Abstract
The use of tin oxide (SnO2) with high theoretical capacity in practical application as the anode material of lithium-ion batteries (LIBs) has been limited due to its large volume expansion and fast capacity decay. To address this problem, we proposed the synthesis of encapsulating SnO2 nanoparticles in the channels of nitrogen-doped carbon nanotubes (SnO2-in-NCNTs) by capillary force. The confined spaces of NCNTs not only restrict the particle size of SnO2, but also effectively buffer the volume change during lithiation/delithiation processes. In addition, the conductive NCNTs also ensure the effective contact of the electrolyte to the electrode surface, facilitating both ion and electron transfer. When applied to LIBs, the SnO2-in-NCNTs possess high reversible capacities of 961.8 mAh g−1 at 0.1 A g−1 and 326.3 mAh g−1 at 10 A g−1. Moreover, they exhibited superior cyclic stability with a capacity retention of 96% at 5 A g−1 after 500 cycles. This work provides a simple and effective strategy for performance improvement of SnO2-based anode materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries (LIBs) are the predominant energy storage devices in modern portable electronic devices and electric vehicles. However, the traditional graphite anodes which are limited by a low theoretical capacity of 372 mAh g−1 cannot satisfy the next-generation LIBs with higher energy and power densities.1,2,3 Various alternative materials with high reversible capacity, including metals, alloys, and metallic oxides/sulfides, have been investigated extensively.4,5,6,7,8 Among them, tin oxide (SnO2) has attracted great attention in view of its abundance, high theoretical capacity (1494 mAh g−1) and low working potential.9,10,11 Nevertheless, the practical application of SnO2 anode in LIBs is still impeded by its inherent disadvantages. For instance, the severe pulverization and serious capacity fading due to large volume expansion (> 300%) during lithiation/delithiation processes lead to rapid capacity fading. In addition, the SnO2 with poor conductivity is not conducive to electron transmission, which results in poor rate performance.12,13,14,15
To conquer these defects, reducing the size of SnO2 can relieve the mechanical stress and thus inhibit the tendency to fracture and crack.16,17 Furthermore, such design will generally promote the high reversibility and realize the long cycling stability of SnO2. Despite partial problems that can be solved, the intrinsic low conductivity of SnO2 still exists. One effective strategy is to combine nanoscale SnO2 with an electrically conductive carbonaceous matrix. Among these carbon materials, carbon nanotubes (CNTs) have received wide attention due to their high electrical conductivity, large surface area and mechanical stability.18,19,20,21 Liu et al.22 synthesized SnO2/carbon nanotube hairball composites with SnO2 nanoparticles homogeneously anchored on the surface of CNTs, achieving high lithium storage properties and an excellent high-rate capability. However, the SnO2 nanoparticles inevitably fall off from CNTs during long cycles, resulting in deterioration of the electrochemical performance. Embedding SnO2 nanoparticles into carbon nanotubes is a potential solution to solve such problems. Previous studies have proved that the wet chemical method utilizing the capillary force is a convenient method to achieve such a structure.23,24,25 The capillary effect mainly depends on the inner diameter of CNTs and surface tension. Guo et al.26 proved that the addition of polyvinylpyrrolidone (PVP) could decrease the surface tension, thus increasing the effect of capillary action to fill the inner hollow cavities with GeO2/NaCl in an aqueous solution. Therefore, PVP is expected to be applied to the filling of commercial CNTs with solution.
