Abstract
Plasma electrolytic oxidation (PEO) of AZ91 Mg alloys was performed in ZrO2 nanoparticles containing Na2SiO3-based electrolytes. The phase composition and the microstructure of PEO coatings were analyzed by x-ray diffraction and scanning electron microscopy followed by energy dispersive spectroscopy. Pitting corrosion properties of the coatings were investigated using cyclic polarization and electrochemical impedance spectroscopy tests in a Ringer solution. The results showed the better pitting corrosion resistance of the composite coating, as compared to the oxide one, due to the thickened inner layer and the decrease in the surface defects of the composite coating. Also, the PEO process decreased the corrosion current density from 25.06 µA/cm2 in the Mg alloy to 2.7 µA/cm2 in the oxide coating and 0.47 µA/cm2 in the composite coating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnesium is a light metal with 1.74 g/cm3 density. It has low density, high strength to weight ratio, dimensional stability, high thermal conductivity and good recyclability and machining, leading to the use of it and its alloys in various fields such as aerospace, communication, automotive industries and medical equipment (Ref 1, 2).
In recent years, magnesium alloys have been considered as biomaterials due to their good biodegradable behavior and closeness to natural bone mechanical properties, as compared to other metal implants (Ref 3, 4). The low corrosion resistance of magnesium and its alloys are, however, among the limitations of this alloy. So, creating a protective coating against corrosion has been studied in the past decade by various researchers using various techniques such as conversion coatings, anodizing, electrochemical plating, vapor phase deposition processes and organic coatings (Ref 5, 6).
Plasma electrolytic oxidation (PEO) is a method of oxide layer creation on the light metals surface; it is known as the best commercial method for increasing the corrosion resistance of magnesium alloys due to such advantages as cost-effectiveness and environmentally friendly features (Ref 7,8,9,10,11,12). During this process, which takes place at voltages higher than the oxide breakdown voltage, the discharge phenomenon occurs and plasma is created, leading to the growth of an oxide layer. The oxide layer is made of a thin compacted inner layer with low thickness and high adhesion to the substrate, and an outer porous layer due to the presence of discharge channels; the inner layer with high adhesion to substrate leads to the high corrosion resistance of the oxidized layers (Ref 13,14,15,16).
Strengthening corrosion resistant phases is the method of increasing corrosion resistance in PEO oxide layers. Shchukin et al. (Ref 17) used second phase particles in the oxide layer for the first time, by using the PEO method on the Al alloy surface. Further researches have been carried out using the oxide particles of SiO2, CeO2, TiO2, WC and Si3N4 based on the composite oxide layer formation, through the PEO method (Ref 16,17,18). According to the results of the study done by Lim et al. (Ref 19), the addition of CeO2 particles into plasma electrolytic oxidation electrolyte improved the corrosion resistance of PEO coatings obtained in 30 g/L CeO2 particle-containing electrolytes. The results of other studies have also shown that nanoparticles of the electrolyte solution are absorbed on the sample surface under electrophoretic force, increasing the compaction and adhesion of the PEO oxide layer through placement in the common area of the inner layer and the outer layer, as well as the porosities of the latter. The presence of nanoparticles at high temperatures, due to the plasma process, leads to the formation of corrosion resistant phases that can help to increase the corrosion resistance of coatings in the presence of second phase particles. ZrO2 is an oxide particle used by different researchers in order to create a composite oxide layer on the magnesium alloy surface. Liu investigated ZrO2 particle effects on the corrosion behavior of the PEO coating on the AM30 magnesium alloy surface (Ref 20). According to the results, using ZrO2 particles led to increasing the compaction of the inner layers on the magnesium surface. Investigations on the coating cross section obtained in the electrolyte solution of K2ZrF6 also showed that the oxide layer contained an inner compacted and sticky layer to the substrate; the compaction was reinforced due to the presence of oxide particles. Polarization investigation showed that the presence of ZrO2 in the PEO oxide layer led to the improved uniform corrosion behavior of the magnesium alloy. Results obtained by Lee regarding the electrochemical behavior of PEO oxide layers containing ZrO2 nanoparticles on the AZ91 alloy surface indicated the presence of ZrO2 nanoparticles within the coating outer layer and the change of nanoparticles phase from a monoclinic to a tetragonal one; finally, the presence of nanoparticles in the coating led to the improved corrosion behavior (Ref 21). According to the results of the study carried out by Gnedenkov et al. (Ref 22), regarding the fabrication of PEO coatings on the surface of the Mg alloy, using ZrO2 nanoparticles enhanced the morphological characteristics; in particular, the porosity was decreased and the thickness and resistivity were increased up to two orders of the magnitude of the porousless sublayer, in comparison with the base PEO layer. Results of other similar researches show the best effect of ZrO2 on the corrosion properties of PEO coatings (Ref 23, 24).
