Abstract
We report on white-light polymer light-emitting diodes based on the blue polymer poly(dibenzothiophene-S,S-dioxide-co-dioctyl-2,7-fluorene) (PFSO10) and poly[bis(4-phenyl)(4-butylphenyl)amine](Poly-TPD). Through incomplete energy transfer from the blue polymers to low-energy exciplex, we realized a white-light emission based on two high-energy blue polymers, which proved a simple and effective method to appreciate the white-light emission. The exciplex device presented a white-light emission with a color rendering index (CRI) of 49 and Commission Internationale de L’Eclairage (CIE) coordinates of (0.38, 0.41) by optimizing the blended ratio of PFSO10 and Poly-TPD. In order to obtain a broader white-light emission, the red phosphorescent material, Ir(piq)3 , was doped into an emissive layer to improve the red light emission. The maximum luminous efficiency of 1.7 cd/A was realized after optimizing the device process. Compared to the exciplex device using PFSO10:Poly-TPD = 70:30 as the emissive layer, a 40% improvement from the maximum luminous efficiency was achieved. Through optimizing the thermal temperature, the CRI of 89 and CIE coordinates of (0.38, 0.41) were obtained when the thermal temperature was 140°C. These observations demonstrate that utilizing incomplete energy transfer from a high-energy emitter to a low-energy exciplex can be an effective strategy to realize a high-quantity white-light emission.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
White polymer light-emitting diodes (WPLEDs) based on solution processing technology have attracted scientific and industrial attention due to their great potential for applications in flat-panel displays and solid-state light sources.1,2,3,4,5,6 To obtain an efficient white-light emission, the emissive layer needs to be comprised of certain proportions of two complementary colors (blue with orange or yellow) or three primary colors (blue, green, and red), simultaneously.7,8,9,10,11,12,13,14 Therefore, different approaches have been employed to realize white-light emissions, such as chemically grafting light-emitting units into polymer chains, or physically blending monochromic polymer materials.15,16,17,18,19,20 It is worth noting that the intermolecular excited state (exciplex), which is formed between the hole-dominant emitter and the electron-dominant emitter, can realize a broad emission profile.21,22,23 Therefore, exciplex can be worked as a low-energy emissive species to compensate for high-energy blue emissions to obtain a high color rendering index (CRI) of white-light emission.24,25
Recent progress in exciplex presents a range of novel sorts, which was focused on developing highly effective luminous material and their light-emitting mechanism.26,27,28 Wang et al. reported a series of highly efficient green-emission TADF exciplex systems based on an benzimidazole-triazine-based electron acceptor, PIM-TRZ and di-[4-(N,N-ditoly-amino)-phenyl]cyclohexane (TAPC), which showed low Vons (2.3 V), and high efficiencies of 39.7~60.1 cd A−1 for CE, 53.2 ~ 80.1 lm W−1 for PE, and 12.5~18.4% for EQE.29 Subsequently, Zhao et al. successfully developed efficient solution-processed ternary exciplex emitters by incorporating a novel triphenylamine derivative, 4-(9-(perfluoropyridin-4-yl)-9H-fluoren-9-yl)-N,N-diphenylaniline (TPA-3), as electron donor. The OLED device based on the TPA-3:9PhFDPhTz/PO-T2T ternary exciplex successfully achieved a higher EQE of 24%.30 The previous results indicated that exciplex emissions have achieved significant progress. However, most of the reported exciplex were focused on developing monochromatic light emitters.31 There is rare investigation about white-light exciplex emitters which can be a simple and effective method to realize high-quality white-light devices.32,33 Therefore, exploiting new white-light exciplex can promote the development of white-light devices.
In this study, we have realized a high CRI white-light emission by using an exciplex emission, which is formatted between the blue polymer poly[(dibenzothiophene-S,S-dioxide)-co-(dioctyl-2,7-fluorene)] (PFSO10) and poly[bis(4-phenyl)(4-butylphenyl)amine] (Poly-TPD). The electron-withdrawing moiety of the dibenzothiophenen-S,S-dioxide(SO) unit from the PFSO10 and the electron-donating moiety of the triphenylamine (TPA) unit from the Poly-TPD have been used to realize exciplex by physical blending, due to their appropriate frontier molecular orbital energy levels. Therefore, the complementary white-light emission was achieved from incomplete energy transfer from high-energy blue polymers to the low-energy exciplex. The prepared WPLEDs based on such exciplex emissions as low-energy band exhibited a maximum luminous efficiency of 1.01 cd/A and the CIE coordinates of (0.35, 0.40). After introducing red phosphorescent material Ir(piq)3, the WPLEDs achieved a CRI of 89 and the CIE coordinates of (0.38, 0.41) by optimizing the annealing temperature.