Herein, we propose a simple strategy to synthesize the SnO2 nanoparticles encapsulated in CNTs by capillary force. The results reveal that PVP can effectively reduce surface tension of the PVP/ethanol solution, which is conducive to solution filling in CNTs. Finally, the SnO2/nitrogen-doped carbon nanotubes (SnO2-in-NCNTs) were obtained after a two-step calcination. The conductive NCNTs ensure the effective contact of the electrolyte to the electrode surface, facilitating both ion and electron transfer. In addition, the confined space of NCNTs restricts the particle size of SnO2. Moreover, there are sufficient void spaces between the adjacent particles in the NCNTs, which can accommodate the volume change of SnO2 during the lithiation/delithiation processes. Consequently, the resulting SnO2-in-NCNTs exhibit enhanced cycling stability (483.6 mAh g−1 after 500 cycles at 5 A g−1) and better rate performance (326.3 mAh g−1 at 10 A g−1) in comparison with SnO2 nanoparticles dispersed on the surface of the same CNTs (SnO2-out-NCNTs).
Experimental
Materials
Multiwalled carbon nanotubes with 5–10 nm inner diameter were purchased from Aladdin, polyvinylpyrrolidone (PVP, MW = 10,000) was purchased from Adamas, stannous chloride dihydrate (SnCl2·2H2O, 99%) was purchased from Adamas, and absolute ethanol (EtOH, ≥ 99.7%) was purchased from Greagent. All the chemicals and reagents were used without any further processing.
Synthesis of SnO2-in-NCNTs, SnO2-out-NCNTs, SnO2-in-CNTs
Typically, 100 mg of CNTs and 30 mg of PVP were dispersed in 100 mL of absolute ethanol. After sonication for 1 h, 40 g of SnCl2·2H2O was added into the above solution and stirred for 10 h. The mixture was further filtered and washed with ethanol solution. Then, the precipitates were dried in an oven at 60°C for 12 h. Subsequently, the dried precipitates were pyrolyzed in a tube furnace at 400°C for 2 h under Ar atmosphere (ramp rate: 5°C min−1), and then heated under 500°C in NH3 atmosphere and maintained for 0.5 h. Afterwards, the system was naturally cooled to room temperature, forming the product of SnO2-in-NCNTs. As a control, the SnO2-out-NCNTs were prepared by the same process without addition of PVP, while the SnO2-in-CNTs were obtained without the subsequent NH3 atmosphere annealing compared with the preparing processes of SnO2-in-NCNTs.
Characterization
The morphology and structure of the samples were characterized with a field-emission scanning electron microscope (SEM, FEI Nova NanoSEM 450) and a transmission electron microscope (TEM, FEI Talos F200s). X-ray diffraction (XRD) was analyzed by Bruker D8 Advance x-ray powder diffractometer using Cu Kα (λ = 0.154 nm) radiation operated at 40 kV and 40 mA. The content of SnO2 in the composites was determined by thermogravimetric (TG) analysis on a Netzsch STA449F5 simultaneous thermal analyzer. The ex situ Raman spectra were obtained by an iHR550 Raman microscope with a 532-nm laser. Brunauer–Emmett–Teller (BET) specific surface area was obtained from N2 isotherms by using a Micromeritics ASAP 2010 analyzer. X-ray photoelectron spectroscopy (XPS) was performed with a Thermo Scientific EscaLab 250Xi spectrometer using an Al Kα x-ray source (hν = 1468.6 eV) to detect the surface chemical states. The oxygen vacancy concentration was characterized using electron paramagnetic resonance (EPR) was obtained on a 100G-18 KG/EMX-8/2.7 (Bruker).
Electrochemical Measurements
To prepare the working electrodes, 80% active material, 10% carbon black and 10% poly(vinyldifluoride) were homogeneously mixed in N-methyl-2-pyrrolidinone solvent. Then, the resultant slurry was uniformly coated on Cu foil and dried at 120°C for 12 h under vacuum conditions. The loading of active materials is 0.8—1 mg cm−2 and the electrochemical properties of the as-prepared electrodes were measured using coin-cell batteries (CR2016) assembled in an Ar-filled glove box with lithium foil as the counter electrode and polypropylene membrane (Celgard 2400) as the separator. The electrolyte was 1 M LiPF6 (1 M) in a mixture of ethylene carbonate (EC)/diethyl carbonate (DEC) (1:1 in volume)/5 wt% fluoroethylene carbonate (FEC). The cycled electrodes were first washed by DMC solvent and then dried at 100°C for 2 h for characterization. Galvanostatic charge–discharge experiments were conducted in the voltage range of 0.01 to 3 V (vs. Li+/Li) by a LAND CT2001A battery tester over a series of specific current densities at room temperature. The cyclic voltammetry (CV) measurements (at various scanning rates from 0.2 to 1.0 mV s−1) and electrochemical impedance spectroscopy (EIS) over a frequency range from 100 kHz to 0.01 Hz were obtained with an Autolab PGSTAT302N electrochemical workstation.
