Abstract
In order to characterize the interfacial behavior of brazed joints and offer theoretical basis for the applications of TiZrCuNi-based composite fillers, Cf/SiC composite and TC4 were brazed by TiZrCuNi filler, and the microstructures of joints versus temperature and versus holding time were systematically studied in this paper. The mechanical properties of brazed joints were measured and analyzed. The results showed that Ti(Zr)C, Ti5Si3, Ti2Cu, TiNi, TiZrCu2, Ti2(Cu,Ni) and Ti(s,s) were the predominant compounds in the joints. Brazing temperature had a distinct effect on the microstructures of joints: with the increase of brazing temperature, the structure of brazed joints was reduced from four parts to three parts, and the wavy reaction layer became continuous and much thicker. While holding time had a similar but weaker effect on microstructures: with the extension of holding time, the reaction layer became thicker, but it was difficult to induce the decrease in the structural parts of joint. The thickness of reaction layer determined the mechanical properties of joints. The results were beneficial for the selection of reinforced phases and the design of composite fillers to obtain better mechanical performances. When the brazing temperature was 940 °C and the holding time was 25 min, the maximum shear strength of brazed joints attained a value of 143.2 MPa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon fiber-reinforced SiC (Cf/SiC) ceramic matrix composites have attracted great interest in aeronautical turbines and advanced rocket propulsion thrust chambers for their outstanding high-temperature properties, such as excellent resistance to wear and oxidation (Ref 1-3). In many engineering applications, Cf/SiC composites are usually joined to high-temperature metals, especially titanium alloys, to fabricate complex large-scale structural components, because of their inherently hard and brittle characteristics (Ref 4, 5). TC4, a type of α + β titanium alloy, is widely used in the aerospace field, due to its excellent properties, like low density and heat resistance (Ref 6, 7). To enhance their potential for practical applications, it is essential to achieve robust and reliable joining and integration between Cf/SiC composite and TC4.
Brazing (Ref 8-10) is a relatively simple and cost-effective joining method, which has been applicable to a wide range of ceramics and composites. One major consideration in Cf/SiC composites/TC4 brazing is the residual stresses, which is caused by mismatch in the coefficient of thermal expansion (CTE) between Cf/SiC composites and TC4. This issue can impair the mechanical properties of the joints significantly. To overcome this problem, low CTE reinforcements in the joining materials have been applied in the brazing. Xiong et al. (Ref 11) studied the active brazing technology of Cf/SiC composite and Ti-6Al-4V using TiC particle-reinforced Ag-Cu-Ti filler metal. The TiC particles reduce the CTE of brazing alloy, resulting in the improvement of the joint strength. But the joints brazed by Ag-Cu-Ti filler must serve under 700 °C, which greatly limits the applications of Cf/SiC composites at high temperature. Cui et al. (Ref 12) introduced W particle-reinforced TiZrCuNi active filler to braze Cf/SiC composite to Ti-6Al-4V. It was discussed that the dispersion of W particles in the interlayer could effectively relieve residual stresses by the low CTE and load transfer mechanism. It was also demonstrated that TiZrCuNi filler exhibited excellent wettability and spreadability on Cf/SiC composites and the joints had superb high-temperature properties. However, the interfacial behavior and the interfacial microstructures adjacent to Cf/SiC composite have not been reported in detail in Ref 12, which is critical to ceramic matrix composites (CMCs) brazing. As known, the appropriate interfacial reaction between Cf/SiC composite and filler is one of the key factors for improving the mechanical properties of brazed joints: if limited, the insufficient bond occurred; if over-reaction, the intrinsic brittleness of reaction layer can impair the mechanical properties of joints. Therefore, the research on interfacial behavior has great significance for the development of active composite filler composed of TiZrCuNi and reinforced phase (such as SiC, ZrC and graphic).
In this paper, Cf/SiC composite and Ti-6Al-4V were brazed by TiZrCuNi filler under different conditions. The microstructures of the brazed joints, especially interfacial microstructures, were discussed in detail to investigate the interfacial behavior adjacent to Cf/SiC composite. Moreover, the correlation between the microstructure and the mechanical properties was analyzed. This research would lay the foundation for the further study of composite or reactive-composite brazing using TiZrCuNi-based composite fillers.
