Abstract
The corrosion inhibition effect of phenanthroline (Phen) and its cobalt complex (CoPhen) on the corrosion of carbon steel in sulphuric acid solutions was studied using potentiodynamic polarization and electrochemical impedance spectroscopy techniques at 20, 30, and 40 °C. Scanning electron microscopy techniques were used to characterize the mild steel surface. The results indicate that the compounds inhibit the corrosion of mild steel in H2SO4 solutions through a predominant physical adsorption following the Langmuir adsorption isotherm. Polarization curves suggest that the complex and ligand are mixed-type inhibitors. The efficiency of the inhibitors is concentration- and temperature-dependent and follows the trend CoPhen > Phen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acid solutions are widely used in industry for acid pickling of iron and steel, chemical cleaning and processing, ore production, and oil well acidification. Due to the aggressiveness of these acid solutions against metallic materials such as carbon steels, the use of corrosion inhibitors is usually required to minimize the corrosion attack. A number of compounds have been explored as corrosion inhibitors and most of the well-known inhibitors are organic compounds containing nitrogen, sulfur, and oxygen atom in their molecular structures. Among them, nitrogen-containing heterocyclic compounds are considered to be effective corrosion inhibitors on steel in acid media (Ref 1). These compounds function as inhibitors by adsorption on the metal surface through the nitrogen heteroatom, the triple or conjugated double bonds, and/or aromatic rings in their molecular structures. Also, some organic (coordinating) compounds could react with metal ions in solution resulting from corrosion process to form insoluble complexes that may inhibit the corrosion reaction (Ref 2). These ligands and their complexes as corrosion inhibitors have not been fully explored.
A known coordinating organic compound is 1,10-phenanthroline (hereafter abbreviated Phen). The study of Phen and its derivatives as corrosion inhibitors is of significant interest because of their structures which suggests a number of possible mechanisms by which they can interact with the metal surface (Ref 3). Phen is a tricyclic aromatic bidentate N,N-heterocyclic ligand known to form strong complexes or chelate substances with most metal ions (Ref 4). It has been reported to behave as a predominantly cathodic corrosion inhibitor for mild steel in H2SO4 solutions (Ref 5), and as a mixed-type inhibitor in HCl and chloride containing solutions (Ref 6-8). Being a strong complexing ligand for metals, chemisorption on the metal surface via the electron donor properties of the nitrogen atoms as well as the π-electron of the aromatic ring has also been suggested (Ref 3, 5). Phen could also form insoluble metal complexes on the surface of the metal which further inhibits corrosion. However, there are no reports on the corrosion inhibition of its metal complexes. Phen derivatives have also been acknowledged as efficient corrosion inhibitors for steel in acidic media (Ref 3, 6, 9, 10).
In the literature, there appears to be very few works on the corrosion inhibition behavior of metal complexes in acidic media. Rangelov and Mircheva (Ref 2) reported the complexes of tetramethyldithio-oxamide with Fe(II), Co(II), Ni(II), and Sn(II) as corrosion inhibitors of steel in 1 M H2SO4 solutions at a temperature range of 20-80 °C. Zn(II) complex of pyridoxal-(4-methylthiosemicarbazone) was reported by Ita and Offiong (Ref 11) as an effective corrosion inhibitor for mild steel in HCl solutions. Khaled et al. (Ref 12) investigated the corrosion inhibiting properties of 1,4,8,11-tetraazacyclotetradecane, piperidine-, 2-, 3-, and 4-methylpiperidine-dithiocarbamates as well as their corresponding complexes with Co(III) ion on mild steel on iron in 0.1 M HClO4 and the inhibition mechanisms for the complexes and the amino-ligands are proposed to be mixed-type and cathodic-type, respectively. Ruthenium-ligand complex (Ref 13), cobalt-N,N′-bis (salicylaldehyde)-1,3-diaminopropane complex (Ref 14), and acetylacetonate complexes of Zn(II), Mn(II), Co(II), and Cu(II) (Ref 15) have been shown to be cathodic-type inhibitors for steel in acidic media. Aytac (Ref 16) reported the inhibition ability of Co(II), Ni(II), and Cu(II) complexes of -Br and -OCH2CH3-substituted Schiff bases as corrosion inhibitors for aluminium in 0.1 M HCl, while the complexes of Co(II), Ni(II), Cu(II), and Zn(II) with 2-acetylthiophene benzoylhydrazone (Ref 17) and Co(II), Ni(II), Cu(II), and Zn(II) with 2-acetylthiophene benzoylhydrazone (Ref 18) were reported to show appreciable corrosion inhibition properties for mild steel in 1 M HCl solutions. Generally, the metal complexes were reported to be more effective in inhibiting acidic corrosion than their respective ligands (Ref 11, 12, 14, 16-18). The increased efficiency of the metal complexes compared to their respective ligands has been attributed to the low water solubility, high molecular weight, and molecular planarity of the complexes (Ref 2, 17), and the stabilizing chelate effect of the ligands (Ref 12). Complex formation between the complexes and corrosion products resulting in the precipitation of insoluble compounds on the surface of the metal has also been proposed as a possible mechanism for the corrosion inhibition of metal by complexes (Ref 17).
