Abstract
CuO films with Zr doping were successfully fabricated on substrates of soda-lime glass (SLG) using a spin-coating method at various doping concentrations. X-ray diffraction (XRD) patterns for pure and Zr-doped CuO thin films indicated that all thin CuO films have a monoclinic polycrystalline nature, with two maximum peaks (−111) and (111). The dislocation density values of the (−111) and (111) planes are increased from 13.4 × 1014 to 34.9 × 1014 m−2 and from 26 × 1014 to 42.7 × 1014 m−2, respectively, owing to the expansion of structural parameters with Zr dopant content. Scanning electron microscopy (SEM) indicated nanostructure particles uniformly distributed on all thin-film surfaces without any agglomerated nanostructure particles. The thickness of CuO films in conjunction with Zr doping is approximately 460 nm. The EDX spectrum of pure CuO in thin film contains Cu and O elements; 1%, 2%, and 3% Zr-doped CuO thin films contain Zr, Cu, and O elements, as expected. Atomic force microscopy (AFM) figures indicate that the surface topologies of thin films are uniformly distributed. Ultraviolet–visible spectroscopy (UV–Vis) measurements of the thin films revealed that the transmittance increased from 25% to 45% in the visible range with increasing Zr concentration at room temperature. The energy band gap increased from 1.67 to 2.03 eV with increasing Zr concentration. At room temperature, a Hall effect system was used to investigate the electrical parameters, including carrier concentration, resistivity, conductivity type, and hole mobility of the CuO films with Zr doping. Conductivity type conversion was observed with 2% and 3% Zr-doped CuO, and confirmed by capacity–voltage \((C-V\)) measurements. The charge-carrier concentration of the samples ranged from 1.08 × 1016 to 5.06 × 1018 cm−3 with Zr doping. Thus, the optical and electrical properties of CuO thin film such as the band gap energy, transmittance, and carrier mobility can be modified.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, transition metal oxide films in the nanoscale range have attracted significant research attention for their fundamental and technological features. Copper oxide exhibits two dissimilar forms, cuprous oxide (Cu2O) and cupric oxide (CuO). Of the nanoscale transition metal oxides, CuO exhibits monoclinic p-type conductivity for depositing a variety of devices with a band gap energy of 1.2–1.9 eV.1,2 Cu2O (cubic) also exhibits p-type conductivity, with an energy band gap of 2.1–2.6 eV.3 Ghisjen et al.4 reported that p-type conductivity arises from holes in the valence band caused by fabricating parameters and doping and annealing effects. They also demonstrated that CuO exhibits strong deviations owing to electron interactions in the open-shell d bands. CuO can also exhibit n-type conductivity related to fabrication parameters.5 Lu et al.6 reported that CuO films have n-type resistivity of 0.19 Ω cm and band gap energy of approximately 1.58 eV with O2 pressure greater than 0.24 Pa. CuO films have also attracted research interest in nanoscience and technology fields, including perovskite solar cells,7 lithium ion batteries,8 cross-point memory applications,9 CO2 sensing applications,10 H2 detection applications,11 and supercapacitor applications12 because they possess important electrical, optical, structural, and magnetic properties compared to conventional bulk materials. Copper oxide may have direct and indirect band gap energy values3 that are greater than those for Cu2O; deposition techniques strongly influence the energy band gap values. Kuhaili showed that cupric oxide is the stable phase for temperatures less than 300°C at dissimilar partial pressures of oxygen, and presented an equilibrium phase diagram for the copper–oxygen (Cu–O) system in air less than 1000°C.13 Cu2O is a thermodynamically stable phase only in partial pressures of oxygen greater than 650°C.14 Nonetheless, a nanocrystalline Cu2O phase was produced by annealing Cu thin films in air below 300°C; a single monoclinic phase of CuO was similarly produced from thin films by annealing between 350°C and 450°C.15
Shape-controlled topologies have a significant effect on transition metal oxide film properties in the nanoscale region. With the excellent physical and chemical properties of CuO nanostructured thin films, many metals can be used as doping elements to improve the crystalline thin film properties. Gülen et al.16 reported through UV–Vis analysis that Mn added to CuO films changed the transmittance and energy band gap value. They also indicated that the catalytic properties of CuO can improve by introducing Mn. Chiang et al.17 reported that Li-doped CuO film increased the photocurrent density and photon-to-hydrogen conversion efficiency compared to undoped CuO, owing to a surge in the electrical conductivity of the thin film. Shaikh et al.18 demonstrated that Ru-doped CuO film plays a vital role in improving a green chemistry approach for more efficient supercapacitors. Huang et al.19 reported that Ag particles loaded on CuO nanosheet arrays affected the electrical conductivity of the electrode and increased electrical interaction in the substrate using a template-free fabrication technique and silver mirror reactions. Many techniques have been used to grow CuO film, including spray pyrolysis,20 sol–gel spin-coating,21 plasma evaporation,22 thermal decomposition,23 and chemical deposition.24 With an adjustable fabrication rate and substrate temperature, spin-coating is a more effective technique for transition metal oxide thin films than other fabrication methods. Recently, transition metal oxides (Ni, Ru, Fe, Cd) have been used to improve the crystal structure and optical properties of pure CuO films.18,25,26 The physical and chemical properties of CuO thin films have been reported21,27,28; however, Zr-doped CuO thin films fabricated by spin-coating have not been studied comprehensively.