Experimental
The PLEDs were made by a well-established procedure. An ultrasonic bath was used to refresh ITO glass substrates (sheet resistance of 15–20 Ω/sq) with acetone, detergent, de-ionized water, and isopropanol. Then, a vacuum at 80°C was employed to dry the ITO substrates. After oxygen plasma treatment, a 40-nm-thick PEDOT:PSS was spin-coasted on an ITO substrate, then dried on a hot plate at 120°C for 20 min. The PFSO10, Poly-TPD, and PFSO10:Poly-TPD blending were deposited from p-xylene to give a uniform film. The emissive layer (EML) was thermally annealed fr 10 min on a hot plate at different temperatures. Finally, the cathode of CsF (1.5 nm)/Al (100 nm) was thermally evaporated at a pressure of about 2 × 10–4 Pa. The active area was 0.16 cm2 , which was defined with a shadow mask. The thickness of the cathode was monitored by an STM-100/MF Sycon quartz crystal.
Photoluminescence (PL) spectra of the PLEDs device were measured on a spectro-fluorimeter. Atomic force microscopy was employed to examine the morphology of the blended film. The descriptions were verified in tapping mode on a Seiko SPA 400, provided with an SPI 3800 probe station. A Keithley 236 source measurement unit and a calibrated silicon photodiode were used to log the luminous efficiency–current density (LE–J) luminance characteristics of the PLEDs. The brightness was calibrated by a PR-705 Spectra Scan spectrophotometer (Photo Research), with simultaneous acquisition of the electroluminescence (EL) spectra and Commission Internationale de l’Eclairage (CIE) coordinates, driven by a Keithley model 2400 voltage–current source. The device fabrication process has been described previously. The PLEDs were encapsulated with a UV-cured epoxy resin.
Results and Discussion
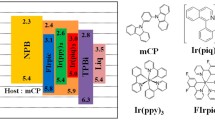
Figure 1 shows the chemical structure of PFSO10, Poly-TPD (see in Fig. 1a), the device structure, and the energy levels of WPLEDs based on an exciplex-emissive layer (see Fig. 1b). It was noted that the Poly-TPD has a very low highest occupied molecular orbital level of − 5.03 eV, which traps the holes from the anode and resists the electrons from the cathode.34 The PFSO10 shows the lowest unoccupied molecular orbital level of − 2.95 eV, which can efficiently trap the electrons from the cathode and block the holes.35 It is worth pointing out that there is a comparatively high barrier for both hole and electron injection between these polymers.36 Such a large barrier can efficiently restrict the holes and electrons in the interface between the PFSO10 and Poly-TPD, which is favorable for the formation of exciplex.
To identify the formation of exciplex between the polymers PFSO10 and Poly-TPD, we first recorded the PL spectra of the blended film of PFSO10 and Poly-TPD. Figure 2a shows the PL spectra of PFSO10, Poly-TPD, and the blended film based on PFSO10:Poly-TPD, with the weight ratio of 50:50. It can be found that the maximal emission peaks of PFSO10 and Poly-TPD were, respectively, at 448 nm and 426 nm, and showed pure blue emissions. In contrast, a fresh low-energy emission sign at 475 nm appeared from the PL spectrum of the blended film, presenting broad spectra dual peaks. The high-energy emission peak at 426 nm from Poly-TPD has been wiped out. These results indicate that the exciplex was effectively obtained from the blended film, and that the incomplete energy transfer from the blue polymer to the exciplex realizes a broad emission band, which is favorable for high-quality white-light emissions.