Results and Discussion
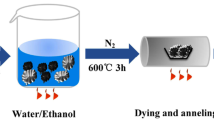
Figure 1 schematically illustrates the synthesis of SnO2-in-NCNTs via the wet chemistry method. Typically, the PVP was first introduced into the ethanol solution with CNTs evenly dispersed to reduce the surface tension and thus ensure the adequate wettability of CNTs. The SnCl22H2O was then added into the above solution with constant stirring, and the SnCl2/PVP mixed solution was infiltrated into the tube of CNTs through capillary force. Finally, the as-prepared precursor was pyrolyzed by a two-step process in a tube furnace to obtain the SnO2-in-NCNTs. During the annealing process under an NH3 atmosphere, the CNTs were doped with nitrogen successfully. As a control, the SnO2-out-NCNTs were prepared by the same processes just without the addition of PVP. In order to clarify the role of PVP in the solution, the solution tension of pristine ethanol and the ethanol solution with PVP were measured. As shown in Fig. S1, the PVP can effectively reduce surface tension of the PVP/ethanol solution, which similarly is conducive to the solution filling in NCNTs. It is obvious that the PVP plays a key role in the capillary force enhancement.
The morphology and microstructure of as-prepared SnO2-in-NCNTs and SnO2-out-NCNTs were characterized by SEM and TEM. As shown in Fig. 2a, SnO2-in-NCNTs show a smooth surface of NCNTs while the SnO2-out-NCNTs show clustered nanoparticles attached to the external surfaces of NCNTs (Fig. 2b). The TEM images of SnO2-in-NCNTs are subsequently presented in Fig. 2c-d. Figure 2c shows that the morphology of commercial NCNTs is still well retained and the channels of NCNTs are filled with aligned nanoparticles (~ 5 nm). High-resolution TEM (Fig. 2d) reveals that the lattice spacing of the particles is 0.34 nm, which is assigned to the SnO2 (110) plane, as is further validated in the fast Fourier transform (FFT) image (inset of Fig. 2c).9 Nevertheless, as shown in Fig. S2, most of the SnO2 nanoparticles in SnO2-out-NCNTs are dispersed on the outside of the NCNTs with larger grain diameter.