Results of some other studies show an increase in the corrosion current density of PEO coatings containing nanoparticles (Ref 25,26,27), while some other researches reveal a decrease in the corrosion current density of PEO coatings containing nanoparticles (Ref 16, 17, 19). While a few works have been conducted to study the PEO coating formed in an electrolyte containing ZrO2 nanoparticles on the AZ91 magnesium alloy, in this study, coating was done in a silicate-based electrolyte solution containing ZrO2 nanoparticles using the constant current method; the effects of ZrO2 nanoparticles on the pitting corrosion behavior of the composite coating were investigated using the cyclic polarization technique and electrochemical impedance spectroscopy in a Ringer’s solution to find whether using these particles could have a negative or positive effect on the corrosion behavior of PEO coatings on the AZ91 magnesium alloy; this was done in order to evaluate the application of MgO/ZrO2 composite coatings in manufacturing the magnesium implants.
Materials and Methods
Rectangular samples (10 × 10 × 3 mm3) of the AZ91 magnesium alloy (9.05% Al, 0.65% Zn, 0.21% Mn, 0.02% Si, 0.002% Fe, 0.0005% Ni, 0.002% Cu) in the cast ingot form were used as the substrate. The samples were ground with a series of silicon carbide waterproof abrasive papers up to 1200 grits. Thereafter, the samples were ultrasonically cleaned in acetone, rinsed in distilled water and finally dried in the warm air. In the PEO process, the samples were used as the anode and a 316 stainless steel sheet with dimension of 40 × 25 × 1 mm3 was employed as the cathode. The applied current was done using a power supply of Ruyingaran Sanaat Co, the model IRN6000, and the electrolyte temperature was kept at less than 35 °C during the coating process. Coating was done using the constant current mode, based on a range of effective parameters including duty cycle, current density, oxidation time and wt.% of nanoparticles (0.3, 0.6, 0.9 and 1.2 g/L) in a 0.025 M Na2SiO3_5H2O/0.05 M KOH electrolyte solution with the pH = 12.5. Experiments were designed using Taguchi method and the corrosion behavior of the coatings was evaluated and the coating with the lowest corrosion rate was selected as the optimal one. Finally, the oxide coating was created in a silicate electrolyte solution with the pH = 12.5, the current density of 160 mA/Cm2, the duty cycle of 50% and the frequency of 100 Hz; the composite coating was created in similar conditions using 0.9 g/L of ZrO2 nanoparticles in the electrolyte solution. For simplicity, these two types of coatings were named oxide and composite coatings. A time of 4 min was considered for coatings growth in uniform ignition conditions in order to grow the oxide layer according to another research in this area (Ref 21, 28, 29). Figure 1 shows the TEM image of ZrO2 nanoparticles. Voltage variations versus oxidation time were recorded during the coating process; after the coating process, the samples were washed with distilled water and dried by warm air.
X-ray diffraction experiment was done using Philips, PW3040 model, x-ray diffractometer in order to identify phases of the coating. VEGA, TESCAN-LMU model, SEM scanning electron microscope was used in order to measure the thickness and study the morphology of the coating at the surface and cross section. Given the insulative nature of coatings, a thin layer of gold was used on the surface in order to create surface conductivity. Electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization tests were performed using the Princeton applied (EG & G), the model 2273, Potentiostat machine. All electrochemical measurements were taken with a conventional three-electrode cell (Flat cell) consisting of a platinum electrode as the counter electrode, a saturated calomel electrode (SCE) as the reference electrode and PEO coatings as the working electrode; this was done in a Ringer solution at room temperature and after 20 min of an initial delay to stabilize the open-circuit potential (OCP). Cyclic polarization tests with the speed of 1 mV/S and in the range of E I = −250 mv versus ocp were started and continued to E v = 1200 mv versus ocp and ended in E f = −250 mv versus ocp. The EIS measurements were taken at OCP with the AC amplitude of 5 mV and the frequency range of 10−2 to 10−5 Hz. Corrosion parameters of repassivation potential (E rp), corrosion potential (E corr), corrosion current density (I corr), anodic Tafel slope (β a) and cathodic Tafel slope (β c) were obtained using Corr view software. Electrochemical impedance data were analyzed by the Z.View software, and the data were matched using Z.Sim 3.2 software in order to determine the equivalent circuit.
