Abstract
Spark plasma sintering (SPS) has been recognized, in the recent past, as a very useful method to produce metal matrix composites with enhanced mechanical and wear properties. Obviously, the materials’ properties are strongly related to the reinforcement types and percentages as well as to the processing parameters employed during synthesis. The present paper examines the effect of 2 wt.% of Al2O3 nanoparticles on mechanical and microstructural behaviors of Al-based metal matrix composites produced via SPS. The composite mechanical properties were evaluated through micro-, nanoindentation and tensile tests. The microstructural evolution was studied through scanning electron microscopy observations. It was found that the addition of nanoparticles produces the reduction of materials porosity and the improvement of mechanical properties in SPSed materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, nanocomposite has received considerable attention thanks to their unique mechanical properties that are reachable through the addition of low reinforcement volume factions. (Ref 1). The reinforcement of the aluminum ductile matrix with stronger and stiffer second-phase reinforcements such as oxides, carbides, borides and nitrides provides considerable improvements in physical and mechanical properties of nanocomposites (Ref 2).
It is well known that the amount, the size and the distribution of hard particles throughout the matrix have a crucial influence on the final properties of nanocomposites (Ref 1-4). The strength of aluminum matrix composite can be improved about 20% by decreasing the reinforcement particle size from micron to nanosize (Ref 3). As a matter of fact, the yield and tensile strength of Al-1vol.% Si3N4 (15 nm) nanocomposite resulted higher with respect to those belonging to Al-15vol.%SiCp (3.5 μm). On the other hand, by adding ceramic nanoparticles, elastic modulus and hardness can be increased (Ref 5). However, homogeneous dispersion of ceramic nanoparticles in the matrix is difficult to achieve. Agglomeration of nanoparticles within the grains and grain boundaries of aluminum matrix becomes more impressive as the reinforcement content exceeds 4% (Ref 5, 6). Generally, nanoparticles tend to agglomerate into coarse clusters during liquid metallurgy processing. This can be attributed to the poor wettability. In order to overcome these obstacles, a variety of processing routes have been successfully used to produce aluminum matrix nanocomposites including casting, melting infiltration and powder metallurgy (PM) technologies (Ref 7). However, mechanical milling and consequent alloying are very affordable processes for homogenous dispersing ex situ nanoparticles (Ref 8, 9). In addition, powder metallurgy (PM) processes are ideal for a rapid production of near-net-shape components characterized by complex geometries.
Aluminum-based metal matrix composites reinforced with micro- or nanosized ceramic particles offer excellent promising potentials in terms of mechanical and wear properties. Various methods are reported in the literature on the different processing routes available to produce aluminum-based composites reinforced with ceramic particles (Ref 10-13). Among these methods, spark plasma sintering has been recognized, in the recent past, as a very useful tool to produce metal matrix composites with enhanced mechanical properties by employing lower temperatures and short sintering times (Ref 14-16). In addition, this technique allows to produce nanostructured materials characterized by very high mechanical properties if compared to their microcrystalline counterparts. Obviously, mechanical properties strongly depend on the materials porosity that should be reduced in order to avoid undesirable properties (Ref 17). Experimental evidences demonstrate the strong effect of pressure variation during sintering on the nanostructured materials properties (Ref 18). Spark plasma sintering (SPS) is used for consolidating nanocrystalline materials with reduced grain growth and clean grain boundaries thanks to the effective interface formation (Ref 19). These composites are characterized by excellent mechanical properties at room and very high temperatures (Ref 20). The strengthening is governed by the Orowan mechanism due to the ceramic particles presence and by the Hall-Petch effect due to grain refinement (Ref 21). Further enhancement in mechanical properties can be obtained by employing high-entropy alloys particles as reinforcement (Ref 22, 23). Obviously, the SPSed composite properties are strongly dependent on the reinforcing particles dimensions and process parameters (Ref 24, 25). SPS has also been employed to produce composites with in situ formed reinforcement leading to a strong reduction in the materials porosity levels (Ref 26, 27). The effect on microstructural properties of microcrystalline alumina powders is shown for composites produced via SPS with different reinforcement percentages (Ref 28). The aim of the present paper is to describe the potential of producing aluminum-based metal matrix composites with nanometric ceramic reinforcement via SPS and to show the mechanical and microstructural behavior of composites with respect to SPS pure aluminum.