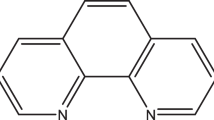
In order to contribute to the scarcity of information in the literature on the corrosion inhibitory effects of complexes and their respective ligands, and to elucidate a possible mechanism for the corrosion inhibition of ligands and their respective complexes, the present work is aimed at studying the corrosion inhibitory properties of 1,10-Phen and its cobalt complex (CoPhen): tris(l,10-Phen) cobalt(III) (NO3)3 (hereafter abbreviated CoPhen) on mild steel in H2SO4 at different temperatures. The molecular structures of Phen and CoPhen are shown in Fig. 1(a) and (b), respectively. Electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization methods were employed to evaluate the corrosion rate of the mild steel and inhibition efficiency of the inhibitors. The morphology of the steel surface was examined by scanning electron microscope.
Experimental
Materials Preparation
N80 carbon steel cut from its parent pipe was used as the test material for these experiments, whose chemical composition is shown in Table 1. The steel sheet was cut into coupons of dimension 1 cm × 1 cm × 0.8 cm. The coupons were embedded in two-component epoxy resin and mounted in a PVC holder. A copper wire was soldered to the rear side of the coupon as an electrical connection. The surfaces of specimens were carefully ground with silicon carbide abrasive paper up to 800-grit, rinsed with distilled water, and degreased with acetone.
Experiments were undertaken in stagnant aerated 0.1 M H2SO4 solutions in the absence and presence of different concentrations of Phen and CoPhen at 20, 30, and 40 °C ± 1 °C. The temperature was maintained by placing the cell on a thermostat water bath. Electrochemical measurements were made using a PARSTA® 2273 electrochemical measurement system connected to a computer and a JSM-6360LV Scanning electron microscope was used for the corrosion surface morphology observation. CoPhen was prepared according to published procedures (Ref 19). All chemicals used were of Analytical reagent quality.
Electrochemical Measurements
Experiments were conducted using a conventional three-electrode cell assembly with the counter electrode made of a platinum foil and the reference electrode being a saturated calomel electrode (SCE) connected to the cell externally through a Luggin capillary tube (Ref 20) positioned close to the working electrode to minimize the ohmic potential drop. The glass cell was filled with 250 mL of the test solution and the working electrode was immersed for 30 min prior to each experimental measurement to attain a steady state (Ref 21, 22). EIS measurements were taken over the frequency range of 100 kHz-10 mHz with a signal amplitude perturbation of 5 mV at the corrosion potential.
The potentiodynamic polarization sweeps were conducted at a sweep rate of 0.5 mV/s. The same cell and system were used as in the EIS method. However, the solution and metal coupon were changed after each sweeps. Each experiment was carried out at least twice where a good reproducibility was attained.