Materials and Methods
Three-dimensional carbon fiber-reinforced SiC matrix (3D-Cf/SiC) composite used in this investigation was fabricated by the Polymer Infiltration Pyrolysis (PIP) with the Chemical Vapor Deposition (CVD). It had a density of 1.8 g/cm3, porosity of 10-15 vol.% and carbon fiber volume fraction of 45-50%. The chemical composition of TC4 was Ti-6Al-4V (wt.%). The Cf/SiC composite and TC4 were sectioned into two kinds of pieces with sizes of 5 mm × 5 mm × 5 mm and 10 mm × 10 mm × 3 mm, respectively. The surfaces to be joined were ground by 400 grit silicon carbide papers for Cf/SiC composite and 150 grit for the TC4. They were then ultrasonic cleaned in ethanol and dried below 50 °C prior to brazing.
The filler powder was Ti-13Zr-21Cu-9Ni (wt.%) with an average size of 300 mesh. It was turned into a paste by alcohol and placed between Cf/SiC composite and TC4, as shown in Fig. 1(a). The assemblies of Cf/SiC composite and TC4 were brazed at 920-960 °C for 15-35 min in a vacuum furnace with vacuum level better than 6 × 10−3 Pa in order to suppress undesirable reactions with oxygen. The heating rate was 15 °C/min. Then, all samples were furnace-cooled to the room temperature.
Subsequent to brazing, the brazed joints were sectioned, mounted in epoxy, ground and polished, and examined using SEM coupled with EDS on a LEO-1450 system for analyzing the microstructures of the joints. In order to analyze the reaction layer between Cf/SiC composite and braze more reasonably, we define the average thickness (d) of reaction layer as follows:
where A is the area of reaction layer in the SEM micrograph, and L is the length of reaction layer in the same micrograph. Moreover, the difference between maximum thickness and average thickness is the upper bound of error, and the difference between minimum thickness and average thickness is the lower bound on error. The Cf/SiC composite was removed completely and grinded appropriately before the joints were analyzed by x-ray diffraction (XRD) with Cu Kα radiation. The shear strength testing was performed at room temperature; the schematic is shown in Fig. 1(b). Each shear strength value of the brazed joints was the average of three measurements in this study.
Results and Discussion
Microstructure of Cf/SiC Composite/TC4 Joint Brazed with TiZrCuNi
Figure 2 shows the SEM images of Cf/SiC composite/TC4 joint brazed by TiZrCuNi filler at 920 °C for 35 min. Table 1 gives the average chemical compositions of brazed joint at the same condition.
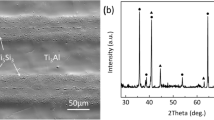
Discontinuity and structural imperfections such as interfacial voids or cracks are not detected at the interfaces. The well-bonded joint reveals that TiZrCuNi filler exhibits good wetting and intimate contact with Cf/SiC composite and TC4. It is noted that a variety of products are generated by reactions at the interfaces, suggesting metallurgical bonding occurred between filler and substrates. The whole joint could be divided into four parts: part I (adjacent to Cf/SiC composite), part II and part III (interlayer), part IV (close to TC4), as shown in Fig. 2(a).
The dark black part I is the reaction layer (marked as A in Fig. 2b) adjacent to Cf/SiC composite with a thickness of 1-2 μm. According to EDS results, it mainly contains Ti and C elements with some Zr and Si. Based on the analysis of XRD patterns (Fig. 3a), it is inferred as Ti(Zr)C with some Ti5Si3.