Figure 2b shows the EL spectra of PFSO10, Poly-TPD, and the blended polymer of PFSO10 and Poly-TPD with different ratios in film. It can be seen that there is a fresh low-energy emission peak at 525 nm from the EL spectrum of the blended film, which achieves a broad emission and realizes white-light emission. Also, the blended ratio of PFSO10:Poly-TPD play s an important role in energy transfer between the high-energy blue polymers and the low-energy exciplex. The emissions originating from PFSO10 and Poly-TPD were obviously quenched after formatting the low-energy exciplex state, which proved the incomplete energy transfer from high-energy PFSO10 and Poly-TPD to the exciplex state. Furthermore, the emission intensities of PFSO10 and Poly-TPD were gradually decreased with increasing the ratio of the exciplex. These discoveries indicate that the EL spectra of exciplex can be adjusted by tuning the mixture ratios. The exciplex devices exhibited effective white-light emissions, which embraced the high-energy and low-energy emissions corresponding to the PFSO10 and the formed exciplex, respectively. This observation indicates that incomplete energy transfer occurred between the PFSO10 and the formed exciplex, which provides a novel approach to achieving white-light emissions.37
The investigation of the EL performance of the blended films was carried out by fabricating devices with the architecture of ITO/PEDOT:PSS (40 nm)/EML (80 nm)/CsF (1.5 nm)/Al (100 nm), where the EML is the PFSO10, Poly-TPD, and PFSO10:Poly-TPD blended film with blend ratios of 50:50, 70:30, or 90:10. The recorded luminance–voltage (L–V) characteristics and LE–J characteristics are illustrated in Fig. 3, and the related device properties are set out in Table I. It can be seen that the turn-on voltage (Von, symbolized as the device with a luminance of 1 cd m−2) of the exciplex devices are slightly decreased from 3.7 V for PFSO10 and 5.2 V for Poly-TPD to 3.3 V. The current density was decreased with increasing the blend ratio of PFSO10 due to its higher injected barrier (see Fig. S1). The device performances based on the blended films as EML depends on the blend ratio of PFSO10 and Poly-TPD, which showed a maximum luminous efficiency (LEmax) of 1.01 cd A−1 and a maximum luminance (Lmax) of 2029 cd m−2. The EL profiles of the blended film achieved a broadened emission due to the incomplete energy transfer of the blue polymer to the low-energy exciplex, which is similar to the PL spectra of the blended films.
To comprehend the influence of the operating voltages on the emissions from the exciplex, the EL spectra at various driving voltages of the device based on PFSO10:Poly-TPD = 70:30 as the emissive layer were recorded. It is worth noting that the relative intensity of the low-energy exciplex emission was decreased with increasing the driving bias. Considering that the blended film is processed from solution, the potential phase separation with relatively large domain size may occur due to the dissimilar polarity of the two polymers.38 This phase separation may be unfavorable for the immigration of excitons and the energy transfer from bulk excitons to the restricted exciplex formed at the heterojunction interface, thus resulting in the visible voltage-dependent EL spectra of the blended film (Fig. 4).39
Although the exciplex device shows a broad emission band to realize the white-light emission, this emission is not a relatively ideal white light due to the lack of a red-light emission.40,41,42 In order to achieve a more ideal white-light emission, the red phosphorescence material ,Ir(piq)3 , was doped into the blended film as EML. Figure 5 shows the EL spectrum of WPLEDs based on PFSO10:Poly-TPD:Ir(piq)3 = 70:30:0.1 as EML (see Fig. 5). It is noted that the EL spectrum of the exciplex device with Ir(piq)3 shows a white-light emission with the maximum emission peak at 615 nm, which is attributed to the Ir(piq)3. The EL spectrum with doping of Ir(piq)3 became much broader than that of the PFSO10:Poly-TPD blended film, indicating that effective energy transfer has occurred from the PFSO10:Poly-TPD blended film to the Ir(piq)3, which improves the white-light quality.43
To explore the influence of the operating voltages on the emissions from the exciplex, the EL spectra of devices based on PFSO10:Poly-TPD:Ir(piq)3 = 70:30:0.1 were investigated under different voltages. It can be seen that the relative intensity of the exciplex emission was increased with improving the operating bias. Considering the lower energy-level of Ir(piq)3, it can be realized that the Ir(piq)3 would preferentially trap the carrier through the trap effect in this blended system. With increasing the operating voltages, the trapping carrier of the Ir(piq)3 easily reached saturation due to its low doping ratio. Therefore, the excess carrier would be trapped in the exciplex, leading to increasing the relative intensity of the exciplex with increasing the work voltage (Fig. 6).