Results and Discussion
Potentiodynamic Polarization Measurements
The potentiodynamic polarization curves for mild steel, measured after reaching steady state, in aerated 0.1 M H2SO4 solutions in the absence and presence of different concentrations of Phen and CoPhen at 20 °C are shown in Fig. 2(a) and (b), respectively. Inspection of these figures shows that the addition of Phen and CoPhen has a pronounced inhibitive effect on the anodic and cathodic part of the polarization curves, while the corrosion potential (E corr) is only slightly shifted. According to Riggs (Ref 23), the classification of a compound as an anodic or cathodic inhibitor is feasible when the open circuit potential(OCP) displacement is at least 85 mV in relation to the blank solution’s value. Banerjee and Misra’s (Ref 5) work on Phen as corrosion inhibitor for mild steel in 1 N H2SO4 obtained similar results but with slight displacement of E corr in the negative direction (a shift less than 20 mV) and proposed that Phen is a mixed-type inhibitor. From the results obtained in this work, Phen and CoPhen decrease both the anodic dissolution of mild steel and the hydrogen evolution reaction to varying extents, but it does not cause an appreciable change on OCP values for a reasonable classification based on OCP results. This clearly shows that Phen and CoPhen can be classified as mixed-type corrosion inhibitors. This is in agreement with the proposal of Li et al. (Ref 8). It, therefore, suggests that the inhibiting action of the compounds occurred by simple blocking of the available anodic and cathodic sites on the metal surface, thus lowering the corrosion rate. The cathodic inhibitive effect of CoPhen seems more pronounced than that of Phen. The electrochemical parameters, corrosion potential E corr, and corrosion current density i corr obtained from the polarization curves are given in Table 2. i corr values were estimated by extrapolation of the cathodic region to E corr. A similar method has been previously employed for non-Tafel dependence curves (Ref 24). The data revealed that the corrosion current density (i corr) decreases with increasing inhibitors concentration and no definite trend was observed in the shift of E corr values in the presence of the various concentrations of the inhibitors. The negligible effect of Phen and CoPhen on E corr is an indication that the compounds can be used as pickling inhibitors (Ref 14, 25). It is clear from the potentiodynamic experiments that the presence of Phen and CoPhen in the acid solutions decreases the corrosion rate (i.e., i corr decreases) and that CoPhen is a more effective corrosion inhibitor than Phen. From the values of i corr, the inhibition efficiency (%η) was determined from the following equation:
where i corr and \( i_{\text{corr}}^{\text{o}} \) are inhibited and uninhibited corrosion current densities, respectively. The values of the calculated inhibition efficiency are also shown in Table 2 and were observed to increase with inhibitor concentrations to a maximum value at a concentration of 100 mg/L of the inhibitors. This suggests that the inhibitor species are probably adsorbed on the mild steel/solution interface where the adsorbed species mechanically screen the metal surface from the action of the corrosive medium (Ref 26). Comparing the inhibition efficiencies shows that CoPhen is a more effective inhibitor than Phen at the studied temperatures.
To investigate the effects of temperature on the corrosion and corrosion inhibition process, further potentiodynamic polarization curves were taken at inhibitor concentration of 100 mg/L at 30 and 40 °C. The polarization parameters obtained from the curves and the inhibition efficiency are given in Table 2. The data in Table 2 reveal that increase in temperature increases the values of the corrosion current densities and decreases the inhibition efficiency of the compounds. Such behavior has been interpreted on the basis that an increase in temperature resulted in the desorption of some adsorbed inhibitor molecules leading to a decrease in the inhibition efficiency which is suggestive of a physical adsorption mechanism. For a chemical adsorption mechanism, the inhibition efficiency increases with increase in temperature.
The apparent activation energies (E a) for the corrosion of N80 mild steel in 0.1 M H2SO4 in the absence and presence of the inhibitor were calculated from the condensed Arrhenius equation as follows:
where I corr is the corrosion current density, A is the frequency factor, R is the gas constant, and T is the absolute temperature. The E a values can be calculated from the slope of the lg(I corr) versus 1/T plots (Fig. 3). The calculated E a value in the absence of inhibitor (blank) is 65.02 kJ/mol, and in the presence of Phen and CoPhen E a values of 79.06 and 129.78 kJ/mol are obtained, respectively. The increase in the E a values in the presence of inhibitor is an indication of a strong inhibitive action for the studied compounds by increasing the energy barrier (minimum energy for corrosion reactions) for the corrosion process and also emphasizes a dominant physical nature of the adsorption mechanism (Ref 27-29) as also suggested by the trend of inhibition efficiencies with temperature.