Part II mainly consists of dark gray (marked by B) and white (marked by C) zones, as shown in Fig. 2(b). According to the EDS results (Table 1), zone B is rich in Ti, Cu and Ni. Combined with Ti-Cu-Ni (Ref 13) ternary phase diagram (Fig. 4a), B is determined to be a mixture of Ti2Cu + TiNi, which has been proven by the XRD pattern in Fig. 3(b). The EDS results indicate that zone C is composed of Ti, Zr, Cu and Ni. In order to analyze this phase, Cu and Ni atoms are considered as same elements, since Ni can be the substitute for Cu in the Cu-Ni intermetallic compound lattice (Ref 14). Therefore, combined with Ti-Zr-Cu (Ref 15) ternary diagram (Fig. 4b) and XRD results, zone C should be a mixture of TiZrCu2 and Ti2(Cu,Ni) compounds. Part II is the microstructure of residual Ti-Zr-Cu-Ni filler material. Compared with part II, the dark block phases with some white dots (marked by D) are detected in part III, as shown in Fig. 2(c). This phenomenon is related to the diffusion of Ti atoms from TC4 into interlayer. In the zone adjacent to TC4, Ti content has increased due to Ti atomic diffusion. So, during the freezing, β-Ti compounds separate out firstly and then decompose into α-Ti and Ti2Cu compounds. Consequently, D is mainly Ti solid solution.
A bright dendritic phase is shown in part IV (Fig. 2c), indicating that the formation of diffusion layer between interlayer and TC4. Ti2Cu phase is formed at TC4 alloy side due to the Cu elements diffusion and the higher Ti content in the base metal. The closer distance nearby TC4, the lower concentration of the Cu element, which makes the formation of Ti(s,s) solution easily. Therefore, after cooling down, the part IV is composed of coarse Ti2Cu phase and a eutectoid microstructure of (α-Ti + fine Ti2Cu), consistent with Ref 12.
Interfacial Behavior and Microstructures Of Joints Brazed Under Different Parameters
Effect of Brazing Temperature
The microstructures of the brazed joints versus brazing temperature are shown in Fig. 5. Obviously, from Fig. 5(a), (b), and (c), with the increase of temperature, the original microstructure of filler materials (part II) gradually disappears in the joint, and the structure of interlayer reduces from four parts to three parts. More Cu atoms diffuse into TC4 and more Ti atoms migrate into interlayer, and the thickness of diffusion layer (part IV) thus increases greatly. Accordingly, Ti content in interlayer rises, leading to the precipitation of black block α-Ti compounds. As shown in Fig. 5(b) and (c), the black block phase distributes uniformly in part III and its content increases gradually with the elevated temperature. Moreover, the island-shape Ti(s,s) compounds in part III increase and connect to the diffusion layer of Ti(s,s) + Ti2Cu.
From Fig. 5(d), (e), and (f), the wavy reaction layer (part I) gradually becomes continuous and smooth. Meanwhile, the thickness of reaction layer increases significantly. According to Arrhenius law, diffusion coefficient (D) increases with the elevated temperature (T). When the brazing temperature rises, more Ti atoms will migrate into the interfacial zone adjacent to Cf/SiC composite. The Ti contents in the interfacial zone adjacent to Cf/SiC composite (t = 25 min) at 920, 940 and 960 C are 62, 68 and 74 at.%, respectively. Apparently, Ti content in interfacial zone adjacent to Cf/SiC composite has been increased with the elevated brazing temperature. If the liquid is considered to be the ideal solution, the chemical potentials of titanium in liquid (μ Ti) can be written as:
where μ Ti * is the chemical potential of the component in the standard state, a Ti is activity of titanium, and x Ti is mole fraction of titanium in the molten filler alloy adjacent to Cf/SiC composite. R is the gas constant, and T is the absolute temperature.
The value of μ Ti * is determined by the following equation (Ref 16):
where T represents the absolute temperature.
According to Eq 2 and 3, the chemical potentials of titanium in liquid were calculated. As shown in Fig. 6, with the rising temperature, the mole fraction of titanium is improved from 0.62 to 0.74, and the value of μ Ti has been improved from 4185 to 4569 J/mol. This means the interfacial reactions are much easier to occur, and the degree of interfacial reactions increases dramatically.