Results and Discussion
Voltage: Time Response
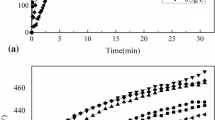
Variations of voltage versus oxidation time (V-T) are shown in Fig. 2 during the growth of the composite and the oxide coating.
As shown in Fig. 2, three areas are shown in the V-T curve during the coating growth. A rapid increase in voltage with time occurred in stage 1. In fact, this stage is known as the anodic oxidation stage and magnesium dissolution is done during Reaction 1 before reaching the breakdown voltage; due to silicate electrolyte solution, a thin protective layer during Reactions 2 and 3 was formed on the surface (Ref 10).
At this stage, composite and oxide coatings reached the breakdown voltage after 46 and 51 s, respectively. Moreover, the breakdown voltage of oxide and composite coatings was 182 and 233 volts, respectively. Therefore, nanoparticles effects at the first stage of coating increased the breakdown voltage by 28%. Since the formation of Mg(OH)2 and Mg2SiO3 layers led to the increased voltage (Ref 30), nanoparticles could cause a delay in the formation of these two phases and increase the breakdown voltage.
In the second stage of the coating process, oxide coating reached the sustainable potential of 440 volts after 164 s from the ignition time. However, a composite coating needs 189 s in order to be sustained. The required 15% more time for the stable potential from the ignition time could be due to the presence of nanoparticles in the electrolyte and its effects on the coating growth. In the second stage of curve, the white sparks that appeared on the surface reflected the start time of the coating growth. The first protective layer was demolished at this stage, and during the Reaction 4, oxygen anions were absorbed into the substrate through the discharge channels due to the effect of the strong electric field, which was 106 V/cm, on discharge channels and the corrosion resistant oxide layer (periclase) during the reaction with the magnesium ions (Ref 31).
According to the high temperature of the plasma process (Ref 10), the forsterite phase could be formed during periclase phase reactions of 5 and 6 in the presence of the magnesium silicate.
The presence of ZrO2 nanoparticles at this stage could lead to the formation of new phases. As shown in Fig. 3, XRD analysis results showed MgO, Mg2SiO4 phases in both composite and oxide coatings and Mg2Zr5O12 and ZrO2 phases in the composite coating.
The Reactions 4 to 6 are endothermic reactions done by the plasma discharge process (Ref 10). ZrO2 nanoparticles presence at this stage, as a heat absorbing foreign agent, could lead to reduced temperatures and a decreased MgO oxide layer formation rate during the Reaction 5, thereby affecting the Reaction 6. Also, due to the negative surface charge of nanoparticles on the one hand and the electrophoresis force effect on the other, the absorption of negative ions by nanoparticles and the formation of negatively charged colloids were possible, thereby moving toward anode through the electrophoresis force and being absorbed on its surface (Ref 21).
Figure 4 shows the SEM image of the surface and the cross section of the oxide and the composite coating.
As shown in Fig. 4, the cross section of both oxide and composite coatings was made of two layers with a different nature. A layer is the interface between the coating and the substrate that is thin, compacted and free from the defect inner layer; and the other one is the outer layer located over the coating with a porous nature and high thickness. In fact, these pores are discharge channels created during the plasma process and the molten magnesium flows from the substrate to the coating surface through these channels. Figure 6 shows the placement of ZrO2 nanoparticles in the coating pores with the EDS analysis of A and B points. According to this figure, nanoparticles were present in the outer layer and the inner layer was free from these particles. Table 1 provides the mean thickness of the outer and inner layers in three different points, using the SEM micrograph of the cross section of composite and oxide coatings.