Typical XRD patterns of the SnO2-in-NCNTs and SnO2-out-NCNTs are shown in Fig. 3a. The diffraction peaks for two samples match well with SnO2 (PDF #41–1445).27 The mass content of SnO2 in the two hybrids are both calculated to ~ 58 wt% based on the TG analysis (Fig. 3b). Furthermore, the XPS was studied to investigate the chemical bonding of the two as-synthesized samples. Figure S3a presents the survey XPS spectra of SnO2-in-NCNTs and SnO2-out-NCNTs, suggesting the presence of Sn, O, C and N. In the Sn 3d spectra (Fig. S3b) of the two hybrids, two peaks at 487.1 eV and 495.6 eV belong to Sn 3d5/2 and Sn 3d3/2, which suggests the presence of Sn4+ in the SnO2. The C1s spectrum of SnO2-in-NCNTs can be fitted into three peaks (Fig. 3c), among which the peaks at 284.8 eV and 288.8 eV correspond to C–C/C = C and O = C–OH, respectively. Another peak at 286.1 eV belongs to Sn–O-C, which is considered beneficial for keeping a highly stable structure during cycle processes.28 The C 1s spectrum of SnO2-out-NCNTs is provided in Fig. S4, and the weaker Sn–O-C bond is shown in SnO2-in-NCNTs which indicates an inferior connection of SnO2 and NCNTs. The nitrogen content is 2.1% according the XPS analysis and the N 1s XPS spectrum of SnO2-in-NCNTs can be resolved into pyridinic N, pyrrolic N and graphitic N, respectively, centered at 398.5 eV, 399.9 eV and 401.1 eV (Fig. S5).29 The N2 isotherms and pore size distribution are shown in Fig. 3d, and obvious type-H3 hysteresis rings can be observed when the relative pressure P/P0 is high. The specific surface area of SnO2-in-NCNTs (87.9 m2 g−1) is lower than SnO2-out-NCNTs (132.1 m2 g−1) owing to homogeneous filling of the ultrafine SnO2 nanoparticles into NCNTs. EPR analysis was performed on the two samples (Fig. S6), and both of the EPR spectra present EPR activity, proving the presence of an oxygen vacancy (VO), which is an effective way to improve the Li+ storage kinetics.27,30,31,32
The as-prepared hybrids were evaluated as anode materials for LIBs, and the electrochemical performance of cyclic voltammetry was first evaluated. According to Fig. 4a and b, there are two reduction peaks for SnO2-in-NCNTs and SnO2-out-NCNTs in the first cathodic scan. The reduction peak at ~ 0.8 V is related to the formation of SEI film and the conversion reaction of SnO2 to Sn and Li2O.33,34,35 The peak around 0.1 V is due to the formation of LixSn alloy. There are three oxidation peaks at ~ 0.49, 1.2 and 1.85 V in the first delithiation stage. The oxidation peak at ~ 0.52 V is the dealloying reaction of SnO2. The peaks at ~ 1.26 and 1.84 V correspond to the conversion reaction from Sn to SnO2, respectively. An additional oxidation peak at ~ 2.49 V is observed in the SnO2-in-NCNTs, indicating its higher reversibility. The initial three charge–discharge profiles of the SnO2-in-NCNTs, SnO2-out-NCNTs at current density of 0.1 A g−1 with voltage range from 0.01 to 3.0 V are presented in Fig. S7. Obviously, the electrochemical behavior is consistent with that observed on the CV curves. The obtained specific charge capacity of SnO2-in-NCNTs is 961.8 mAh g−1 with higher coulombic efficiency (68.7%), which is higher than SnO2-out-NCNTs (833.2 mAh g−1, 58.3%). Furthermore, the advantage of nitrogen doping can be verified by comparing with undoped sample (SnO2-in-CNTs). The rate performance was evaluated for all samples at 0.1 A g−1, 0.2 A g−1, 0.5 A g−1, 1 A g−1, 2 A g−1, 5 A g−1 and 10 A g−1. the SnO2-in-NCNTs performs the best at any rate (Fig. 4c). More impressively, after high-current density measurement, the capacity of the SnO2-in-NCNTs can recover to its initial value at 0.1 A g−1, indicating a high reversibility of the composite. The capacity contribution of SnO2 is calculated under various current densities (Fig. 4d, Fig. S9). For example, in the first cycle the capacity of SnO2 can be calculated to 1412.1 mAh g−1 in SnO2-in-NCNTs according to the capacity contribution of NCNTs (Fig. S8), which is almost equal to the theoretical capacity of SnO2 and much higher than 1249.8 mAh g−1 in SnO2-out-NCNTs. Furthermore, the capacity contributed by SnO2 in SnO2-out-NCNTs decays rapidly with increasing current density. The EIS of SnO2-in-NCNTs, SnO2-in-CNTs and SnO2-out-NCNTs before cycling are provided in Fig. S10. The charge transfer resistance (Rct) of SnO2-in-NCNTs is smaller than that of SnO2-out-NCNTs and SnO2-in-CNTs, indicating the higher charge transfer and good electrical conductivity of SnO2-in-NCNTs.36,37 The cycling performance at a current density of 0.2 A g−1 is displayed in Fig. S11, where the SnO2-in-NCNTs enable a respectable capacity retention after 100 cycles. However, the SnO2-out-NCNTs show an inferior cycling stability. Furthermore, the cycling performance of the electrode after 20 cycles of activation is shown in Fig. 4e. Even at a higher current density of 5 A g−1, it still maintains a capacity of 483.6 mAh g−1 after 500 cycles. The CV measurement at various scan rates is studied to analyze the reaction kinetics (Fig. S12). At a scan rate of 1 mV s−1, the capacitive contribution ratio of SnO2-in-NCNTs is 64.5%. Benefitting from the fast capacitance-controlled lithium storage process, the SnO2-in-NCNTs exhibit excellent fast charge–discharge capability.