Effect of Holding Time
Compared with brazing temperature, holding time has a weaker effect on the microstructure of joints. The extension of holding time does not change the configuration of brazed joints: at lower brazing temperature (T = 920 °C), all interlayers contain four parts, as shown in Fig. 7. Though the reaction layer becomes thicker with the extension of time, it is still wavy shape. At higher brazing temperature (T = 940 and 960 °C), the interlayers all contain three parts, as shown in Fig. 8 and 9, and the reaction layers are continuous.
In the case of lower brazing temperature (T = 920 °C), Ti atoms, which diffuse into part II, are few, so the original filler (part II) is left. The activity of Ti in the interfacial zone decreases ascribe to the progress of reactive wetting, so the interfacial reactions gradually reduce. It is well known that TiC has a very small critical nucleation size of precipitates and randomly distributes in the front of the solid-liquid interface. Thus, fewer interfacial products form wavy reaction layer between Cf/SiC composite and interlayer. Ti atomic diffusion is accelerated by the elevated temperature (T = 940 °C), resulting in the appearance of few black block Ti(s,s) in part III. Meanwhile, the reaction layer becomes continuous in some positions, as shown in Fig. 8(a) and (b). In this case, more Ti atoms diffuse into the interlayer due to the prolonged holding time. So the quantity and shape of block Ti(s,s) increase, and the whole reaction layer becomes continuous and thicker. Similarly, the interlayers at higher brazing temperature (T = 960 °C) all contained three parts, as shown in Fig. 9. As analyzed above, the interfacial reactions are enhanced by rising temperature and lots of compounds nucleate and grow at interface, leading to the formation of continuous and smooth reaction layer. With the extension of the reactive time, the reaction layer becomes thicker significantly.
Overall, with the enhanced brazing parameters (include brazing temperature and holding time), all reaction layers become thicker, as shown in Fig. 10. Obviously, the effect of holding time is weaker than that of brazing temperature. From Fig. 10(b), when the layer thickness is about 2 μm, it is hard to be thicker by improving temperature or adding time. What is more, it is too thick for reaction layer to bear the large residual stresses, caused by the mismatch in coefficient of thermal expansion. Micro-cracks thus will be generated in some zones of the layer due to the intrinsic brittleness of carbide. Additionally, compared with Fig. 10(a) and (b), the error decreases significantly, indicating that the reaction layer becomes continuous with the improvement of brazing parameters. This is mainly caused by the increase of Ti diffusion rate.
Mechanical Performance of Brazed Joints
The effects of brazing temperature and holding time on shear strength of brazed joints are shown in Fig. 11. The shear strength of brazed joints reaches its maximum (143.2 MPa) with the brazing temperature at 940 °C and the holding time of 25 min. In order to investigate the relationship between brazing parameters (including brazing temperature and holding time) and microstructure, the fracture microstructures of joints were analyzed.
In the case of weaker parameters (e.g., 920 °C, 15 min), the shear strength of joints is low (88.71 MPa). This is attributed to the insufficient interfacial reactions between the filler alloy and the based material, arising from the less atomic diffusion. As seen from Fig. 7(d), reaction layer is extremely thin at the interface between Cf/SiC composite and filler, resulting in only a few carbon fibers pulled out (see Fig. 12b). The fracture morphology (marked by zone C) in Fig. 12(c) shows the characteristic of brittle failure behavior. As analyzed in Sect. 3.1, the residual filler retained in part II is mainly composed of brittle (Ti,Zr)2Cu compounds. Besides, there are large residual thermal stresses caused by the different CTE of base materials. The cracks thus are formed during cooling, as shown in Fig. 12(c), which will decrease the shear strength of the joints.
With the increase of brazing parameters (e.g., 940 °C, 25 min), the shear strength of brazed joints increases significantly (143.2 MPa). More Ti atoms diffuse to the Cf/SiC composite/TiZrCuNi interface, and more interfacial reactions occur. Thus, the thickness of reaction layer increases, which is beneficial for improving the interfacial bonding. Apparently, from Fig. 13(b), a part of carbon fibers is pulled out and some honeycomb pits are left during the shear testing. In the brazing process, the molten filler penetrated into the cracks or voids of Cf/SiC composite by capillary and retained in the Cf/SiC composite during freezing (denoted by C in Fig. 13c). The fracture morphology of region C shows river pattern named tearing ridge. It indicates that the fracture possesses some plastic, which is beneficial for the shear strength improvement (Ref 12). Besides, more Ti(s,s) are formed in the interlayer due to the increase of Ti content, contributing to the joint strength enhancement.