As illustrated by the EDX elemental maps of the cross-sectional PEO composite coating in Fig. 5, it was observed that the distribution of the element O was similar to that of Si and these elements were distributed uniformly. From the element Zr, which originated from ZrO2, it was found that the distribution in the outer layer was higher than that in other regions. It should be noted that there were red areas within the substrate and the inner layer that could be caused by the spread of zirconia particles in the process of sample surface preparation.
As given in Table 1 and Fig. 4, the increased thickness of the inner compacted layer and the reduced thickness of the porous outer layer ultimately decreased the thickness of the composite coating, as compared to the oxide coating obtained by using ZrO2 nanoparticles in the electrolyte. Also, the range of changes in the coating thickness was decreased from 4.9 micron in the oxide coating to 2.4 micron in the composite coating, representing an increase in the uniformity of the coating in the presence of ZrO2 nanoparticles.
The growth of the inner layer was toward the substrate through oxygen penetration, and the growth of the outer layer was from the substrate toward the coating, with the leakage of molten magnesium through discharge channels and quenching on the surface (Ref 8). According to the electrophoretic absorption of nanoparticles on the anode surface and the reduced onset of ignition, as well as the growth mechanism of the outer layer from the substrate toward the electrolyte, nanoparticles were in direct contact with the outer layer and could not move and be absorbed in the inner layer. So it could be used as an external factor and as a barrier against the withdrawal of molten magnesium, thereby reducing the growth of the outer layer. As shown in Fig. 3(c) and (d), the composite coating was highly compacted, as compared to the oxide coating, and the discharge channels size was reduced. In fact, as shown in Fig. 6, there were some ZrO2 nanoparticles which settled in the discharge channels, decreasing the diameter of the micropores from 5.49 µm in the oxide coating to 3.78 µm in the composite coating. Also, the thickness of the inner layer in the composite coating was increased, as compared to the oxide coating; these results were in accordance with other studies in this field (Ref 20, 22, 24, 32), showing improvement in the morphology of the PEO coating in the presence of ZrO2 nanoparticles.
Given that the constant current was used for the coating growth in this study, changes in coating resistance were in line with voltage variations. Due to the insulation nature of the oxide layer, with the growth of it, the resistance and voltage of the coating were increased. Coating resistance of the composite coating was increased, as compared to the oxide coating; thus, it needed more time to achieve a sustainable potential, probably due to the presence of nanoparticles in the coating and its increased compactness. These factors could delay the discharge process and reduce the growth rate of the coating and increase the required time to stabilize the potential up to 15%. The results of the research conducted on adding oxide particles to the electrolyte solution indicated this voltage change versus time (Ref 19, 20, 25, 26).
In the third stage of the chart, sparks density could be decreased; the formation of thick orange sparks would be due to the coating growth and its increased current resistance. Then, coating resistance could be increased at this stage and passing current would be reduced; so the discharge channels of the coating could be reduced and fewer sparks of larger size would be formed, resulting in uniform voltage values.
Electrochemical Corrosion Tests
To evaluate the corrosion resistance of the specimens, their electrochemical properties were considered. Corrosion potential (E corr) and corrosion current density (I corr) were used to determine the corrosion behavior of the specimens. Generally, high E corr and low I corr suggest good corrosion resistance. The potentiodynamic polarization curves of bare Mg, oxide and composite coatings in the Ringer solution are shown in Fig. 7. Polarization curves data are shown in Table 2.
As given in Table 2, the presence of PEO coatings on the magnesium surface led to the reduction in the corrosion potential, indicating the reduced tendency to corrosion in the presence of the oxide coating. The created oxide and composite coatings on the magnesium alloy surface reduced the corrosion current density from 25.06 to 2.7 µA/cm2 on the oxide coating and to 0.47 µA/cm2 on the composite coating; this reduction in corrosion rate was due to the presence of ZrO2 nanoparticles in the oxide coating. Comparing polarization resistance values indicated the increased polarization resistance of the composite coating, as compared to the oxide one.
According to polarization curves, the cathodic behavior of all three samples was similar, indicating the identical cathodic reactions mechanism. On the other hand, the anodic behavior of oxide and composite coatings was different from that of the uncoated magnesium, thereby showing the difference in the anodic mechanism.