The excellent electrochemical performance of the SnO2-in-NCNTs electrodes can be attributed to the effective confined synthesis of SnO2 nanoparticles in NCNTs. The advantages of such rationally designed structure are as follows: (1) The nitrogen doping in CNTs and VO greatly improve the low electrical conductivity of SnO2. (2) The 3D channel carbon skeleton structure cross-linked by NCNTs ensures the transport of ions and electrons, which are favorable for the excellent rate performance.38 (3) Moreover, the confined space of NCNTs channels can restrict the particle size of SnO2 and the sufficient void space in NCNTs can accommodate the large volume change during cycling. Therefore, the resultant electrode can effectively improve the poor kinetics of pure SnO2 and maintain high structural integrity of the hybrids, resulting in high reversible capacities, superior rate capability and long cycle life.
In order to confirm the structural integrity associated with cyclic stability, we employed HRTEM measurement to explore the morphology change of SnO2-in-NCNTs and SnO2-out-NCNTs after 100 cycles at 0.2 A g−1. As shown in Fig. 5a, the nanoparticles remain encapsulated within the tube in SnO2-in-NCNTs. The fast Fourier transform (FFT) image (inset of Fig. 5a) matches well with the (110) and (211) facets of SnO2. However, the SnO2-out-NCNTs (Fig. 5b) suffers from particle agglomeration and pulverization. Figure S13 displays the EIS spectra of the two hybrids after cycles. The radius of SnO2-in-NCNTs is smaller than SnO2-out-NCNTs, which is mainly related to the cracking, exfoliation of SnO2 and the thickening of the SEI film in the SnO2-out-NCNTs during the cycling. Different structural evolutions of the two SnO2-based hybrids can be better illustrated in Fig. 5c. When SnO2 nanoparticles are embedded in the channel NCNTs, the NCNTs can effectively restrict the particle size of SnO2 and accommodate the volume expansion of SnO2 during lithiation/delithiation processes. In addition, mechanical stress is relieved during cycling due to expansion along the available void space of the tube. Therefore, the design that SnO2 encapsulated in carbon nanotubes can effectively stabilize the structural change and ensure a stable cycle ability.
Conclusion
In conclusion, the SnO2-in-NCNTs with SnO2 nanoparticles encapsulated in carbon nanotubes were successfully fabricated via capillary force. With the addition of PVP, the surface tension of the PVP/ethanol solution can be decreased, thus increasing the efficiency of capillary force. The introduction of VO in SnO2 and the highly conductive NCNTs can greatly improve electron transfer in the hybrids. In addition, the sufficient voids between particles can effectively alleviate volume expansion during the lithiation/delithiation processes. Moreover, the carbon nanotubes can assemble into interconnected networks, which is beneficial to the transfer of ions and electrons. As a result, the SnO2-in-NCNTs demonstrate a superior cycling stability and high-rate capability. The capacity is well-maintained after 500 cycles at 5 A g−1 and the capacity can still reach 326.3 mAh g−1 even at 10 A g−1. The present work opens up an efficacious avenue to develop high-performance anode materials with encapsulated structure for LIBs.