However, with the further increase of brazing parameters (such as 960 °C, 35 min), the shear strength of brazed joints greatly decreases (86.97 MPa). The fracture morphology of the joints is mainly composed of TiC and Ti5Si3 compounds (see Fig. 14a). The fracture surface of the joint is flush, and very few honeycomb pits and extracted carbon fibers are observed. When brazing parameters are relatively stronger, much more brittle compounds are formed at interface, such as TiC (CTE, 7.4 × 10−6 °C−1) and Ti5Si3 (CTE, 11 × 10−6 °C−1), due to the more intensive Ti atomic diffusion, which leads to the over-reaction between Cf/SiC composite and filler. The fractographic analysis is in good agreement with the microstructure analysis in Fig. 9(f). Due to the different CTE between Cf/SiC composite (3.0 × 10−6 °C−1) and TC4, large residual thermal stresses will be formed during cooling down. So, the micro-cracks mainly propagate along the reaction layer adjacent to Cf/SiC composite, as shown in Fig. 14(b).
Noticeably, the shear strength of joints obtained at 920 °C increases with the extension of holding time (see Fig. 11). At lower temperature, Ti exhibits a lower diffusion rate, so that few Ti atoms diffuse into interlayer from TC4. While with holding time prolonging, the diffused Ti atoms will accumulate to a certain degree. Compared with the diffusion layer in Fig. 7(c) and 8(a), as holding time lasts over 35 min, it can be inferred that black block Ti(s,s) begins to appear in part II, implying part II-to-part III translation. Meanwhile, more interfacial reactions will occur, leading to the appearance of continuous reaction layer. Though the contact area between Cf/SiC composite and interlayer has been increased due to the wavy reaction layer, which is beneficial for improving the bond strength. But, under the large residual stresses, the thicker and more continuous reaction layer is prone to crack, even break, due to the intrinsic nature of brittle compounds. Thus, brazed at 920 °C for more than 35 min, the shear strength value is deduced to be less than 150 MPa.
In summary, the shear strength of the joints is mainly affected by the reaction layer adjacent to Cf/SiC composite, which will have poor mechanical properties due to too thin or too thick reaction layer. In addition, appropriate Ti(s,s) in the interlayer can present certain plasticity and improve the shear strength of the joints.
Conclusion
Reliable brazing of Cf/SiC composite and TC4 alloy was achieved by using TiZrCuNi filler. The interfacial microstructures and interfacial behavior were analyzed systematically. The relationship between microstructure and mechanical properties of joints was discussed in detail. The typical microstructure is determined to be Cf/SiC composite/Ti(Zr)C + Ti5Si3/Ti2Cu + TiNi + TiZrCu2 + Ti2(Cu,Ni)/TiZrCu2 + Ti2(Cu,Ni) + Ti(s,s) + TiNi + Ti2Cu/Ti(s,s) + Ti2Cu/TC4. Brazing temperature has a distinct effect on microstructures of brazed joints: with the increase of brazing temperature, the constitution of joints reduces from four parts to three parts, and the wavy reaction layer becomes continuous and much thicker; while holding time has a similar but weaker effect on microstructures of brazed joints: with the extension of holding time, interfacial reaction layer becomes thicker, but the constitution of joints does not change. Appropriate interfacial reaction is one key factor to affect the mechanical properties of brazed joints: both too thin and too thick reaction layers will cause the sharp decrease of mechanical properties. The formation of black Ti(s,s) is beneficial for improving the joint plasticity and strength. The highest average shear strength reaches 143.2 MPa when brazed at 940 °C for 25 min.
