is working in an acidic atmosphere. In the process of electrochemical corrosion, Al, as the cathode, participated in the reaction due to the lower electrode potential. The Al metal near the interface was corroded away, and a large area of the discontinuous Al metal appeared. As a result, it was exposed to more corrosion in comparison with the copper side. The corrosion depth was approximately 2 mm, as shown in Fig. 4(b). Figure 4(d) is the plan view of the corrosion region. The component analysis showed that a layer of impurities was attached to the surface.
Phase Evolution After Failure
The comparative interfacial microstructures and the composition analysis of the cathode conductive head Al/Cu interface before and after use for different periods are shown in Fig. 5. The microstructure observation samples were cut from the center of the sample. It was observed that the thickness of the transition layer in different samples did not significantly increase. However, the cracks increased sharply in the local compound regions on the interface as the usage time increased. There was a massive crack that appeared in the local metallurgical bond areas on the interface after use for 480 h, as shown in Fig. 5(c). EDS spot scan analysis was used to determine the compound. On the interface of the unused joint, it indicated that the majority of the interface regions were the solid solution phases, which were near the matrix materials in the region of point 01 and 03 in Fig. 5(a), and the atom ratio of Al/Cu in the region of point 02 is close to 4:9 (possible phase Al4Cu9) in the center of the compound. On the interface of the joint that was used for 240 h, the majority of the compound regions were intermetallics (possible phase in the region of point 05 is Al2Cu and point 06 is AlCu) and a small number of solid solution phase remained, as shown in Fig. 5(b). Based on the atom ratios in the region of Fig. 5(c), the compound regions on the interface of the joint used for 480 h were all intermetallics of Al2Cu and AlCu. It can be speculated that the phase transition occurred during the cathode conductive head usage.
In order to further determine the phase transformation on the Al/Cu interface of the cathode conductive head, XRD analyses were carried out to detect the interface phases on the fracture surface. The results are shown in Fig. 6, and the corresponding fracture surfaces are shown in Fig. 8(b-d). It was observed that the phase type changed during the cathode conductive head use. The only weak diffraction peak of Al4Cu9 was found in the unused sample. The diffraction peak of Al2Cu and AlCu was found in the sample used for a different time. It can be speculated that the solid solution phase and Al4Cu9 in the unused joint were transformed into Al2Cu and AlCu in the process of use.
The Al/Cu binary phase diagram indicates that there are five equilibrium phases, Al2Cu, AlCu, Al3Cu4, Al2Cu3 and Al4Cu9. In this study, only Al2Cu, AlCu and Al4Cu9 were found. The effective heat of formation (EHF) model has been used to predict the formation of the first phases in many binary systems (like M-Al). The calculated results indicated that the Al2Cu has the maximum negative EHF, which was expected to form first in the diffusion zone as a result of thermodynamics combined with kinetic theory (Ref 26). The effective Gibbs free energy change of formation (ΔG ei) replaced the effective heat of formation, which can more reasonably predict the formation sequence (Ref 27). It suggested that the effective Gibbs free energy change of formation (ΔG ei) of the Cu/Al intermetallics was ΔG eAl2Cu < ΔG eAl4Cu9 < ΔG eAlCu in the thermodynamic equilibrium (Ref 28).
However, during explosion welding, phase formation on the Al/Cu interface is a non-equilibrium process. The materials near the interface have simultaneously encountered high temperature and severe plastic deformation. The atom diffusion was activated not only by temperature but also by the severe plastic deformation. The non-equilibrium microstructure, such as a supersaturated solid solution and Al4Cu9 phase, appeared in the system far from the thermodynamic equilibrium state. In the process of subsequent electricity use, the phase transitions occurred due to the interface temperature rise of the resistance heating, such as with saturated solid solutions of Al(Cu)/Cu(Al) + Al4Cu9 → Al2Cu + AlCu. The interface system tends to favor the thermodynamic equilibrium state.
Interface Microhardnesses, Shear Strength and Conductivity
The microhardnesses across the interface were tested. The interface microhardness distributions of different joints are shown in Fig. 7(a). The hardness value increased significantly after use for 240 and 480 h. This is consistent with the transformation of the intermetallics. The corresponding microstructures of the test positions, observed by optical microscopy, are shown in Fig. 7(b-d). The size of the indentation could partly reflect the value of the hardness when the test pressure was consistent.