Electrochemical Impedance Spectroscopy Measurements
Measurements were undertaken to assess the impedance parameters of the mild steel/electrolyte interface in the presence of different concentrations of Phen and CoPhen. The recorded EIS spectra (Nyquist plots) for the mild steel in 0.1 M H2SO4 solutions in Fig. 4 represent the results obtained in the absence and presence of Phen (Fig. 4a) and CoPhen (Fig. 4b) at 20 °C. The Nyquist plots for the blank tests shown in Fig. 4 are used as reference for results with inhibitors at various concentrations. The spectra in the blank solution and in the presence of inhibitors show a depressed capacitive loop at the higher frequency range followed by an inductive loop that is observed in the lower frequency region. The similarity in the profile of the plots suggests similar metal dissolution mechanism in both systems. The presence of the high frequency loops have been attributed to the double-layer capacity in parallel with the charge-transfer resistance (R ct) (Ref 30) and the inductive loop to the relaxation process obtained by adsorption species on the metal surface (Ref 31-35) and/or to the re-dissolution of the passivated surface at low frequencies (Ref 36). Inspection of Fig. 4 reveals that increase in concentration of Phen and CoPhen results in increase in the capacity loop diameter of the Nyquist plots without affecting their characteristics which is an indication of the inhibition of the corrosion process. At higher temperature (30 and 40 °C), the capacity loops for both blank and inhibited systems are observed to have similar characteristics but smaller diameters (compared with those at lower temperature). This indicates that temperature accelerates the dissolution rate of the metal in the absence and presence of the inhibitors.
The impedance spectra were analyzed by fitting to the equivalent circuit model. The intersection of the capacitive loop with the real axis represents the charge-transfer resistance (R ct) of the corrosion product films and the solution enclosed between the working electrode and the reference electrode, R s (Ref 35, 37, 38). R ct represents the charge-transfer resistance whose value is a measure of electron transfer across the surface and is inversely proportional to corrosion rate (Ref 39). Thus, the R ct values were calculated from the difference in impedance at lower and higher frequencies, as suggested by Tsuru et al. (Ref 40) and the values given in Table 3. It is observed from Table 3 that R ct increases with increase in the concentration of the inhibitors. A large R ct value is associated with a slower corroding system. The characteristic frequencies f max were obtained from the semicircle maxima and used to calculate the associated capacitance (C dl) from the equation (Ref 41)
where Y o and n are the CPE constant and exponent, respectively. The value of the double-layer capacitance depends on many variables including electrode potential, temperature, ionic concentrations, types of ions, oxide layers, electrode roughness, impurity adsorption, etc. (Ref 42). Table 3 indicated that by increasing the concentration of Phen and CoPhen, C dl values decrease. The decrease in C dl, which results from local dielectric constant decrease and/or an increase in the thickness of the electrical double layer, suggests that these molecules act by adsorption on the metal/solution interface (Ref 43).
From the values of R ct, the inhibition efficiency (%η) was determined from the equation
where R ct(b) and R ct(inh) are the uninhibited and inhibited charge-transfer resistance, respectively. The inhibition efficiency values are observed from Table 3 to increase with inhibitor concentrations and decrease with temperature. At all concentrations and temperatures, CoPhen was observed to perform better than Phen. The order of the inhibition efficiency is in satisfactory correlation for both electrochemical methods (Tables 2, 3).