References
T. Ishikawa, S. Kajii, K. Matasanaga, and T. Hogani, A Tough, Thermally Conductive Silicon Carbide Composite with High Strength up to 1600 °C in Air, Science, 1998, 282, p 1295–1297
G. Boitier, J.L. Chermant, and J. Vicens, Multiscale Investigation of the Creep Behavior of a 2.5D Cf/SiC Composition, J. Mater. Sci., 1999, 34, p 2759–2767
R. Naslain, Design, Preparation and Properties of Non-oxide CMCs for Application in Engines and Nuclear Reactors: An Overview, Compos. Sci. Technol., 2004, 64, p 155–170
M. Singh and R. Asthana, Brazing of Advanced Ceramic Composites: Issues and Challenges, Ceram. Trans., 2007, 198, p 9–14
R. Asthana and M. Singh, Active Metal Brazing of Advanced Ceramic Composites to Metallic Systems, Advances in Brazing: Science, Technology and Applications, D.P. Sekulic, Ed., Woodhead Publishing Limited, Cambridge, 2013, p 323–360
W. Guo, L. Wang, Y. Zhu, and P.K. Chu, Microstructure and Mechanical Properties of C/C Composite/TC4 Joint with Inactive AgCu Filler Metal, Ceram. Int., 2015, 5, p 7021–7027
I.P. Semenova, G.I. Raab, E.R. Golubovskiy, and R.R. Valiev, Service Properties of Ultrafine-Grained Ti-6Al-4V Alloy at Elevated Temperature, J. Mater. Sci., 2013, 48, p 4806–4812
G.B. Lin, J.H. Huang, and H. Zhang, Joints of Carbon Fiber-Reinforced SiC Composites to Ti-Alloy Brazed by Ag-Cu-Ti Short Carbon Fibers, J. Mater. Process. Technol., 2007, 189, p 256–261
B. Chen, H.P. Xiong, X. Wu, Joining of Cf/SiC Composite with AuNi(Cu)-Cr Brazing Fillers and Interfacial Reactions, Weld. World, 2016, 60, p 813–819
Y.Z. Liu, L.X. Zhang, and C.B. Liu, Brazing C/SiC Composites and Nb with TiNiNb Active Filler Metal, Sci. Technol. Weld. Join., 2011, 16, p 193–198
J.H. Xiong, J.H. Huang, and Z.P. Wang, Joining of Cf/SiC Composite to Ti Alloy Using Composite Filler Materials, Mater. Sci. Technol. Lond., 2009, 25, p 1046–1050
B. Cui, J.H. Huang, and C. Cai, Microstructures and Mechanical Properties of Cf/SiC Composite and TC4 Alloy Joints Brazed with (Ti-Zr-Cu-Ni) + W Composite Filler Materials, Compos. Sci. Technol., 2014, 97, p 19–26
H. Zhang, Y. He, and F. Yang, Thermodynamic Assessment of Cu-Ni-Ti Ternary System Assisted with Key Measurements, Thermochim. Acta, 2013, 574, p 121–132
X.R. Song, H.J. Li, and V. Casalegno, Microstructure and Mechanical Properties of C/C Composite/Ti6Al4V Joints with a Cu/TiCuZrNi Composite Brazing Alloy, Ceram. Int., 2016, 5, p 6347–6354
G. Effenberg, SpringerMaterials, http://materials.bibliotecabuap.elogim.com/msi/docs/sm_msi_r_10_019042_01. Accessed 29 Apr 2016
N. Frage, L. Levin, E. Manor, R. Shneck, and J. Zabicky, Iron-Titanium-Carbon System. I. Equilibrium Between Titanium Carbide (TiC x ) of Various Stoichiometries and Iron-Carbon Alloys, Scripta Mater., 1996, 7, p 791–797
Acknowledgments
The research was supported by the National Natural Science Foundation of China (No. 51175037), People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, D., Huang, J., Cui, B. et al. Interfacial Behavior and Its Effect on Mechanical Properties of Cf/SiC Composite/TiAl6V4 Joint Brazed with TiZrCuNi. J. of Materi Eng and Perform 26, 1114–1121 (2017). https://doi.org/10.1007/s11665-017-2571-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-017-2571-4