Generally, there is a layer of Mg(OH)2 on the magnesium alloy surface that has a porous nature, with weak adhesion to the substrate (Ref 32). By increasing the anodic potential, Cl− corrosive ions could easily pass the porous layer and dissolve the substrate. Oxide layer formation on the magnesium surface led to 89% reduction in the corrosion current density, indicating the role of the coating in preventing the diffusion of the corrosive ions. On the other hand, the composite layer increased the corrosion resistance of magnesium up to 11%, as compared to the oxide layer. In fact, the presence of the compacted and thicker inner layer, placement of ZrO2 nanoparticles in the porous outer layer, the increased coherence of the coating surface and the reduced diameter of the discharge channel in composite coatings led to the reduced penetration power of corrosive Cl− ions to the substrate. Creation of Mg2SiO4, Mg2Zr5O12 and ZrO2 phases in discharge channels and the coating surface as the second phase resulted in the strengthened composite behavior of the oxide layer and prevention of the aggressive ions penetration through discharge channels, as well as the improved corrosion behavior due to the high chemical stability (Ref 33, 34).
Cyclic polarization curves in the Ringer solution could be used to distinguish the pitting corrosion resistance of the coatings. When the reverse curve was scanned back on the tip of the forward curve, the pitting corrosion occurred. The area of the closed curve is an opposite measure of the pitting corrosion resistance. E rp value of both oxide and composite coatings was less than E corr value, indicating that the passive layer was not reformed and had susceptibility to pitting. Given the disposition of the composite coating versus the oxide one toward the left side of the polarization chart, the presence of nanoparticles in the coating led to the reduced oxide coating tendency to pitting. The positive hysteresis of both oxide and composite curves indicated that the broken passive film was not reformed and there was pitting possibility. The hysteresis loop area of the oxide coating was less than that of the composite coating; as a result, pitting was decreased in the presence of ZrO2 nanoparticles and the pitting corrosion resistance performance of the composite coating was the best. In the stage 2 of the coating growth (Fig. 1), the molten magnesium flow from the discharge channels into the coating surface and its contact with the electrolyte led to quenching the molten magnesium and obtaining the residual stress in the oxide structure (Ref 35). Figure 8 shows the SEM pictures of pitting in the composite and oxide coatings after the cyclic polarization test.
As shown in Fig. 8, the uniformity of coating after the polarization test in the composite coating was higher than that of the oxide coating. Also, the diameter of the biggest pit on the composite coating, in comparison with the oxide coating, was decreased by about 300 µm, which could be due to the higher resistance of the composite coating.
EIS test was carried out to further understand the corrosion behavior and the associated deterioration process of the oxide and composite coatings. Figure 10 shows the results of EIS test in Nyquist and bode plot forms. As shown in Fig. 9(b), for the oxide and composite coatings, in the Nyquist diagram, there was a straight line with the angle of about 45° in the low frequency stage, showing that the Warburg diffusion participated in the anodic reaction. This occurred when electrochemical reaction was fully controlled by the diffusion phenomenon; so Warburg element showed that corrosion behavior was due to electrolytes diffused in the micro-holes of oxide and composite coatings.
As shown in Fig. 10 and the results of other similar studies (Ref 36), the uncoated magnesium curve shows a capacitive behavior in a wide range of frequencies. Figure 10(b) shows that the uncoated magnesium impedance was about 1000 Ω cm2 at low frequencies, while the impedance of oxide and composite coatings was 10,000 Ω cm2 at unique frequencies. This indicated that PEO coating resistance was about 10 orders of the uncoated magnesium. The bode phase angle plot in Fig. 10(c) shows that there were two time constants for the PEO coatings and only one time constant for the Bare Mg in the Ringer solution. We could employ many equivalent circuit models by using Z.Sim 3.2 Software to simulate the Mg/coating/Ringer solution system. Our experimental data were found to best fit the model reported in Fig. 9.