References
M. Armand and J.M. Tarascon, Building Better Batteries. Nature 451, 652 (2008).
L.A. Ellingsen, C.R. Hung, G. Majeau-Bettez, B. Singh, Z. Chen, M.S. Whittingham, and A.H. Stromman, Nanotechnology for Environmentally Sustainable Electromobility. Nat. Nanotechnol. 11, 1039 (2016).
G. Derrien, J. Hassoun, S. Panero, and B. Scrosati, Nanostructured Sn-C Composite as an Advanced Anode Material in High-Performance Lithium-Ion Batteries. Adv. Mater. 19, 2336 (2007).
X.Y. Li, Y.M. Chen, H.T. Wang, H.M. Yao, H.T. Huang, Y.W. Mai, N. Hu, and L.M. Zhou, Inserting Sn Nanoparticles into the Pores of TiO2−x-C Nanofibers by Lithiation. Adv. Funct. Mater. 26, 376 (2016).
K. Ma, Y. Liu, H. Jiang, Y.J. Hu, R. Si, H.L. Liu, and C.Z. Li, Multivalence-Ion Intercalation Enables Ultrahigh 1T Phase MoS2 Nanoflowers to Enhanced Sodium-Storage Performance. CCS Chem. 2, 1472 (2020).
Y.R. Dong, Yu. Liu, Y.J. Hu, K. Ma, H. Jiang, and C.Z. Li, Boosting Reaction Kinetics and Reversibility in Mott-Schottky VS2/MoS2 Heterojunctions for Enhanced Lithium Storage. Sci. Bull. 65, 1470 (2020).
J.G. Zhu, D.L. Chao, W.L. Xu, M.H. Wu, and H.J. Zhang, Microscale Si-based Anodes: Fundamental Understanding and Industrial Prospects for Practical High-energy Lithium-Ion Batteries. ACS Nano 15, 15567 (2021).
C. Hu, Y.J. Hu, A.P. Chen, X.Z. Duan, H. Jiang, and C.Z. Li, Atomic Interface Catalytically Synthesizing SnP/CoP Hetero-Nanocrystals within Dual-Carbon Hybrids for Ultrafast Lithium-Ion Batteries. Engineering (2022). https://doi.org/10.1016/j.eng.2021.11.026.
Y. Liu, C. Hu, L. Chen, Y.J. Hu, H. Jiang, and C.Z. Li, Confining Ultrahigh Oxygen Vacancy SnO2 Nanocrystals into Nitrogen-Doped Carbon for Enhanced Li-Ion Storage Kinetics and Reversibility. J. Energy. Chem. 69, 450 (2022).
C. Hu, L. Chen, Y.J. Hu, A.P. Chen, L. Chen, H. Jiang, and C.Z. Li, Light-Motivated SnO2/TiO2 Heterojunctions Enabling the Breakthrough in Energy Density for Lithium-Ion Batteries. Adv. Mater. 33, 2103558 (2021).
C. Hu, L. Chen, Y.J. Hu, A.P. Chen, L. Chen, H. Jiang, and C.Z. Li, Optimizing SnO2-x/Fe2O3 Hetero-Nanocrystals Toward Rapid and Highly Reversible Lithium Storage. Small 17, 2103532 (2021).
H.B. Wu, J.S. Chen, H.H. Hng, and X. Wen Lou, Nanostructured Metal Oxide-Based Materials as Advanced Anodes for Lithium-Ion Batteries. Nanoscale 4, 2526 (2012).
D. Larcher, S. Beattie, M. Morcrette, K. Edstroem, J.C. Jumas, and J.M. Tarascon, Recent Findings and Prospects in the Field of Pure Metals as Negative Electrodes for Li-Ion Batteries. J. Mater. Chem. 17, 3759 (2007).