Materials and Methods
The studied composites were produced by employing high-purity aluminum powders (ECKA, Germany) with an average size of 30 μm and alpha alumina powders (Evonik Industries, Germany) with an average size and specific area of 30 nm and 70 m2 g−1, respectively. Mechanical milling was carried out in a high-energy ball mill equipment (Retsch, Germany). The synthesis process was started by blending Al powders and ceramic reinforcements of nanometric size. Powder blends were then subjected to planetary ball milling under argon atmosphere with the following parameters: charge ratio: 10:1 (wt.); powder mass: 15 g; mass of balls: 150 g; ball diameter: 15 and 10 mm; ball material: hardened stainless steel; no. of balls: three balls with 15 mm and five balls with 10 mm diameter; vial speed: 250 rpm; and vial material: hardened stainless steel. To prevent severe cold welding, stearic acid (2 wt.%) was added to the mixture during ball milling. The constituent powders were milled continuously for 2 h. Prior to SPS, ball-milled blended powders were kept in a glow box in order to minimize the oxidation effects and/or other potential atmospheric contamination.
Samples were sintered by SPS technique using a Model 10 Ton equipment manufactured by KHPF Inc. (Iran). The desired amount of milled powder blend (approximately 15 g) was loaded into a graphite die of 40 mm in diameter. The graphite die was ISG-M303 with a thermocouple hole drilled into the punch. For the sintered pellets removal, a graphite paper sheet is placed between the punch and the powder as well as between the die and the powder in order to prevent sticking due to the possible reaction between the powder and graphite die/punch. The sintering heating and cooling rates were 75 and 30 °C/min, respectively. Pulsed DC current, with a 36 ms on and 8 ms off profile, was applied.
The materials’ density was measured following the ASTM B962-14 standard. After polishing, the specimens were first weighed in air and then after immersion in distilled water. The aluminum matrix nanocomposite density (\({\rho_{\text{AMNC}}}\)) was calculated as [A/(A − B)] × ρ o, where A is the mass [g] of the composite in air, B is the mass [g] of the same composite in distilled water, and \(\rho_{0}\) [g/cm3] is the density of the distilled water (i.e., the density of distilled water at room temperature is 0.998 g/cm3). The density values were obtained by averaging five measurements per sample.
The absolute samples porosity was calculated as p = (ρ th−ρ AMNC)/ρ th, where p is the absolute porosity [%],\(\rho_{\text{th}}\) and \({{\rho }_{\text{AlMNC}}}\) [g/cm3] are theoretical and effective densities, respectively. To accurately compare the extent of densification that occurred during sintering, the theoretical density was calculated by taking into account the rule of mixtures as ρ th = ρ mVm− ρ r, f V r, f, where \(\rho_{\text{m}}\) and \(\rho_{r \cdot f}\) [g/cm3] are the densities of the matrix and of the reinforcement, respectively; \(V_{\text{m}}\) and \(V_{r \cdot f}\) [cm3] are the volume fractions of matrix and of the reinforcement, respectively.
The statistical analysis of the particle size and porosity distributions of SPSed specimens was performed by optical microscopy and image analysis (Image J software).
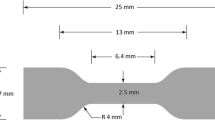
Mechanical properties of AMNCs were determined by nano- and microindentation tests at room temperature. The microhardness, using the Rockwell H scale, was carried out using Akashi apparatus with a 50-N load for 15 s, while the indentation tests were performed using an Anton Paar nanoindenter model TTX-NHT2. The applied maximum load was 500 mN and the dwell time was 5 s for each indent. The tests were performed in load control. Seven indentations, per each sample, were performed, and the average values were calculated. The microhardness measurements were taken on five randomly selected points for each specimen to obtain an average value of hardness. The specimens for uniaxial tensile tests were prepared by employing elecrodischarge machining (EDM) according to ASTM E8 for small size specimens. The specimens had a gauge length of 6.4 mm (with square section), 25 mm overall length and 4 mm thickness. The tensile tests were conducted at room temperature with initial strain rate of 10−4 s−1 using a Hounsfield H50KS machine. The total elongation of the samples was measured from the difference between the samples lengths before and after testing.