The systematic error in the results was fitted, as shown in Table 3, suggesting that this model presented a reliable explanation of the electrochemical system. In this model, R s is the electrolyte resistance, and CPEdl represents the double-layer capacitance of the constant phase element at the electrolyte/coating interface at high frequency, which is generally employed to replace the pure capacitance in view of the porous/rough nature of the electrode in the electrochemical process; also, R t represents charge transfer resistance processes occurring within the pores of the coating and on the surface. Also, CPEfilm and Rfilm are used, in parallel, to describe the complicated Mg/composite coating interface response at low frequencies. A more general constant phase element (CPE) was used instead of a capacitive element and the formula Y CPE(w) = 1/Z CPE = Q a(jw)n, as proposed by Zoltowski (Ref 37), was employed for the admittance, Y, of the CPE element (Fig. 9).
Table 3 shows that R coat was higher than R polar. This means that coating resistance could be regarded as a major factor in the corrosion protection of the magnesium alloy. It could be seen that the coating resistance of the composite coating was higher than that of the oxide one; the higher polarization resistance in the composite coating demonstrated that the composite coating/substrate interface acted as a good barrier inhibiting the penetration of the aggressive electrolyte to the metal surface, possibly due to the high chemical stability of ZrO2 (Ref 37). On the other hand, as given in Table 3, it could be seen that Warburg impedance of the composite coating was higher than that of the oxide one. It should be noted that embedding ZrO2 nanoparticles into the coating resulted in the decrease in the thickness of the nanoporous film, as indicated by the increase of CPE2.
According to scanning electron micrographs of the oxide and composite coatings surface (Fig. 4), the reduced diameter of surface holes, the decreased cracks of the coating surface and placement of nanoparticles within coating porosities and the created barrier against the passage of corrosive ions could be taken as the most important changes due to ZrO2 nanoparticles. So diffusion of corrosive ions in the composite coating was reduced toward the substrate, which could be a reason for the reduced corrosion behavior of the composite coating, as compared to the oxide one. These results were consistent with those of another research using oxide nanoparticles in the electrolyte solution (Ref 18, 19, 23,22,23,24,25,26).
Conclusions
-
1.
Using ZrO2 nanoparticles in the electrolyte solution led to the increased compaction and the reduced thickness of the oxide coating. On the other hand, examining the coating surface morphology reflected the reduced surface defects and the decreased diameter of the discharge channel at the composite coating surface, as compared to the oxide coating.
-
2.
Generating Mg2SiO4, Mg2Zr5O12 and ZrO2 phases in the discharge channels and the coating surface as the second phase led to the strengthened composite behavior of the oxide coating; this, in turn, prevented the penetration of the aggressive ions from discharge channels and improved the corrosion behavior of the composite coating, as compared to the oxide coating, due to high chemical stability.
-
3.
The reduced hysteresis loop area of the composite coating, as compared to the oxide coating, reflected the reduced tendency to pitting in the composite coating.
-
4.
Using this coating could be useful in the development of AZ91 alloys in various fields such as medical equipment that suffer from some restrictions due to corrosion.