To investigate the EL properties of the devices upon incorporation of the red phosphorescence material, Ir(piq)3, WPLEDs with the device construction of ITO/PEDOT:PSS (40 nm)/EML (80 nm)/CsF (1.5 nm)/Al (100 nm) were fabricated, in which the EML is the PFSO10:Poly-TPD:Ir(piq)3 blended film with a blended ratio of 70:30:0.1. Figure 7 shows the L–V and LE–J characteristics of the devices under different thermal annealing temperatures. We noted that the thermal annealing temperature slightly influenced the Von and luminance of the exciplex WPLEDs. However, there is a distinct impact on the luminous efficiency, CRI, and CIE, and the results are summarized in Table II. The current density was increased with improving the annealing temperature from 100°C to 160°C, then the current density was obviously decreased with further enhancing the annealing temperature to 180°C (see Fig. S2). The device with thermal annealing at 100°C exhibited the maximum efficiency (LEmax) of 1.7 cd/A. The LEmax was slightly decreased with increasing the thermal annealing temperature from 100°C to 180°C. Through optimizing the thermal annealing temperature, the exciplex devices achieved the best excellent CRI of 89 and CIE of (0.38, 0.41), which is near to the ideal white-light emission. It is worth noting that the EL spectra of the high-energy region attributed to PFSO10 is increased with increasing the work voltage, which is favorable for obtaining a high CRI. This phenomenon is similar to the PFSO10:Poly-TPD blended film. The phase separation phenomenon from PFSO10:Poly-TPD:Ir(piq)3 becomes more significant with the increasing thermal annealing temperature, thus resulting in the visible voltage-dependent EL spectra of the blended film.39
In order to obtain insights into the effects of the thermal annealing temperature on the film morphology, tapping mode atomic force microscopy (AFM) measurements were conducted for the blended film of PFSO10:Poly-TPD:Ir(piq)3 = 70:30:0.1. The film was fabricated by spin-coating on the top of the PEDOT:PSS-modified ITO electrode, which was thermally annealed under 100°C, 120°C, 140°C, 160°C, and 180°C. As shown in Fig. 8, the root mean square (RMS) roughness of the blended films with 100°C, 120°C, 140°C, 160°C, and 180°C thermal annealing were 1.59 nm, 1.52 nm, 1.46 nm, 1.23 nm, and 1.16 nm, respectively. The RMS value of the blended films was slightly decreased with increasing the thermal annealing temperature. Furthermore, the pores of the blended film were obviously decreased and the size of the pores grew with increasing the thermal annealing temperature. These phenomena may be attributed to the different polarity between PFSO10 and Poly-TPD. With increasing the thermal annealing temperature, the polymer chain of the blended film was rearranged and phase separation appeared. The adjacent pores of the blended film became fused, which led to a decrease in the pore numbers of the blended film and increased its size. These phenomena were favorable for the phase separation phenomenon of the blended film, resulting in significantly dissimilar EL spectra with different annealing temperatures.
Conclusions
We have developed white-light emissions by employing exciplex formatting from the blue polymers PFSO10 and Poly-TPD. Of particular interest is that broadened emissions were realized with incomplete energy transfer from the high-energy blue polymers to the low-energy exciplex. The WPLEDs based on the blended exciplex material realized the maximum luminous efficiency of 1.7 cd A−1 with CIE color coordinates of (0.44, 0.42). The device achieved a high color rending index of 89 and a CIE of (0.38, 0.41) by optimizing the annealing temperature, which is close to the CIE (0.33, 0.33) of an ideal white-light source. These observations indicate that development of incomplete energy transfer exciplex emitters can be a novel and promising strategy to attain broad white-light emissions.
Data Availability
All data included in this study are available upon request by contact with the corresponding author.
References
C.W. Tang and S.A. VanSlyke, Organic electroluminescent diodes. Appl. Phys. Lett. 51, 913 (1987).
D.W. Zhang, M. Li, and C.F. Chen, Recent advances in circularly polarized electroluminescence based on organic light-emitting diodes. Chem. Soc. Rev. 49, 1331 (2020).