Adsorption Consideration
Based on the results obtained in this work and the suggested adsorption mechanism for the inhibition of Phen and CoPhen on mild steel in 0.1 H2SO4 solutions, attempts were made to fit the experimentally derived data (for surface coverage θ, where θ = η%/100) from the polarization measurements to some adsorption isotherms. The best fit was obtained with the Langmuir isotherm (Fig. 5) for Phen and CoPhen, respectively, suggesting that the experimental data follow the Langmuir isotherm and confirm the adsorption of the inhibitors molecules on the metal surface. The slight deviation of the slope from unity may be attributed to the interactions between the adsorbed species on the surface of the metal as well as changes in the adsorption heat with increasing surface coverage. The Langmuir isotherm may be formulated as
where c is the concentration of the inhibitor and k (determined from the intercept of the plot in Fig. 5) is the adsorption coefficient whose values indicate the binding power of the inhibitor to the steel surface. The calculated values of k show that CoPhen (with k value of 25,000 M−1) is more efficiently adsorbed on the surface of the metal and thus a better inhibitor than Phen (with k value of 5000 M−1) which is in good agreement with the experimentally determined inhibition efficiency trend. k is related to the standard free energy of adsorption \( \Delta G_{\text{ad}}^{\text{o}} \) by the equation
where R is the universal gas constant, T is the absolute temperature, and the value 55.5 is the concentration of water in solution in mol/L. The calculated values of \( \Delta G_{\text{ad}}^{\text{o}} \) are −30.54 and −34.46 kJ/mol for Phen and CoPhen, respectively. The negative values of \( \Delta G_{\text{ad}}^{\text{o}} \) indicate a spontaneous adsorption stable layer of Phen and CoPhen on the surface of the metal under the experimental conditions. Generally, values of \( \Delta G_{\text{ad}}^{\text{o}} \) up to −20 kJ/mol are consistent with physisorption, while values more negative than −40 kJ/mol involve sharing or transfer of electrons from the inhibitor molecules to the metal surface to form a co-ordinate type of bond (chemisorption) (Ref 44, 45). The \( \Delta G_{\text{ad}}^{\text{o}} \) values obtained are more negative than −20 kJ/mol but greater than −40 kJ/mol. This may also indicate that the adsorption process under the present experimental conditions involves both physisorption and chemisorption. However, the trend of inhibition efficiency with temperature indicates that electrostatic interactions (physisorption) play a dominant role in the adsorption process. A similar observation has been reported for N containing heterocyclics in acid media (Ref 46, 47). Phen and CoPhen have lone pair of electrons on the nitrogen atoms (for Phen) and π-electrons in aromatic ring, and the iron has incomplete d-orbitals, which determines its role as an acceptor of electrons, fulfilling the conditions required for the formation of a chemisorptive bond. Thus, it is admissible to assume that in the present experimental conditions chemisorption may be slow, so that physisorption dominates.
Morphological Studies
Morphological studies of the mild steel surfaces after 2 h of immersion in 0.1 M H2SO4 solutions in the absence and presence of 100 mg/L Phen and CoPhen at room temperature were carried out using scanning electron microscopy (SEM). In the uninhibited solution, a rough surface is observed due to acid corrosion (Fig. 6a). Figure 6(b) and (c) show the SEM micrographs of the mild steel surface exposed to 0.1 M H2SO4 solutions containing 100 mg/L Phen and CoPhen, respectively. The micrographs show a morphology with no pits and cracks except polishing lines indicating that the corrosion damage is substantially reduced.
A closer look at the surface of the metals shows a slight evidence of the presence of the adsorption film. The film formed on the surface of the metal in the CoPhen-inhibited system seems comparatively more compact than that formed in the Phen-inhibited system. This shows that Phen and CoPhen are adsorbed on the surface of the mild steel and form protective films that inhibit the corrosion of the steel in 0.1 M H2SO4 solution, and that CoPhen is a better inhibitor than Phen.
Adsorption Mechanism
The effectiveness of the compounds as inhibitors can be ascribed to the adsorption of the molecules on the metal surface. Figure 1 gives the molecular structures of the two tested inhibitors. Both compounds have nitrogen heterocyclic ring, but the presence of three nitrogen heterocyclic rings in CoPhen structure may contribute more adsorption sites for the interaction of the molecule on the mild steel surface making CoPhen a better inhibitor. In addition to the nitrogen heterocyclic ring, there are negatively charged Cl− and positively charged complex ion in CoPhen. In acidic solution, the metal surface can be positively charged; CoPhen was more effective than Phen because the compound can be adsorbed on the mild steel surface by electrostatic interaction between the negative charge on the metal surface (as a result of the specific adsorption of Cl−) and the positive charge on the complex cation.
Conclusions
1,10-Phen and its CoPhen inhibit the corrosion of N80 carbon steel in 0.1 M H2SO4, and the inhibiting properties are concentration- and temperature-dependent. The presence of Cl− in CoPhen structure contributes an adsorption site for the electrostatic interaction of the molecule on the mild steel surface making CoPhen a better inhibitor. The trends of inhibition efficiency with temperature as well as values of kinetic and activation parameters for the corrosion inhibition processes indicate that the adsorption process under the present experimental conditions is predominantly physisorption with minor occurrence of chemisorption. The adsorption of the compounds obeys Langmuir’s adsorption isotherm.