X-ray diffractometry (XRD) was employed to identify the existing phases of samples before and after SPS process. A Philips diffractometer (40 kV) with Cu Kα radiation with λ = 0.15406 nm over 20-100° 2θ was used for measurements. XRD scans were performed with a step size of 0.05° and a dwell time per step of 2 s. The grain size of Al was estimated from broadening of XRD peaks using Williamson-Hall Eq 1.
where \(\beta_{s}\) is full width at half maximum of the diffraction peak, \(\theta\) is the diffraction angle [°], λ is the x-ray wavelength, d is the crystallite size [nm], and ε is the lattice strain.
Microstructural and fracture surfaces observations were performed with a PHILIPS XL30 and ZEISS EVO40 scanning electron microscope (SEM) equipped with energy-dispersive spectroscopy (EDS).
Results and Discussion
Materials Preparation
The aluminum and nanosized alumina particles aspects are shown in Fig. 1.
The mixture powders and mold were heated from ambient temperature to the sintering temperature of 550 °C, with mentioned heating rate and then holding at that temperature for 5 min (Fig. 2). The gas removal takes place during the initial stages of heating in SPS. A uniaxial pressure of 50 MPa was applied throughout the entire heating and cooling cycle. The samples were then furnace-cooled up to room temperature in vacuum atmosphere.
Figure 3(a) depicts the data recorded during SPS sintering of nanocomposite . The electrical power sharply increases, and then, it remains constant and finally it decreases immediately in the last stage of the sintering process. The generated heat is normally higher with respect to that belonging to other conventional techniques. A high electrical power minimizes the time that a material spends at a temperature at which coarsening mechanisms, like surface diffusion, are active. Figure 3(b) illustrates the temperature and the punch displacement as a function of the sintering time. The densification behavior of Al-2wt.%Al2O3 nanocomposite can be observed through the punch displacement. The positive punch displacement values correspond to the sample shrinkage. The maximum displacement of the powder particles takes place around 6 mm during three time stages: 0-188, 218-320 and 410-690 s. Throughout the rest of the time, no shrinkage takes place. Rearrangement of powder particles, due to the initial press, localized deformation, bulk deformation and extensive sintering by mass transport phenomenon (Ref 29, 30) take place in the I, II and III regions.
Displacement increases with temperature and time up to 225 °C and 188 s, respectively. This behavior can be attributed to the rearrangement of coarse particles, to the gas removal and to the pores size reduction (Fig. 4). A possible explanation for the high displacement rate is ascribed to the high applied pressure.
In fact, the rearrangement of the aluminum particles is due to the pressure application that governs the relative motion between the powder particles (Ref 31, 32). Then, with increasing temperature and time (region II), densification increases with densification rate in comparison with the previous stage. This is due to the localized deformation at the particles contact points. During this stage, many necks between the surface of aluminum particles take place. This mechanism is a consequence of the mass transport phenomena such as grain boundary and volume diffusion. In fact, the localized deformation and the consequent necking formation result in a slight densification. On the other hand, the presence of the alumina nanoparticles enhances the rearrangement and the local deformation of the aluminum particles.
The maximum displacement takes place at the end of region II. It could be due to the high displacement rate of region I. The initial rearrangement of the aluminum particles, in the early stage, is followed by the flow of fine particles into the voids that are generated between the aluminum particles. Finally, the main shrinkage (region III) starts at 400 °C and ends at 550 °C. In this stage, the incipient propagation of deformation within the core of the aluminum particles starts. Bulk deformation and extensive sintering by mass transport phenomenon cause more coalescence between aluminum particles, especially in the necked areas. Diffusion is one of the main reasons for the lowest densification rate of region III. By holding the pressure at the maximum temperature, there was no distinguished change in displacement and displacement rate. It means that the sintering process is completed. The materials were produced into disks of 10 mm in thickness and 40 mm in diameter.