J.M. Sun, P. Cai, X.W. Zhang, A.H. Liang, L.J. Zhang, and J.W. Chen, Synthesis, characterization and device application of a novel blue-emitting copolymer incorporating fluorene and benzothiazole backbone units. Opt. Mater. 98, 109443 (2019).
L. Ying, C.-L. Ho, H.B. Wu, Y. Cao, and W.-Y. Wong, White polymer light-emitting devices for solid-state lighting: materials, devices, and recent progress. Adv. Mater. 26, 2459 (2014).
J.F. Liang, L. Ying, F. Huang, and Y. Cao, Recent advances in high performance solution processed WOLEDs for solid-state lighting. J. Mater. Chem. C. 4, 10993 (2016).
J. Xu, L. Yu, Z.Z. Sun, T. Li, H.B. Chen, and W. Yang, Efficient, stable and high color rendering index white polymer light-emitting diodes by restraining the electron trapping. Org. Electron. 84, 105785 (2020).
X. Guo, C.J. Qin, Y.X. Cheng, Z.Y. Xie, Y.H. Geng, X.B. Jing, F.S. Wang, and L.X. Wang, White electroluminescence from a phosphonate-functionalized single-polymer system with electron-trapping effect. Adv. Mater. 21, 3682 (2009).
S.Y. Shao, and L.X. Wang, Through-space charge transfer polymers for solution-processed organic light-emitting diodes. Aggregate 1, 45 (2020).
B.H. Zhang, G.P. Tan, C.-S. Lam, B. Yao, C.-L. Ho, L.H. Liu, Z.Y. Xie, W.-Y. Wong, J.Q. Ding, and L.X. Wang, High-efficiency single emissive layer white organic light-emitting diodes based on solution-processed dendritic host and new orange-emitting iridium complex. Adv. Mater. 24, 1873 (2012).
Z.M. Zhong, Y.W. Ma, H.L. Liu, F. Peng, L. Ying, S.R. Wang, X.G. Li, J.B. Peng, and Y. Cao, Improving the performance of blue polymer light-emitting diodes using a hole injection layer with a high work function and nanotexture. ACS Appl. Mater. Interfaces 12, 20750 (2020).
K.Q. He, X.D. Wang, J.T. Yu, H.G. Jiang, G.S. Xie, H. Tan, Y. Liu, D.G. Ma, Y.F. Wang, and W.G. Zhu, Synthesis and optoelectronic properties of novel fluorene-bridged dinuclear cyclometalated iridium (III) complex with A-D–A framework in the single-emissive-layer WOLEDs. Org. Electron. 15, 2942 (2014).
F. Peng, W.K. Zhong, Z.M. Zhong, T. Guo, and L. Ying, Improving the electroluminescent performance of blue light-emitting polymers by side-chain modification. ACS Appl. Mater. Interfaces. 12, 8495 (2020).
Q. Wang, D.G. Ma, J.Q. Ding, L.X. Wang, Q.Q. Qiao, H.P. Jia, B.E. Gnade, and J. Hoshikawa-Halbert, An efficient dual-emissive-layer white organic light emitting-diode: Insight into device working mechanism and origin of color-shift. Org. Electron. 19, 157 (2015).
N. Sun, C.M. Jiang, D.C. Tan, X.G. Cao, S. Bi, and J.H. Song, A color-tunable and high-effective organic light-emitting diode device with forward-inverse structure as intelligent lighting display. J. Mater. Sci: Mater Electron. 32, 22309 (2021).
N. Sun, C. Jiang, Q. Li, S. Bi, and J. Song, Performance of OLED under mechanical strain: a review. J. Mater. Sci: Mater Electron. 31, 20688 (2020).
J. Xu, F. Peng, Z.Z. Sun, L. Yu, W. Yang, and Y. Cao, Near-infrared polymer light-emitting diodes based on an inverted device structure. J. Mater. Chem. C 7, 12114 (2019).
M.X. Wang, X.Z. Wei, W.X. Zhang, H.C. Zhao, Y.L. Wu, Y.Q. Miao, H. Wang, and B.S. Xu, Fluorene-containing polyhedral oligomericsilsesquioxanes modified hyperbranched polymer for white light-emitting diodes with ultra-high color rendering index of 96. J. Solid State Chem. 298, 122122 (2021).