P. Meduri, C. Pendyala, V. Kumar, G.U. Sumanasekera, and M.K. Sunkara, Hybrid Tin Oxide Nanowires as Stable and High Capacity Anodes for Li-Ion Batteries. Nano Lett. 9, 612 (2009).
H.P. Cong, S. Xin, and S.H. Yu, Flexible Nitrogen-Doped Graphene/SnO2 Foams Promise Kinetically Stable Lithium Storage. Nano Energy 13, 482 (2015).
W.S. Kim, Y. Hwa, J.H. Jeun, H.J. Sohn, and S.H. Hong, Synthesis of SnO2 Nano Hollow Spheres and Their Size Effects in Lithium Ion Battery Anode Application. J. Power Sources 225, 108 (2013).
P. Wu, N. Du, H. Zhang, J. Yu, and D. Yang, CNTs@SnO2@C Coaxial Nanocables with Highly Reversible Lithium Storage. J. Phys. Chem. C 114, 22535 (2010).
D. Wang, J. Yang, X. Li, D. Geng, R. Li, M. Cai, T.K. Sham, and X. Sun, Layer by Layer Assembly of Sandwiched Graphene/SnO2 Nanorod/Carbon Nanostructures with Ultrahigh Lithium Ion Storage Properties. Energy Environ. Sci. 6, 2900 (2013).
X.S. Zhou, Z.H. Dai, S.H. Liu, J.C. Bao, and Y.G. Guo, Ultra-Uniform SnOx/Carbon Nanohybrids toward Advanced Lithium-Ion Battery Anodes. Adv. Mater. 26, 3943 (2014).
C.R. Ma, W.M. Zhang, Y.S. He, Q. Gong, H.Y. Che, and Z.F. Ma, Carbon coated SnO2 Nanoparticles Anchored on CNT as A Superior Anode Material for Lithium-ion Batteries. Nanoscale 8, 4121 (2016).
C.H. Xu, J. Sun, and L. Gao, Synthesis of Multiwalled Carbon Nanotubes That Are Both Filled and Coated by SnO2 Nanoparticles and Their High Performance in Lithium-Ion Batteries. J. Phys. Chem. C 113, 47 (2009).
M. Liu, S. Zhang, H.C. Dong, X. Chen, S. Gao, Y.P. Sun, W.H. Li, J.Q. Xu, L.W. Chen, A.B. Yuan, and W. Lu, Nano-SnO2/Carbon Nanotube Hairball Composite as a High-Capacity Anode Material for Lithium Ion Batteries. ACS Sust. Chem. Eng. 7, 4195 (2019).
Y.J. Chen, C.L. Zhu, and T.H. Wang, The Enhanced Ethanol Sensing Properties of Multi-Walled Carbon Nanotubes/SnO2 Core/Shell Nanostructures. Nanotechnology 17, 3012 (2006).
E. Dujardin, T.W. Ebbesen, H. Hiura, and K. Tanigaki, Capillarity and Wetting of Carbon Nanotubes. Science 265, 1850 (1994).
P.M. Ajayan, and S. Iijima, Capillarity-Induced Filling of Carbon Nanotubes. Nature 361, 333 (1993).
H.P. Guo, B.Y. Ruan, L.L. Liu, L. Zhang, Z.L. Tao, S.L. Chou, J.Z. Wang, and H.K. Liu, Capillary-Induced Ge Uniformly Distributed in N-doped Carbon Nanotubes with Enhanced Li-Storage Performance. Small 13, 1700920 (2017).
R. Jia, J.L. Yue, Q.Y. Xia, J. Xu, X.H. Zhu, S. Sun, T. Zha, and H. Xia, Carbon Shelled Porous SnO2-δ Nanosheet Arrays as Advanced Anodes for Lithium-Ion Batteries. Energy Storage Mater. 13, 303 (2018).