Porosity
Densification, or consolidation, refers to the porosity removing from a powder compact in order to form a robust and pore-free material (usually 95-98% of the theoretical density). Generally, a powder compact initially has two distinct forms of porosity, known as inter- and intraparticles porosity. As mentioned, particle rearrangement, localized deformation and bulk deformation are the main mechanisms acting during the powder densification that usually takes place in sequence during the sintering temperature increase. Additionally, thermal and non-thermal effects can affect the densification process. In addition, it is expected that the use of high-density current can induce electro-migration effects or current-enhanced mass transport (Ref 33). The SEM microstructure of the studied nanocomposite is shown in Fig. 5.
Hence, all these mechanisms can affect the microstructure and mechanical properties of SPSed samples, especially densification. A significant increase in density is recorded when the alumina nanoparticles are added into aluminum matrix. In fact, the pure aluminum milled for 2 h and consolidated at 550 °C for 5 min showed a relatively density of 95.86%; the SPSed nanocomposite containing 2 wt.% of alumina nanoparticles showed a density of 98,75%. The theoretical density is 2.71 g/cm3 for pure aluminum and 2.735 g/cm3 for the Al-2wt.%Al2O3 nanocomposite; both values were calculated by the rule of mixtures. The real measured density for pure aluminum and Al-2wt.%Al2O3 nanocomposite was 2.61 and 2.67 g/cm3, respectively. The difference between theoretical and real density is attributed to the porosity existence within aluminum matrix. The porosity of pure aluminum is around 4.14%, while it is reduced to 1.25% for Al-2wt.%Al2O3 nanocomposite. This aspect is related to the role of alumina nanoparticles in filling aluminum interparticles spaces by alumina nanoparticles. In fact, the alumina nanoparticles can locate within pores between aluminum particles and consequently leads to the decrease of the porosity level. It is worth noting that the distribution of alumina nanoparticles in the aluminum matrix can affect the porosity level. It is found that the alumina nanoparticles distribution in Al-2wt.%Al2O3 nanocomposite, for a content lower than 2 wt.%, is homogeneous.
Generally, powder compaction containing ceramic and carbide nanoparticles is more difficult with respect to the compaction of micron-sized powders of the same metal. This is because the plasticity in nanocrystalline samples requires order of magnitude larger stresses; in addition, spring-back is more pronounced (Ref 34, 35). Maximum densification is achieved when the mass transport mechanisms take place. Mass transport mechanisms, which are involved in SPS sintering, are volume diffusion, evaporation, surface diffusion, power-law creep and grain boundary diffusion. Here, only grain boundary and volume diffusion cause densification, while evaporation and surface diffusion are responsible for the neck formation as well as the particles coarsening. The surface diffusion has a low activation energy, and so, it is more active at lower temperature than grain boundary or volume diffusion (Ref 36). The high heating rate increases the duration of evaporation and surface diffusion. It causes an increase in sinterability and the densification capability by grain boundary diffusion, volume diffusion and power-law creep. The driving force for sintering is usually affected by the applied pressure. It is well known that an increase in pressure has a remarkable effect on the density of nanocomposite fabricated via powder metallurgy route. By increasing pressure up to 30 MPa, the powders rearrangement or sliding phenomena cause elimination of open pores. With further pressure increasing up to 50 MPa, in addition to holding of powder mixture in sintering temperature, the elimination of the open pores is achieved. Consequently, a consolidation enhancement is observed. In fact, the open pores’ dimensions are reduced through the first stage; during the following processing stages, both mass transportation and lactation of alumina nanoparticles within closed pores cause the acceleration of the pores elimination.
X-rays Diffraction
X-ray diffraction (XRD) analysis was carried out for the as-received powders and before/after SPS of pure Al and Al-Al2O3 nanocomposite. The characteristic XRD peaks indicate the presence of the Al as well as Al2O3 phases and the absence of Al4C3 carbides in all the specimens (Fig. 6).
The peaks intensity in XRD pattern is affected by the strain and the crystallinity of materials. Residual strain after powder milling affects the peaks intensity. The addition of alumina nanoparticles to aluminum particles decreases the crystallite size of aluminum. The presence of nanoparticles enhances the work-hardening rate of the matrix particles and consequently the grain refining process in the aluminum compacts. On the other hand, after SPS of pure aluminum and Al-2wt.%Al2O3 nanocomposite, a negligible grain growth for aluminum particles is observed. It can be ascribed to the high temperature employed during sintering process and to the consequent formation and growth of necking between aluminum particles.