X. Zeng, J. Luo, T. Zhou, T.H. Chen, X. Zhou, K.L. Wu, Y. Zou, G.H. Xie, S.L. Gong, and C.L. Yang, Using ring-opening metathesis polymerization of norbornene to construct thermally activated delayed fluorescence polymers: high-efficiency blue polymer light-emitting diodes. Macromolecules 51, 1598 (2018).
X. Jiang, H. Lin, C. Xue, G. Zhang, W.L. Jiang, and G.Z. Xing, Undoped highly efficient green and white TADF-OLEDs developed by DMAC-BP: manufacturing available via interface engineering. J. Mater. Sci. Mater. Electron. 31, 19136 (2020).
J. Hu, Q. Li, S.Y. Shao, L.X. Wang, X.B. Jing, and F.S. Wang, Single white-emitting polymers with high efficiency, low roll-off, and enhanced device stability by using through-space charge transfer polymer with blue delayed fluorescence as host for yellow phosphor. Adv. Opt. Mater. 8, 1902100 (2020).
Q. Wang, Q.S. Tian, Y.L. Zhang, X. Tang, and L.S. Liao, Single white-emitting polymers with high efficiency, low roll-off, and enhanced device stability by using through-space charge transfer polymer with blue delayed fluorescence as host for yellow phosphor. J. Mater. Chem. C 7, 11329 (2019).
Z.K. Fan, N.Q. Li, Y.W. Quan, Q.M. Chen, S.H. Ye, Q.L. Fan, W. Huang, and H. Xu, A solution-processable triphenylamine-fluorene host for exciplex based white phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2, 9754 (2014).
J.F. Guo, Y.G. Zhen, H.L. Dong, and W.P. Hu, Recent progress on organic exciplex materials with different donor-acceptor contacting modes for luminescent applications. J. Mater. Chem. C 9, 16843 (2021).
C.M. Han, R.M. Du, H. Xu, S.Y. Han, P. Ma, J.K. Bian, C.B. Duan, Y. Wei, M.Z. Sun, X.G. Liu, and W. Huang, Ladder-like energy-relaying exciplex enables 100% internal quantum efficiency of white TADF-based diodes in a single emissive layer. Nat. Commun. 12, 3640 (2021).
C. Zhang, Y. Lu, Z.Y. Liu, Y.W. Zhang, X.W. Wang, D.D. Zhang, and L. Duan, A π–D and π–A exciplex-forming host for high-efficiency and long-lifetime single-emissive-layer fluorescent white organic light-emitting diodes. Adv. Mater. 22, 2004040 (2020).
F.J. Wu, X. Zhao, H.Q. Zhu, X.T. Tang, Y.R. Ning, J. Chen, X.L. Chen, and Z.H. Xiong, Identifying the exciplex-to-exciplex energy transfer in tricomponent exciplex-based OLEDs through magnetic field effect measurements. ACS Photonics 9, 2713 (2022).
N. Zhang, C.J. Zheng, Z.P. Chen, J.W. Zhao, M. Zhang, H.Y. Yang, Z.Y. He, X.Y. Du, and S.L. Tao, Improving the efficiency of exciplex based OLEDs by controlling the different configurations of the donor. J. Mater. Chem. C 9, 600 (2021).
M. Zhang, C. Zheng, H. Lin, and S. Tao, Thermally activated delayed fluorescence exciplex emitters for high-performance organic light-emitting diodes. Mater. Horiz. 8, 401 (2021).
B.Y. Liang, J.X. Wang, Z. Cheng, J.B. Wei, and Y. Wang, Exciplex-based electroluminescence: over 21% external quantum efficiency and approaching 100 lm/W power efficiency. J. Phys. Chem. Lett. 10, 2811 (2019).
J.W. Zhao, C.J. Zheng, Y. Zhou, C. Li, J. Ye, X.Y. Du, W. Li, Z.Y. He, M. Zhang, H. Lin, S.L. Tao, and X.H. Zhang, Novel small-molecule electron donor for solution-processed ternary exciplex with 24% external quantum efficiency in organic light-emitting diode. Mater. Horiz. 6, 1425 (2019).