Figure 8 shows the interface shear strength and morphologies of the fractured surfaces of the joints unused and used for different times. The shear strength decreases along with the usage time until failure. This is primarily due to because of the increased defects and formation of brittle intermetallics on the interface. The morphologies of the fractured surfaces have significant differences. The partial cavity fracture shown in Fig. 8(b) is a typical fracture pattern of the unused joint. With the extension of usage time, the microstructures on the interface transformed into intermetallics, which had higher hardness and brittleness. The fracture pattern changed from a partial cavity fracture to quasi-cleavage fracture (Fig. 6d). The changing characteristics were consistent with the microstructures shown in Fig. 5.
The observable electrical conductivity decrease appeared on the Al/Cu interface of the cathode conductive head after use for a certain time. Figure 9 shows the electrical conductivity change near the interface. An eddy current conductivity meter was used to test the interface conductivity. The conductivities changed from approximately 36.5 MS/m (Al metal) to 30-34 MS/m (interface compound), to 58 MS/m of the Cu metal. After use for 480 h, the conductivity on the Al/Cu interface dropped to 30 MS/m, which would lead to resistance heat generation and effect the cathode conductive head use. The reduction in electrical conductivity could be explained by microstructural factors such as cracking, hard brittle phase and porosity on the interface zone. These defects constrict the current flow across the interface, thus considerably reducing the effective contact area. As a result, the electrical conductivity of the total intermetallic layer decreased.
Interface Failure Mechanism Analysis
The phase transformation and sharply increase in cracks on the Al/Cu interface are the two important reasons for cathode conductive head failure. The microstructures of the Al/Cu interface produced by explosive welding in the unused sample are still in the thermodynamic non-equilibrium state. Depending on the large plastic deformation and extremely fast rise/cooling rate, this non-equilibrium structure has a higher internal energy, which can provide a driving force for nucleation and growth of the intermetallic compound. It would be transformed into an intermetallic in the process of sample usage as conductive devices. The phases of the Al/Cu solid solution and Al4Cu9 were less sensitive to fracture and have lower average densities than the other intermetallic compounds (Ref 29). The volumetric decrease during formation causes internal stress.
On the other hand, the thermal stresses and deformations on the interface exacerbated the crack initiation and propagation in the compounds during use. This led to the sharp increase in the interface crack. The interfacial stress analysis was studied by an interface model between two metals with a large difference in the thermal expansion coefficient when there is a large difference in temperature. The schematic of the Al/Cu interface of the cathode conductive head at room temperature and after the heat expansion is shown in Fig. 10. When the heterogeneous composite structure was heated, expansion deformation was mutually restrained. The heterogeneous interface was subjected to high tensile residual stress, and there was an intense gradient of residual stress when heated because of the different thermal expansion coefficient and mutual constraints. Therefore, the compounds would deform and even fracture, as shown in Fig. 10(b). As a result, the interface intermetallic, with higher hardness and low thermal expansion coefficient, will crack to suit the integral deformation.
Conclusions
The failure of the aluminum-copper cathode conductive head is attributed to the deterioration of microstructure and corrosion on the edge of the contact surface. The main results were obtained as follows:
-
(1)
The corrosion occurred on the surfaces of the cathode conductive head and more seriously on the Al matrix near the interface. The cracks increased sharply in the local compound regions on the interface as the usage time was longer, with the thickness of the compound having no significant change.
-
(2)
With the usage time extended, the phase transformation occurred on the interface. The solid solution phase and Al4Cu9 in the joint before use were transformed into Al2Cu and AlCu during the process of their use. The hardness value increased significantly after usage, which is consistent with the transformation of the intermetallic.
-
(3)
The shear strength and morphologies of the fractured surfaces indicated that the fracture mechanism changed from partial cavity fracture to quasi-cleavage fracture. The obvious electrical conductivity decrease appeared on the Al/Cu interface of the cathode conductive head after used for different times.