The measured crystallite size of the materials before and after SPS is shown in Fig. 7. Crystallite sizes of composites are lower with respect to pure Al.
Mechanical Properties
Typical load-displacement curves (often called the P-h curve) for pure Al and Al-2wt.%Al2O3 nanocomposite are shown in Fig. 8. It can be seen that the average indentation depths for pure aluminum and Al-2wt.%Al2O3 nanocomposite were around 7800 and 4900 nm, respectively. The negligible difference observed in these figures indicates that the samples are homogeneous on this probing scale. The probed volume of materials in each of these tests was approximately 1.15 μm3.
The nanoindentation comparison between the pure aluminum and the composite is shown in Fig. 9. The maximum applied load has a direct correlation with the hardness, and thus, the hardness is strongly influenced by the degree of homogeneous alumina nanoparticles distribution, by the grain size and by the defects presence and density such as porosity and dislocations. The hardness difference between the pure aluminum and the Al-2wt.%Al2O3 nanocomposite is underlined by the large difference in the indentation depth. It seems that the slopes of the unloading curves, related to the materials stiffness, are similar for pure aluminum and nanocomposite, while the penetration depths are different. Therefore, the homogeneous distribution of alumina nanoparticles in the pores and the distance between aluminum particles reflect that there was a strong diffusional bonding between particles. In this situation, it is expected that the elastic modulus is high.
The summary of the nanoindentation results for both the materials is listed in Table 1.
It can be observed that for both the specimens, there is a similar independence of Young’s modulus on indentation depth. It was seen that after reaching the maximum load, curves drop down. It could be due to the existence of a considerable amount of porosity in the microstructure of both the materials. The use of sharp diamond indenter causes the creation of cracks beneath the specimen contact surface near the indenter sharp corners. The surface area, under the load-displacement curve for pure aluminum, was larger than that of Al-2wt.%Al2O3 nanocomposite. This represents that the fracture toughness of pure aluminum is larger than that of the Al-2wt.%Al2O3 nanocomposite . The pure aluminum sintered at 550 °C for 5 min has micro- and nanohardness values of 33 and 40 MPa, respectively, while they resulted in 60 and 68 MPa for the nanocomposite. Generally, the achieved nanoindentation values are higher with respect to the microhardness ones. This is due to the indentation size effect (phenomenon of sinking up). The addition of 2 wt.% of Al2O3 nanoparticles leads to an increase in nanohardness and elastic modulus. Elastic modulus increases from 55 (pure Al) to 72 MPa (Al-2wt.%Al2O3 nanocomposite).
Many mechanisms are involved in such a behavior. First of all, the level of alumina distribution and agglomeration affects hardness and tensile strength. Then, the nanoparticles influence the strength mechanisms like Orowan strengthening, Hall-Petch effect and dislocation strengthening (Ref 37-39).
Generally, nanosized alumina particles are located at grain boundaries and also distributed in the grain interiors. This strongly affects those mechanisms related to load transfer and thermal expansion coefficient (CTE) (Ref 40). The agglomeration is minimum. This resulted in a noteworthy distribution of alumina in aluminum matrix. Accordingly, the alumina interparticle distances in the aluminum matrix are lower than that of agglomerated clusters, inducing the increase of particle-dislocation interaction (Ref 41). The enhanced hardness and strength of the Al-Al2O3 nanocomposite can be attributed to the stronger diffusional bonds and to the structural integrity achieved after SPS.
From previous results, it is clear that nanosized alumina particles have a significant effect on tensile and yield strength of nanocomposites. The tensile curves of the two materials are shown in Fig. 10.
The tensile strength of the composite was 77 MPa, while the pure Al one was 61 MPa. The addition of nanoparticles seems to have no influence on the material ductility.