References
F. Bentiss, M. Traisnel, L. Gengembre, and M. Lagrenée, Inhibition of Acidic Corrosion of Mild Steel by 3,5-Diphenyl-4H-1,2,4-Triazole, Appl. Surf. Sci., 2000, 161, p 194–202
S. Rangelov and V. Mircheva, The Influence of Metal Complexes of Tetramethyldithio-Oxamide on the Rate of Acid Corrosion of Steel, Corros. Sci., 1999, 38, p 301–306
V.S. Agarwala, Corrosion Inhibition by Phenanthrolines, Corrosion, 1990, 46, p 376–379
C.R. Luman and F.N. Castellano, Phenanthroline Ligands, Comprehensive Coordination Chemistry II, Elsevier, Oxford, 2003.
S.N. Banerjee and S. Misra, 1,10,-Phenanthroline as Corrosion Inhibitor for Mild Steel in Sulfuric Acid Solution, Corrosion, 1989, 45, p 780–783
A. El Ouafi, B. Hammouti, H. Oudda, S. Kertit, M. Benkaddour, and T. Ben-Hadda, Study of the Inhibiting Power of 2,9-Chloromethyl-1,10-Phenanthroline for the Corrosion of Mild Steel in Molar Hydrochloric Acid Solution at 90 °C, Ann. Chim. Sci. Mater., 2002, 27, p 71–80
G.N. Mu, X. Li, and F. Li, Synergistic Inhibition Between o-Phenanthroline and Chloride Ion on Cold Rolled Steel Corrosion in Phosphoric Acid, Mater. Chem. Phys., 2004, 86, p 59–68
X. Li, L. Tang, L. Li, G. Mu, and G. Liu, Synergistic Inhibition Between o-Phenanthroline and Chloride Ion for Steel Corrosion in Sulphuric Acid, Corros. Sci., 2006, 48, p 308–321
G.M. Schmid and H.J. Huang, Spectro-Electrochemical Studies of the Inhibition Effect of 4, 7-Diphenyl-1,10-Phenanthroline on the Corrosion of 304 Stainless Steel, Corros. Sci., 1980, 20, p 1041–1057
I.B. Obot, N.O. Obi-Egbedi, and A.O. Eseola, Anticorrosion Potential of 2-Mesityl-1H-Imidazo[4,5-f] [1, 10]-Phenanthroline on Mild Steel in Sulfuric Acid Solution: Experimental and Theoretical Study, Ind. Eng. Chem. Res., 2011, 50, p 2098–2110
B.I. Ita and O.E. Offiong, Inhibition of Steel Corrosion in Hydrochloric Acid by Pyridoxal, 4-Methylthiosemicarbazide, Pyridoxal-(4-Methylthiosemicarbazone) and Its Zn(II) Complex, Mater. Chem. Phys., 1997, 48, p 164–169
K.F. Khaled, K. Babic-Samardzija, and N. Hackerman, Cobalt(III) Complexes of Macrocyclic-Bidentate Type as a New Group of Corrosion Inhibitors for Iron in Perchloric Acid, Corros. Sci., 2006, 48, p 3014–3034
M. Benabdellah, R. Touzani, A. Dafali, B. Hammouti, and S. El Kadiri, Ruthenium-Ligand Complex, An Efficient Inhibitor of Steel Corrosion in H3PO4 Media, Mater. Lett., 2007, 61, p 1197–1204
A.M. Abdel-Gaber, M.S. Masoud, E.A. Khalil, and E.E. Shehata, Electrochemical Study on the Effect of Schiff Base and Its Cobalt Complex on the Acid Corrosion of Steel, Corros. Sci., 2009, 51, p 3021–3024
A. Ghanbari, M.M. Attar, and M. Mahdavian, Acetylacetonate Complexes as New Corrosion Inhibitors in Phosphoric Acid Media: Inhibition and Synergism Study, Prog. Color Colorants Coat., 2009, 2, p 115–122
A. Aytac, Cu(II), Co(II) and Ni(II) Complexes of -Br and -OCH2CH3 Substituted Schiff Bases as Corrosion Inhibitors for Aluminium in Acidic Media, J. Mater. Sci., 2010, 45, p 6812–6818