References
B.L. Mordike and T. Ebert, Magnesium: Properties, Applications, Potential, Mater. Sci. Eng., A, 2001, 302, p 37–45
A. Body, U.T.S. Pillai, and B.C. Pai, Developments in Magnesium Alloys for Transport Applications—An Overview, Indian Foundry J., 2011, 57, p 29–37
M.P. Staiger, A.M. Pietak, J. Huadmai, and G. Dias, Magnesum and Its Alloys as Orthopedic Biomaterials: A Review, Biomaterials, 2006, 27, p 1728–1734
N.T. Kirkland, J. Lespagnol, N. Birbilis, and M.P. Staiger, A Survey of Bio-corrosion Rates of Magnesium Alloys, Corros. Sci., 2010, 52, p 191–287
J.E. Gray and B. Luan, Protective Coatings on Magnesium and Its Alloys—A Critical Review, J. Alloys Compd., 2002, 336, p 88–113
H. Hornberger, S. Virtanen, and A.R. Boccaccini, Biomedical Coatings on Magnesium Alloys—A Review, Acta Biomater., 2012, 8, p 2442–2455
R.O. Hussein, X. Nie, and D.O. Northwood, The Application of Plasma Electrolytic Oxidation (PEO) to the Production of Corrosion Resistant Coatings on Magnesium Alloys: A Review, Corros. Mater., 2013, 38(1), p 55–65
A.L. Yerokhin, X. Nie, A. Leyland, A. Matthews, and S.J. Dowey, Plasma Electrolysis for Surface Engineering, Surf. Coat. Technol., 1999, 122, p 73–93
P. Gupta, G. Tenhundfeld, E.O. Daigle, and D. Ryabkov, Electrolytic Plasma Technology, Science and Engineering—An Overview, Surf. Coat. Technol., 2007, 201, p 8746–8760
S.V. Gnedenkov, O.A. Khrisanfova, A.G. Zavidnaya, S.L. Sinebryukhov, V.S. Egorkin, M.V. Nistratova, A. Yerokhin, and A. Matthews, PEO Coatings Obtained on an Mg–Mn Type Alloy Under Unipolar and Bipolar Modes in Silicate-Containing Electrolytes, Surf. Coat. Technol., 2010, 204, p 2316–2322
Y. Gao, A. Yerokhin, and A. Matthews, DC Plasma Electrolytic Oxidation of Biodegradable cp-Mg: In Vitro Corrosion Studies, Surf. Coat. Technol., 2013, 234, p 132–142
Zh. Chen, G. Li, Zh. Wu, and Y. Xia, The Crack Propagating Behavior of Composite Coatings Prepared by PEO on Aluminized Steel During in Situ Tensile Processing, Mater. Sci. Eng., A, 2011, 528, p 1409–1414
R. Arrabal, E. Matykina, F. Viejo, P. Skeldon, and G.E. Thompson, Corrosion Resistance of WE43 and AZ91D Magnesium Alloys with Phosphate PEO Coatings, Corros. Sci., 2008, 50, p 1744
C. Blawert, V. Heitmann, W. Dietzel, H.M. Nykyforchyn, and M.D. Klapkiv, Influence of Process Parameters on the Corrosion Properties of Electrolytic Conversion Plasma Coated Magnesium Alloys, Surf. Coat. Technol., 2005, 200, p 68
J. Liang, L. Hu, and J. Hao, Characterization of Microarc Oxidation Coatings Formed on AM60B Magnesium Alloy in Silicate and Phosphate Electrolytes, Appl. Surf. Sci., 2007, 253, p 4490
H. NasiriVatan, R. Ebrahimi-Kahrizsangi, and M.K. Asgarani, Tribological Performance of PEO-WC Nanocomposite Coating on Mg Alloys Deposited by Plasma Electrolytic Oxidation, Tribol. Int., 2016, 98, p 253–260
G.L. Shchukin, A.L. Belanovich, V.P. Savenko et al., Microplasma Anodization of Aluminium and Its Copper-Containing Alloy in Potassium Hexafluoro Zirconate Solution, Zh. Prikl. Khim., 1996, 69, p 939
J. Liang, L. Hu, and J. Hao, Preparation and Characterization of Oxide Films Containing Crystalline TiO2 on Magnesium Alloy by Plasma Electrolytic Oxidation, Electrochim. Acta, 2007, 52, p 4836–4840
T.S. Lim, H.S. Ryu, and S. Hong, Electrochemical Corrosion Properties of CeO2-Containing Coatings on AZ31 Magnesium Alloys Prepared by Plasma Electrolytic Oxidation, Corros. Sci., 2012, 62, p 104–111
F. Liu, D. Shan, Y. Song, E. Han, and W. Ke, Corrosion Behavior of the Composite Ceramic Coating Containing Zirconium Oxides on AM30 Magnesium Alloy by Plasma Electrolytic Oxidation, Corros. Sci., 2011, 53, p 3845–3852