The enhanced strength of the nanocomposite is attributed to the stronger diffusional bonds and homogeneous distribution of nanosized alumina particles in aluminum matrix during spark plasma sintering. Moreover, the homogeneous distribution of the reinforcements causes a decrease in the alumina particles distances and so a higher energy is required for the dislocations movement. The pure aluminum strength could be a result of the coarse grains structure due to the absence of reinforcement that normally impedes the grain boundaries movement during grain growth.
Fractography
The tensile fracture surfaces of the studied materials are shown in Fig. 11. The pictures show that fracture is mainly due to pores coalescence.
The nucleation of microvoids can be caused by particle cracking or interfacial failure between the alumina particles and the matrix. Microvoids grow during tension; they coalesce when adjacent microvoids link together or the material between microvoids experiences necking. Accordingly, microvoid coalescence leads to fracture. The alumina nanoparticles increase propensity to grain boundary fracture through the formation of continuous brittle phase alone grain boundaries. Actually, pure aluminum fracture is governed by microvoids coalescence.
By adding nanoparticles, the fracture is mainly transgranular because of grain boundary weakening and porosity coalescence. Higher local ductility is experienced by pure aluminum. This behavior is also confirmed by compositional maps performed at SEM (Fig. 12).
Conclusions
The microstructural behavior and the final mechanical properties of aluminum matrix composites with 2 wt.% of nanosized reinforcing Al2O3 particles produced via SPS have been found to be strongly dependent on the alumina blended to pure aluminum before processing. In particular:
-
The material density strongly increases with low percentage addition of nanosized alumina particles.
-
The reduction of porosity shows a noticeable increase in hardness and elastic modulus if compared to the pure SPSed aluminum.
-
Tensile strength also increases even if no differences in materials ductility are recorded.
-
The fracture surface observations show that composite mainly deforms in a transgranular way, while pure aluminum exhibits higher local ductility.
References
D.K. Koli, G. Agnihotri, and R. Purohit, A Review on Properties, Behaviour and Processing Methods for Al-Nano Al2O3 Composites, Procedia Mater. Sci., 2014, 6, p 567–589
M.T. Khorshid, S.J. Jahromi, and M. Moshksar, Mechanical Properties of Tri-Modal Al Matrix Composites Reinforced by Nano-and Submicron-Sized Al2O3 Particulates Developed by Wet Attrition Milling and Hot Extrusion, Mater. Des., 2010, 31(8), p 3880–3884
Z.R. Hesabi, A. Simchi, and S.M.S. Reihani, Structural Evolution During Mechanical Milling of Nanometric and Micrometric Al2O3 Reinforced Al Matrix Composites, Mater. Sci. Eng, 2006, A428(1–2), p 159–168
R. Casati, F. Bonollo, D. Dellasega, A. Fabrizi, G. Timelli, A. Tuissi, and M. Vedani, Ex Situ Al-Al2O3 Ultrafine Grained Nanocomposites Produced Via Powder Metallurgy, J. Alloys Compd., 2014, 615, p S386–S388
S.C. Tjong, Novel Nanoparticle-Reinforced Metal Matrix Composites with Enhanced Mechanical Properties, Adv. Eng. Mater., 2007, 9(8), p 639–652
Y.-C. Kang and S.L.-I. Chan, Tensile Properties of Nanometric Al2O3 Particulate-Reinforced Aluminum Matrix Composites, Mater. Chem. Phys., 2004, 85(2–3), p 438–443