References
H.C. Tseng, C. Hung, and C.C. Huang, An Analysis of the Formability of Aluminum/Copper Clad Metals with Different Thicknesses by the Finite Element Method and Experiment, Int. J. Adv. Manuf. Technol., 2010, 49(9-12), p 1029-1036
X.L. Cui, Y.Y. Wu, X.F. Liu, Q.R. Zhao, and G.J. Zhang, Effects of Grain Refinement and Boron Treatment on Electrical Conductivity and Mechanical Properties of AA1070 Aluminum, Mater. Des., 2015, 86, p 397-403
M. Naseri, M. Reihanian, and E. Borhani, Bonding Behavior During Cold Roll-Cladding of Tri-layered Al/Brass/Al Composite, J. Manuf. Process., 2016, 24, p 125-137
F. Hajializadeh and M.M. Mashhadi, Investigation and Numerical Analysis of Impulsive Hydroforming of Aluminum 6061-T6 Tube, J. Manuf. Process., 2015, 20, p 257-273
R.N. Raoelison, D. Racine, Z. Zhang, N. Buiron, D. Marceau, and M. Rachik, Magnetic Pulse Welding: Interface of Al/Cu Joint and Investigation of Intermetallic Formation Effect on the Weld Features, J. Manuf. Process., 2014, 16, p 427-434
R.N. Raoelison, T. Sapanathan, N. Buiron, and M. Rachik, Magnetic Pulse Welding of Al/Al and Al/Cu Metal Pairs: Consequences of the Dissimilar Combination on the Interfacial Behavior During the Welding Process, J. Manuf. Process., 2015, 20, p 112-127
X. Wu, T. Liu, and W. Cai, Microstructure, Welding Mechanism, and Failure of Al/Cu Ultrasonic Welds, J. Manuf. Process., 2015, 20, p 515-524
H.G. Kim, S.M. Kim, J.Y. Lee, M.R. Choi, S.H. Choe, K.H. Kim, J.S. Ryu, S. Kim, S.Z. Han, W.Y. Kim, and S.L. Lim, Microstructural Evaluation of Interfacial Intermetallic Compounds in Cu Wire Bonding with Al and Au Pads, Acta Mater., 2014, 64(2), p 356-366
H. Xu, C. Liu, V.V. Silberschmidt, S.S. Pramana, T.J. White, Z. Chen, and V.L. Acoff, Behavior of Aluminum Oxide, Intermetallics and Voids in Cu-Al Wire Bonds, Acta Mater., 2011, 59(14), p 5661-5673
T.T. Sasaki, R.A. Morris, G.B. Thompson, Y. Syarif, and D. Fox, Formation of Ultra-Fine Copper Grains in Cu Clad Al Wire, Scr. Mater., 2010, 63(5), p 488-491
M.P. Satpathy and S.K. Sahoo, Microstructural and Mechanical Performance of Ultrasonic Spot Welded Al-Cu Joints for Various Surface Conditions, J. Manuf. Process., 2016, 22, p 108-114
S.J. Chen and X.Q. Jian, Microstructure Evolution During Magnetic Pulse Welding of Dissimilar Aluminium and Magnesium Alloys, J. Manuf. Process., 2015, 19, p 14-21
B.T. Gibson, D.H. Lammlein, T.J. Prater, W.R. Longhurst, C.D. Cox, M.C. Ballun, K.J. Dharmaraj, G.E. Cook, and A.M. Strauss, Friction Stir Welding: Process, Automation, and Control, J. Manuf. Process., 2014, 16, p 56-73
P.R. Guru, M.D. Khan, S.K. Panigrahi, and G.D.J. Ram, Enhancing Strength, Ductility and Machinability of a Al-Si Cast Alloy by Friction Stir Processing, J. Manuf. Process., 2015, 18, p 67-74
S. Lazarevic, S.F. Miller, J.J. Li, and B.E. Carlson, Experimental Analysis of Friction Stir Forming for Dissimilar Material Joining Application, J. Manuf. Process., 2013, 15, p 616-624
Y. Aizawa, J. Nishiwaki, Y. Harada, S. Muraishi, and S. Kuma, Experimental and Numerical Analysis of the Formation Behavior of Intermediate Layers at Explosive Welded Al/Fe Joint Interfaces, J. Manuf. Process., 2016, 24, p 100-106
P. Xue, B.L. Xiao, D.R. Ni, and Z.Y. Ma, Enhanced Mechanical Properties of Friction Stir Welded Dissimilar Al-Cu Joint by Intermetallic Compounds, Mater. Sci. Eng., A, 2010, 527, p 5723-5727