K. Lee, K. Shin, S. Namgung, B. Yoo, and D. Shin, Electrochemical Response of ZrO2-Incorporated Oxide Layer on AZ91 Mg Alloy Processed by Plasma Electrolytic Oxidation, Surf. Coat. Technol., 2011, 205, p 3779–3784
S.V. Gnedenkov, S.L. Sinebryukhon, D.V. Mashtalyar et al., Fabrication of Coatings on the Surface of Magnesium Alloy by Plasma Electrolytic Oxidation Using ZrO2 and SiO2 Nanoparticles, J. Nanomater., 2015, 16, p 1–12
M. Wang, C. Li, and S. Yen, Electrolytic MgO/ZrO2 Duplex-Layer Coating on AZ91D Magnesium Alloy for Corrosion Resistance, Corros. Sci., 2013, 76, p 142–153
K.M. Lee, Y.S. Kim, H.W. Yang et al., Formation of ZrO2 in Coating on Mg–3wt.%Al–1wt.%Zn Alloy Via Plasma Electrolytic Oxidation: Phase and Structure of Zirconia, Mater. Charact., 2015, 99, p 101–108
X. Lu, C. Blawert, Y. Huang, and H. Ovri, Plasma Electrolytic Oxidation Coatings on Mg Alloy with Addition of SiO2 Particles, Electrochim. Acta, 2016, 187, p 20–33
X. Lu, C. Blawert, N. Scharnagl, and K. Kainer, Influence of Incorporating Si3N4 Particles into the Oxide Layer Produced by Plasma Electrolytic Oxidation on AM50 Mg Alloy on Coating Morphology and Corrosion Properties, J. Magnes. Alloys, 2013, 1, p 267–274
Y.M. Wang, F.H. Wang, M.J. Xu, B. Zhao, L.X. Guo, and J.H. Ouyang, Microstructure and Corrosion Behavior of Coated AZ91 Alloy by Micro Arc Oxidation for Biomedical Application, Appl. Surf. Sci., 2009, 255, p 9124–9131
C.E. Barchiche, D. Veys-Renaux, and E. Rocca, A Better Understanding of PEO on Mg Alloys by Using a Simple Galvanostatic Electrical Regime in a KOH-KF-Na3PO4 Electrolyte, Surf. Coat. Technol., 2011, 205, p 4243–4248
Z. Li, Y. Yuan, and X. Jing, Effect of Current Density on the Structure, Composition and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on Mg-Li Alloy, J. Alloys Compd., 2012, 541, p 380–391
R.O. Hussein, X. Nie, and D.O. Northwood, An Investigation of Ceramic Coating Growth Mechanisms in Plasma Electrolytic Oxidation (PEO) Processing, Electrochim. Acta, 2013, 112, p 111–119
P. Su, X. Wu, Y. Guo, and Z. Jiang, Effects of Cathode Current Density on Structure and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings Formed on ZK60 Mg Alloy, J. Alloys Compd., 2009, 475, p 773
S. Wang, N. Si, Y. Xia, and L. Liu, Influence of Nano-SiC on Microstructure and Property of MAO Coating Formed on AZ91D Magnesium Alloy, Trans. Nonferrous Met. Soc. China, 2015, 25, p 1926–1934
A. Roy, S.S. Singh, M. Datta, B. Lee, J. Ohodnicki, and P.N. Kumta, Novel Sol–Gel Derived Calcium Phosphate Coatings on Mg4Y Alloy, Mater. Sci. Eng., B, 2011, 176, p 1679–1689
J.J. Zhuang, Y.Q. Guo, N. Xiang et al., A Study on Microstructure and Corrosion Resistance of ZrO2-Containing PEO Coatings Formed on AZ31 Mg Alloy in Phosphate-Based Electrolyte, Appl. Surf. Sci., 2015, 357, p 1463–1471
C. Suryanarayana and M.G. Norton, X-ray Diffraction A Practical Approach, 1st ed., Springer, Berlin, 1998, p 207
J. Liang, P. Srinivasan, C. Blawert, and W. Dietzel, Comparision of Electrochemical Corrosion Behaviour of MgO and ZrO2 Coatings on AM50 Magnesium Alloy Formed by Plasma Electrolytic Oxidation, Corros. Sci., 2009, 51, p 2483–2492
P. Zoltowski, On the Electrical Capacitance of Interfaces Exhibiting Constant Phase Element Behaviour, J. Electroanal. Chem., 1998, 443, p 149–154
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eslamzadeh, N., Ebrahimi-Kahrizsangi, R., Karbasi, S. et al. An Investigation into the Corrosion Behavior of MgO/ZrO2 Nanocomposite Coatings Prepared by Plasma Electrolytic Oxidation on the AZ91 Magnesium Alloy. J. of Materi Eng and Perform 26, 4255–4264 (2017). https://doi.org/10.1007/s11665-017-2905-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-017-2905-2