G. Sweet, M. Brochu, R. Hexemer, I. Donaldson, and D. Bishop, Consolidation of Aluminum-Based Metal Matrix Composites Via Spark Plasma Sintering, Mater. Sci. Eng., A, 2015, 648, p 123–133
N. Al-Aqeeli, G. Mendoza-Suarez, C. Suryanarayana, and R. Drew, Development of New Al-Based Nanocomposites by Mechanical Alloying, Mater. Sci. Eng., 2008, A480(1), p 392–396
C. Suryanarayana, Synthesis of Nanocomposites by Mechanical Alloying, J. Alloys Compd., 2011, 509, p S229–S234
S.C. Tjong and Z.Y. Ma, Microstructural and Mechanical Characteristics of In Situ Metal Matrix Composites, Mater. Sci. Eng., 2000, R29(3), p 49–113
Y. Peng, M. Zhi, and S.C. Tjong, Structure, Thermal and Mechanical Properties of in Situ Al-Based Metal Matrix Composite Reinforced With Al2O3 and TiC Submicron Particles, Mater. Chem. Phys., 2005, 93(1), p 109–116
R. German, Powder Metallurgy, 2nd ed., Wiley, New York, 1994
F.A. Khalid, O. Beffort, U.E. Klotz, B.A. Keller, P. Gasser, and S. Vaucher, Study of Microstructure and Interfaces in an Aluminium-C60 Composite Material, Acta Mater., 2003, 51(15), p 4575–4582
K. Dash, D. Chaira, and B.C. Ray, Synthesis and Characterization of Aluminium-Alumina Micro- and Nano-Composites by Spark Plasma Sintering, Mater. Res. Bull., 2013, 48, p 2535–2542
E. Ghasali, M. Alizadeh, and T. Ebadzadeh, Mechanical and Microstructure Comparison Between Microwave and Spark Plasma Sintering of Al-B4C Composite, J. Alloys Compd., 2016, 655, p 93–98
E. Ghasali, A. Pakseresht, A. Rahbari, H. Eslami-shahed, M. Alizadeh, and T. Ebadzadeh, Mechanical Properties and Microstructure Characterization of Spark Plasma and Conventional Sintering of AleSiCeTiC Composites, J. Alloys Compd., 2016, 666, p 366–371
C. Wolff, S. Mercier, H. Couque, and A. Molinari, Modeling of Conventional Hot Compaction and Spark Plasma Sintering based on Modified Micromechanical Models of Porous Materials, Mech. Mater., 2012, 49, p 72–91
Z.-F. Liu, Z.-H. Zhang, J.-F. Lu, A.V. Korznikov, E. Korznikova, and F.-C. Wang, Effect of Sintering Temperature on Microstructures and Mechanical Properties of Spark Plasma Sintered Nanocrystalline Aluminum, Mater. Des., 2014, 64, p 625–630
Z.A. Munir, V. Dat, and A. Quach, Grain-Boundary Enthalpies of Cubic Yttria-Stabilized Zirconia, J. Am. Ceram. Soc., 2011, 94(7), p 1–19
K.L. Firesteina, S. Corthay, A.E. Steinman, A.T. Matveev, A.M. Kovalskii, I.V. Sukhorukova, D. Golberg, and D.V. Shtansky, High-Strength Aluminum-Based Composites Reinforced with BN, AlB2 and AlN Particles Fabricated Via Reactive Spark Plasma Sintering of Al-BN Powder Mixtures, Mater. Sci. Eng., A, 2017, 681, p 1–9
N.K. Babu, K. Kallip, M. Leparoux, K.A. AlOgab, X. Maeder, and Y.A. Rojas, Dasilva, Influence of Microstructure and Strengthening Mechanism of AlMg5-Al2O3 Nanocomposites Prepared Via Spark Plasma Sintering, Mater. Des., 2016, 95, p 534–544
Z. Tan, L. Wang, Y. Xue, P. Zhang, T. Cao, and X. Cheng, High-Entropy Alloy Particle Reinforced Al-Based Amorphous Alloy Composite with Ultrahigh Strength Prepared by Spark Plasma Sintering, Mater. Des., 2016, 109, p 219–226
S. Mula, K. Mondal, S. Ghosh, and S.K. Pabi, Structure and Mechanical Properties of Al-Ni-Ti Amorphous Powder Consolidated by Pressure-Less, Pressure-Assisted and Spark Plasma Sintering, Mater. Sci. Eng., A, 2010, 527, p 3757–3763
J. Zhanga, H. Shi, M. Cai, L. Liu, and P. Zhai, The Dynamic Properties of SiCp/Al Composites Fabricated by Spark Plasma Sintering with Powders Prepared by Mechanical Alloying Process, Mater. Sci. Eng., A, 2009, 527, p 218–224