V.P. Singh, P. Singh, and A.K. Singh, Synthesis, Structural and Corrosion Inhibition Studies on Cobalt(II), Nickel(II), Copper(II) and Zinc(II) Complexes with 2-Acetylthiophene Benzoylhydrazone, Inorg. Chim. Acta, 2011, 379, p 56–63
P. Singh, A.K. Singh, and V.P. Singh, Synthesis, Structural and Corrosion Inhibition Properties of Some Transition Metal(II) Complexes with o-Hydroxyacetophenone-2-Thiophenoyl Hydrazone, Polyhedron, 2013, 65, p 73–81
R.P. Sharma, A. Singh, P. Venugopalan, A. Rodríguez-Diéguez, and J.M. Salas, Influence of Carboxylate Counter Anions on the Cationic Framework of 1,10-Phenanthroline Cobalt(III) Complexes: Syntheses, Characterization and Single Crystal Structure Determination of [Co(phen)2CO3]X·nH2O Complexes, Polyhedron, 2012, 47, p 173–181
E.D. Shchukin, I.V. Vidensky, and I.V. Petrova, Luggin’s Capillary in Studying the Effect of Electrochemical Reaction on Mechanical Properties of Solid Surfaces, J. Mater. Sci., 1995, 30, p 3111–3114
A.A. El-Awady, B.A. Abd-El-Nabey, and S.G. Aziz, Kinetic-Thermodynamic and Adsorption Isotherms Analyses for the Inhibition of the Acid Corrosion of Steel by Cyclic and Open-Chain Amines, J. Electrochem. Soc., 1992, 139, p 2149–2154
C. Jeyaprabha, S. Sathiyanarayanan, and G. Venkatachari, Influence of Halide Ions on the Adsorption of Diphenylamine on Iron in 0.5 M H2SO4 Solutions, Electrochim. Acta, 2006, 51, p 4080–4088
O.L. Riggs, Jr., Corrosion Inhibitors, 2nd ed., C.C. Nathan, Houston, 1973
X. Liu, P.C. Okafor, and Y.G. Zheng, The Inhibition of CO2 Corrosion of N80 Mild Steel in Single Liquid Phase and Single/Particle Two-Phase Flow by Aminoe, Corros. Sci., 2009, 51, p 744–751
G. Lyberatos and L. Kobotiatis, Inhibition of Aluminum 7075 Alloy Corrosion by the Concerted Action of Nitrate and Oxalate Salts, Corrosion, 1991, 47, p 820–824
E.A. Noor, Temperature Effects on the Corrosion Inhibition of Mild Steel in Acidic Solutions by Aqueous Extract of Fenugreek Leaves, Int. J. Electrochem. Sci., 2007, 2, p 996–1017
K. Gomma and M.H. Wahdan, Effect of Temperature on the Acidic Dissolution of Copper in the Presence of Amino Acids, Mater. Chem. Phys., 1994, 32, p 142–148
S. Martinez and I. Stern, Thermodynamic Characterization of Metal Dissolution and Inhibitor Adsorption Processes in the Low Carbon Steel/Mimosa Tannin/Sulfuric Acid System, Appl. Surf. Sci., 2002, 199, p 83–89
E.A. Noor and A.H. Al-Moubaraki, Thermodynamic Study of Metal Corrosion and Inhibitor Adsorption Processes in Mild Steel-1-methyl-4[4′(-X)-Styryl Pyridinium Iodides-Hydrochloric Acid Systems, Mater. Chem. Phys., 2008, 110, p 145–154
R.F.A. Jargelius-Pettersson and B.G. Pound, Examination of the Role of Molybdenum in Passivation of Stainless Steels Using AC Impedance Spectroscopy, J. Electrochem. Soc., 1998, 145, p 1462–1469
M. Keddam, O.R. Mattos, and H. Takenouti, Reaction Model for Iron Dissolution Studied by Electrode Impedance, J. Electrochem. Soc., 1981, 128, p 257–266
H.J.W. Lenderink, M.V.D. Linden, and J.H.W. DeWit, Corrosion of Aluminium in Acidic and Neutral Solutions, Electrochim. Acta, 1993, 38, p 1989–1992