L. Sun, H.C. Si, Y.X. Zhang, Y. Shi, K. Wang, J.G. Liu, and Y.H. Zhang, Sn-SnO2 Hybrid Nanoclusters Embedded in Carbon Nanotubes with Enhanced Electrochemical Performance for Advanced Lithium Ion Batteries. J. Power Sources 415, 126 (2019).
C. Weidenthaler, A.H. Lu, W. Schmidt, and F. Schüth, X-ray Photoelectron Spectroscopic Studies of PAN-Based Ordered Mesoporous Carbons (OMC). Micropor. Mesopor. Mater. 88, 238 (2006).
Z.T. Li, Y.F. Dong, J.Z. Feng, T. Xu, H. Ren, C. Gao, Y.R. Li, M.J. Cheng, W.T. Wu, and M.B. Wu, Controllably Enriched Oxygen Vacancies through Polymer Assistance in Titanium Pyrophosphate as a Super Anode for Na/K-Ion Batteries. ACS Nano 13, 9227 (2019).
B. Liu, S. Sun, R.Y. Jia, H.S. Zhang, X.H. Zhu, C.G. Zhang, J. Xu, T. Zhai, and H. Xia, Oxygen-Deficient Homo-Interface toward Exciting Boost of Pseudocapacitance. Adv. Funct. Mater. 30, 1909546 (2020).
Q.M. Gan, H.N. He, K.M. Zhao, Z. He, S.Q. Liu, and S.P. Yang, Plasma-Induced Oxygen Vacancies in Urchin-Like Anatase Titania Coated by Carbon for Excellent Sodium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 10, 7031 (2018).
L.L. Liu, M.Z. An, P.X. Yang, and J.Q. Zhang, Superior Cycle Performance and High Reversible Capacity of SnO2/Graphene Composite as an Anode Material for Lithium-ion Batteries. Sci. Rep. 5, 9055 (2015).
J. Qin, C.N. He, N.Q. Zhao, Z.Y. Wang, C.S. Shi, E.Z. Liu, and J.J. Li, Graphene Networks Anchored with Sn@Graphene as Lithium Ion Battery Anode. ACS Nano 8, 1728 (2014).
X.Y. Shan, Y. Zhong, L.J. Zhang, Y.Q. Zhang, X.H. Xia, X.L. Wang, and J.P. Tu, A Brief Review on Solid Electrolyte Interphase Composition Characterization Technology for Lithium Metal Batteries: Challenges and Perspectives. J. Phys. Chem. C 125, 19060 (2021).
H.G. Wang, Q. Wu, Y.H. Wang, X. Wang, L.L. Wu, S.Y. Song, and H.J. Zhang, Molecular Engineering of Monodisperse SnO2 Nanocrystals Anchored on Doped Graphene with High-Performance Lithium/Sodium-Storage Properties in Half/Full Cells. Adv. Energy Mater. 9, 1802993 (2019).
C.H. Wang, Y.H. Li, F. Cao, Y.Q. Zhang, X.H. Xia, and L.J. Zhang, Employing Ni-Embedded Porous Graphitic Carbon Fibers for High Efficiency Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 14, 10457 (2022).
Z.Y. Pan, J. Ren, G.Z. Guan, X. Fang, B.J. Wang, S.G. Doo, I.H. Son, X.L. Huang, and H.S. Peng, Synthesizing Nitrogen-Doped Core-Sheath Carbon Nanotube Films for Flexible Lithium Ion Batteries. Adv. Energy Mater. 6, 1600271 (2016).
Acknowledgments
This work was supported by the Innovation Program of Shanghai Municipal Education Commission and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Chen, L., Jiang, H. et al. Confined Synthesis of SnO2 Nanoparticles Encapsulated in Carbon Nanotubes for High-Rate and Stable Lithium-Ion Batteries. J. Electron. Mater. 51, 6637–6644 (2022). https://doi.org/10.1007/s11664-022-09975-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09975-z