D. Shimoyama, and F. Jäkle, Controlling the aggregation and assembly of boron- containing molecular and polymeric materials. Aggregate 3, 149 (2022).
J.F. Liang, S. Zhao, X.-F. Jiang, T. Guo, H.-L. Yip, L. Ying, F. Huang, W. Yang, and Y. Cao, White polymer light-emitting diodes based on exciplex electroluminescence from polymer blendeds and a single polymer. ACS Appl. Mater. Interfaces 8, 6164 (2016).
J.F. Liang, Z.J. Zhong, S. Li, X.F. Jiang, L. Ying, W. Yang, J.B. Peng, and Y. Cao, Efficient white polymer light-emitting diodes from single polymer exciplex electroluminescence. J. Mater. Chem. C 5, 2397 (2017).
Z.L. Tseng, W.L. Huang, T.H. Yeh, Y.X. Xu, and C.-H. Chiang, Thermally activated delayed fluorescence in commercially available materials for solution-process exciplex OLEDs. Polymers 13, 1668 (2021).
Y.Y. Li, H.B. Wu, J.H. Zou, L. Ying, W. Yang, and Y. Cao, Enhancement of spectral stability and efficiency on blue light-emitters via introducing dibenzothiophene-S, S-dioxide isomers into polyfluorene backbone. Org. Electron. 10, 901 (2009).
W. Jiang, G.M. Zhao, H.W. Chen, and Y.M. Sun, Novel ternary exciplex system based on TCTA dendrimer with a new linking type amongst various functional donors. J. Mater. Sci. Mater. Electron. 33, 11403 (2022).
Z. Chen, X.-K. Liu, C.-J. Zheng, J. Ye, C.-L. Liu, F. Li, X.-M. Ou, C.-S. Lee, and X.-H. Zhang, High performance exciplex-based fluorescence–phosphorescence white organic light-emitting device with highly simplified structure. Chem. Mater. 27, 5206 (2015).
A.C. Morteani, R.H. Friend, and C. Silva, Exciton trapping at heterojunctions in polymer blendeds. J. Chem. Phys. 122, 244906 (2005).
S.M. Wang, X.D. Wang, B. Yao, B.H. Zhang, J.Q. Ding, Z.Y. Xie, and L.X. Wang, Solution-processed phosphorescent organic light-emitting diodes with ultralow driving voltage and very high power efficiency. Sci. Rep. 5, 12487 (2015).
S.H. Yang, J.P. Wu, T.L. Huang, and B.F. Chung, Tunneling injection and exciton diffusion of white organic light-emitting diodes with composed buffer layers. J. Electron. Mater. 47, 1232 (2018).
M.C. Gather, A. Köhnen, and K. Meerholz, White organic light-emitting diodes. Adv. Mater. 23(2), 233 (2011).
R. Wang, H.Y. Xiang, J.W. Chen, Y. Li, Y.H. Zhou, W.C.H. Choy, Z.Y. Fan, and H.B. Zeng, Energy regulation in white-light-emitting diodes. ACS Energy Lett. 7, 2173 (2022).
Y.W. Liu, X.F. Wei, Z.Y. Li, J.J. Liu, R.F. Wang, X.X. Hu, P.F. Wang, T. Qi, and Y. Wang, Interface exciplex anchoring the color stability of solution-processed thermally activated delayed fluorescent white organic light-emitting diodes. Adv. Opt. Mater. 6, 1800978 (2018).
Acknowledgments
The authors are grateful for financial support from the Guangdong Basic and Applied Basic Research Fund Guangdong Province and Dongguan City United Fund (2019A1515110813); Scientific research funds of QingYuan Polytechnic (QYPT-2022-003).
Author information
Authors and Affiliations
Contributions
J. F. Liang carried out the PLED device fabrication, measurement, and data analysis. T. Wu and J. Chen characterized the morphology of blended film and provided the necessary consultations during the write-up of the present article. The first draft of the manuscript was written by J. F. Liang and both authors commented on previous versions of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests. The authors certify that there is no conflict of interest with any individual/organization for the present work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, J., Wu, T. & Chen, J. Single-Layer Blending White-Light Polymer Light-Emitting Diodes via Exciplex Emission. J. Electron. Mater. 52, 5013–5021 (2023). https://doi.org/10.1007/s11664-023-10470-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10470-2