G.A. Sweet, M. Brochu, R.L. Hexemer, Jr., I.W. Donaldson, and D.P. Bishop, Consolidation of Aluminum-Based Metal Matrix Composites Via Spark Plasma Sintering, Mater. Sci. Eng., 2015, A648, p 123–133
W. Daoush, A. Francis, Y. Lin, and R. German, An Exploratory Investigation on the In Situ Synthesis of SiC/AlN/Al Composites by Spark Plasma Sintering, J. Alloys Compd., 2015, 622, p 458–462
Z. Sadeghian, B. Lotfi, M.H. Enayati, and P. Beiss, Microstructural and Mechanical Evaluation of Al-TiB2 Nanostructured Composite Fabricated by Mechanical Alloying, J. Alloys Compd., 2011, 509, p 7758–7763
K. Mizuuchi, K. Inoue, Y. Agari, T. Nagaoka, M. Sugioka, M. Tanaka, T. Takeuchi, J. Tani, M. Kawahara, Y. Makino, and M. Ito, Processing and Thermal Properties of Al/AlN Composites in Continuous Solid-Liquid Co-Existent State by Spark Plasma Sintering, Compos. B, 2012, 43, p 1557–1563
M.O. Durowoju, E.R. Sadiku, S. Diouf, M.B. Shongwe, and P.A. Olubambi, Spark Plasma Sintering of Graphite-Aluminum Powder Reinforced with SiC/Si Particles, Powder Technol., 2015, 284, p 504–513
S. Diouf and A. Molinari, Densification Mechanisms in Spark Plasma Sintering: Effect of Particle Size and Pressure, Powder Technol., 2012, 221, p 220–227
S. Devaraj, S. Sankaran, and R. Kumar, Influence of Spark Plasma Sintering Temperature on the Densification, Microstructure and Mechanical Properties of Al-4.5wt.% Cu Alloy, Acta Metall. Sin., 2013, 26(6), p 761–771
S. Decker, S. Martin, and L. Krüger, Influence of Powder Particle Size on the Compaction Behavior and Mechanical Properties of a High-Alloy Austenitic CrMnNi TRIP Steel During Spark Plasma Sintering, Metall. Mater. Trans., 2016, A47(1), p 170–177
E.A. Olevsky and L. Froyen, Impact of thermal diffusion on densification during SPS, J. Amer. Ceram. Soc., 2009, 92(s1).
N. Saheb, M.S. Khan, and A.S. Hakeem, Effect of Processing on Mechanically Alloyed and Spark Plasma Sintered Al-Al2O3 Nanocomposites, J. Nanomater., 2015, 16(1), p 377
J.J. Grácio, C.R. Picu, G. Vincze, N. Mathew, T. Schubert, A. Lopes, and C. Buchheim, Mechanical Behavior of Al-SiC Nanocomposites Produced by Ball Milling and Spark Plasma Sintering, Metall. Mater. Trans., 2013, A44(11), p 5259–5269
J. Garay, Current-Activated, Pressure-Assisted Densification Of Materials, Annu. Rev. Mater. Res., 2010, 40, p 445–468
R. Casati, Aluminum Matrix Composites Reinforced with Alumina Nanoparticles, PoliMi Springer Briefs, 2015, doi:10.1007/978-3-319-27732-5_5
F. He, Q. Han, and M.J. Jackson, Nanoparticulate Reinforced Metal Matrix Nanocomposites—A Review, Int. J. Nanoparticles, 2008, 1(4), p 301–309
H. Asgharzadeh, A. Simchi, and H.S. Kim, Microstructural Features, Texture and Strengthening Mechanisms of Nanostructured AA6063 Alloy Processed by Powder Metallurgy, Mater. Sci. Eng., 2011, A528(12), p 3981–3989
K. Deng, J. Shi, C. Wang, X. Wang, Y. Wu, K. Nie, and K. Wu, Microstructure and Strengthening Mechanism of Bimodal Size Particle Reinforced Magnesium Matrix Composite, Compos. A, 2012, 43(8), p 1280–1284
J. Pelleg, Mechanical Properties of Materials, Springer, New York, 2012
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadeghi, B., Shamanian, M., Ashrafizadeh, F. et al. Influence of Al2O3 Nanoparticles on Microstructure and Strengthening Mechanism of Al-Based Nanocomposites Produced via Spark Plasma Sintering. J. of Materi Eng and Perform 26, 2928–2936 (2017). https://doi.org/10.1007/s11665-017-2699-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-017-2699-2