P. Xue, D.R. Ni, D. Wang, B.L. Xiao, and Z.Y. Ma, Effect of Friction Stir Welding Parameters on the Microstructure And Mechanical Properties of the Dissimilar Al-Cu Joints, Mater. Sci. Eng., A, 2011, 528, p 4683-4689
C.W. Tan, Z.G. Jiang, L.Q. Li, Y.B. Chen, and X.Y. Chen, Microstructural Evolution and Mechanical Properties of Dissimilar Al-Cu Joints Produced by Friction Stir Welding, Mater. Des., 2013, 51, p 466-473
T. Saeid, A. Abdollah-zadeh, and B. Sazgari, Weldability and Mechanical Properties of Dissimilar Aluminum-Copper Lap Joints Made by Friction Stir Welding, J. Alloy. Compd., 2010, 490, p 652-655
M. Acarer, Electrical, Corrosion, and Mechanical Properties of Aluminum-Copper Joints Produced by Explosive Welding, J. Mater. Eng. Perfor., 2012, 21(11), p 2375-2379
M.M.H. Athar and B. Tolaminejad, Weldability Window and the Effect of Interface Morphology on the Properties of Al/Cu/Al Laminated Composites Fabricated by Explosive Welding, Mater. Des., 2015, 86, p 516-525
S.Y. Chen, Z.W. Wu, K.X. Liu, X.J. Li, N. Luo, and G.X. Lu, Atomic Diffusion Behavior in Cu-Al Explosive Welding Process, J. Appl. Phys., 2013, 113(4), p 044901-044906
M. Honarpisheh, M. Asemabadi, and M. Sedighi, Investigation of Annealing Treatment on the Interfacial Properties of Explosive-Welded Al/Cu/Al Multilayer, Mater. Des., 2012, 37, p 122-127
M. Asemabadi, M. Sedighin, and M. Honarpisheh, Investigation of Cold Rolling Influence on the Mechanical Properties of Explosive-Welded Al/Cu Bimetal, Mater. Sci. Eng. A, 2012, 558, p 144-149
Y.J. Guo, G.W. Liu, H.Y. Jin, Z.Q. Shi, and G.J. Qiao, Intermetallic Phase Formation in Diffusion-Bonded Cu/Al Laminates, J. Mater. Sci., 2011, 46, p 2467-2473
R. Pretorius, A.M. Vredenberg, and F.W. Saris, Prediction of Phase Formation Sequence and Phase Stability in Binary Metal-Aluminum Thin-Film Systems Using the Effective Heat of Formation Rule, J. Appl. Phys., 1991, 70, p 3636-3646
Y.N. Wei, J.L. Li, J.T. Xiong, and F.S. Zhang, Investigation of Interdiffusion and Intermetallic Compounds in Al-Cu Joint Produced by Continuous Drive Friction Welding, Eng. Sci. Tech. Int. J., 2016, 19, p 90-95
M.H.M. Kouters, G.H.M. Gubbels, and O. Dos Santos, Ferreira, Characterization of Intermetallic Compounds in Cu-Al Ball Bonds: Mechanical Properties, Interface Delamination and Thermal Conductivity, Microelectron. Reliab., 2013, 53, p 1068-1075
Acknowledgments
This work is financially supported by the China Postdoctoral Science Foundation-Funded Project (No. 2016M592823), the National Natural Science Foundation of China (No. 51701154), the National Natural Science Foundation of China (No. 51371139), the Pivot Innovation Team of Shanxi Electric Materials and Infiltration Technique (No. 2012 KCT-25), the Key Research and Development Project of Shaanxi Province (No. 2017ZDXM-GY-033), the Scientific Research Project of Shaanxi Provincial Department of Education (No. 17JK0563) and the Scientific Research Foundation of Xi’an University of Technology [Grant No. 101-451115015].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, Y., Luo, Y., Qu, H. et al. Microstructure Evolution and Failure Analysis of an Aluminum–Copper Cathode Conductive Head Produced by Explosive Welding. J. of Materi Eng and Perform 26, 6158–6166 (2017). https://doi.org/10.1007/s11665-017-3055-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-017-3055-2