M.A. Veloz and I. Gonzalez, Electrochemical Study of Carbon Steel Corrosion in Buffered Acetic Acid Solutions with Chlorides and H2S, Electrochim. Acta, 2002, 48, p 135–144
M.A. Amin, S.S.A. El-Rehim, E.E.F. El-Sherbini, and R.S. Bayyomi, The Inhibition of Low Carbon Steel Corrosion in Hydrochloric Acid Solutions by Succinic Acid: Part I. Weight Loss, Polarization, EIS, PZC, EDX and SEM Studies, Electrochim. Acta, 2007, 52, p 3588–3600
H.H. Hassan, E. Abdelghani, and M.A. Amin, Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by Triazole Derivatives: Part I. Polarization and EIS Studies, Electrochim. Acta, 2007, 52, p 6359–6366
E.M. Sherif and S.-M. Park, Effects of 1,4-Naphthoquinone on Aluminum Corrosion in 0.50 M Sodium Chloride Solutions, Electrochim. Acta, 2006, 51, p 1313–1321
M. Govic, R. Horvat, and M. Metkos-Hukovic, Proceedings of the Eighth European Symposium on Corrosion Inhibitors (8SEIC), Ann. Univ. Ferrara, N.S., Sez. V, Suppl., 1995, p 97.
M.A. Quraishi and J. Rawat, Corrosion Inhibiting Action of Tetramethyl-Dithia-Octaaza-Cyclotetradeca-Hexaene (MTAH) on Corrosion of Mild Steel in Hot 20% Sulfuric Acid, Mater. Chem. Phys., 2002, 77, p 43–47
A.M. Abdel-Gabar, B.A. Abd-El-Nabey, I.M. Sidahmed, A.M. El-Zayady, and M. Saadawy, Inhibitive Action of Some Plant Extracts on the Corrosion of Steel in Acidic Media, Corros. Sci., 2006, 48, p 2765–2779
T. Tsuru, S. Haruyama, and B. Gijutsu, Corrosion Inhibition of Iron by Amphoteric Surfactants in 2 M HCl, J. Jpn. Soc. Corros. Eng., 1978, 27, p 573–581
C.H. Hsu and F. Manfeld, Concerning the Conversion of the Constant Phase Element Parameter Y o into a Capacitance, Corrosion, 2001, 57, p 747–748
K. Babic-Samardzija, C. Lupu, N. Hackerman, A.R. Barron, and A. Luttge, Inhibitive Properties and Surface Morphology of a Group of Heterocyclic Diazoles as Inhibitors for Acidic Iron Corrosion, Langmuir, 2005, 21, p 12187–12196
E. McCafferty and N. Hackerman, Double Layer Capacitance of Iron and Corrosion Inhibition with Polymethylene Diamines, J. Electrochem. Soc., 1972, 119, p 146–154
G. Moretti, F. Guidi, and G. Grion, Tryptamine as a Green Iron Corrosion Inhibitor in 0.5 M Deaerated Sulphuric Acid, Corros. Sci., 2004, 46, p 387–403
L. Xiang-Hong and X. Xiao-Guang, Inhibition Effect of Pyrimidine Derivatives on the Corrosion of Steel in Hydrochloric Acid Solution, Acta Phys., 2013, 29, p 2221–2231
I. Ahamad, R. Prasad, and M.A. Quraishi, Adsorption and Inhibitive Properties of Some New Mannich Bases of Isatin Derivatives on Corrosion of Mild Steel in Acidic Media, Corros. Sci., 2010, 52, p 1472–1481
E.E. Ebenso, I.B. Obot, and L.C. Murulana, Quinoline and Its Derivatives as Effective Corrosion Inhibitors for Mild Steel in Acidic Medium, Int. J. Electrochem. Sci., 2010, 5, p 1574–1586
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, X., Okafor, P.C., Jiang, B. et al. Electrochemical Study on the Inhibition Effect of Phenanthroline and Its Cobalt Complex as Corrosion Inhibitors for Mild Steel. J. of Materi Eng and Perform 24, 3599–3606 (2015). https://doi.org/10.1007/s11665-015-1608-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-015-1608-9