Zr exhibits n-type conductivity. The ionic radius of Zr4+ is equal to that of Zr2+; Zr4+ can easily replace Zr2+ in the crystal lattice. Thus, Zr is a suitable metallic dopant to enhance the characteristic properties of CuO film for photovoltaics. In this study, we investigated the structural, morphological, optical, and electrical properties of Zr-doped CuO using a spin-coating method. Zr-doped CuO thin films were fabricated on a substrate of soda-lime glass (SLG) with different Zr doping ratios (0 at.%, 1 at.%, 2 at.%, and 3 at.% of Zr). Scanning electron microscopy (SEM) and atomic force microscopy (AFM) clarified the change in the surface topology of the obtained films. The structural and optical properties of films with metal doping were investigated by x-ray diffraction (XRD) and ultraviolet–visible (UV–Vis) spectroscopy at room temperature, respectively. Measurements of the Hall effect were used to indicate the electrical properties including carrier concentration and hole mobility. p–n conductivity type was converted through capacity–voltage \(\left(C-V\right) \mathrm{measurements}.\)
Experiment
In this study, Zr-doped CuO thin films with four different weight ratios (0 at.%, 1 at.%, 2 at.%, 3 at.% of Zr) were fabricated via SLG substrate using a solution of 0.1 M copper(II) acetate (Cu(CH3COO)2·H2O) and 0.01 zirconium(IV) ethoxide, Zr(OEt)4 deposited in optimised conditions. Copper(II) acetate (0.910 g) was stirred in ethanol (50 ml) for 18 h at 300 K. Zirconium(IV) ethoxide (0.055 g) was stirred in ethanol (20 ml) for 18 h at 300 K and gradually poured into the copper(II) acetate solution to produce different doping concentrations (0 at.%, 1 at.%, 2 at.%, 3 at.%). The obtained solutions were agitated with magnetic stirring at 300 K for 4 h until a flawless and homogenous blue solution was obtained. In our research, it has been demonstrated that different Zr doping concentrations have different effects on CuO. Before the fabrication process, all substrates were cleaned by boiling in a sufficient quantity of an H2O, NH3, and H2O2 mixture at 105°C and then in a sufficient quantity of an H2O, H2O2, and HCl mixture at 105°C to remove any residual waste. The SLG substrates were washed in deionised water for 3 min and dried. After obtaining the final pure and Zr-doped CuO solutions and cleaning the substrates, thin films with different weight ratios were deposited via spin-coating at 1500 rpm for 63 s in air. The final solutions were deposited onto the SLG substrate layer by layer; all layers were preheated to 240°C for 10 min. The films were annealed at 500°C for 1 h in air. The effects of different Zr doping on CuO structural, morphological, optical, and electrical properties are investigated in this study. The crystal structure and phase purity of all films annealed at 500°C for 1 h by spin-coating were analysed using a Rigaku Ultima III x-ray diffractometer (XRD: monochromatic Cu Kα radiation) in the \(2\theta \) range of 20–80°, step size = 0.02°, step time = 1 s, \(\lambda =1.54187\) Å, and an operating voltage of 40 kV and a current of 30 mA. The surface topology of all films was obtained through SEM and AFM images. The change in the transmittance and band gap energy of the thin films was explored using a UV–Vis spectrophotometer from 300 to 1100 nm at 300 K. The electrical parameters of the films were investigated with a room-temperature HMS 3000 Hall effect measurement system. To determine the electrical conductivity of undoped and Zr-doped CuO thin films, an approximately 1.5 × 1.0 cm2 area was used in four-probe methods. Contacts were prepared using silver paste on the surface of the sample; the distance between the two points was approximately 0.5 cm. \(C-V\) measurements of the device were performed using an HP 4192A LF impedance analyzer. To obtain the heterojunction, a vacuum fabrication technique was used to deposit Al contacts on the p-Si substrate; the formation mechanism is shown in Fig. 1.
Results and Discussion
Structural Analysis
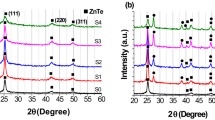
The XRD results for Zr-doped CuO thin films with four different molar concentrations are presented in Fig. 2. The \(2\theta \) angle peaks are at approximately 35.6° and 38.5°; \({d}_{hkl}\) of approximately 2.52 Å and 2.32 Å were associated with (JCPDS 05–6661 for CuO) reflections from the (−111) and (111) planes, respectively. According to the XRD patterns, all thin films had a single-phase monoclinic crystal structure with oxide phases related to the impurity phase because the compound contained zirconium and Cu2O. Obulapathi et al.29 reported comparable XRD data in their study. They demonstrated that the (002) and (111) peak intensity was changed compared to undoped CuO film with the addition of Cr.
Copper oxide films with preferred CuO phase orientation were fabricated successfully and annealed at 500°C using a spin-coating method. Tamuly et al.30 reported seven significant peaks corresponding to (020), (021), (002), (111), (042), (138), and (131) planes. Gopalakrishna et al. used a low-cost spray pyrolysis method and reported eight XRD peaks corresponding to (002), (111), (−202), (020), (202), (−113), (−311), and (113) planes31 at a 0.15 M CuO film concentration.
From the diffraction data, the peak positions for the CuO films shifted slightly with increasing Zr dopant; several additional peaks appear in the XRD spectra for pure CuO film, but they are weak. The intensity of the peaks changed with an increase in the Zr concentration in the film during copper oxide fabrication. These findings (the intensity of peaks, peak broadening, and shift) indicate that tetrahedral site Zr4+ ions were successfully embedded into the CuO lattice.
The crystallite size (\(D\)) of pure and Zr-doped CuO film was examined by determining the angular width of the peak width at half maximum (FWHM) for the two maximum peaks using Eq. 1.32
where λ is Å in the XRD wavelength; \(\theta \) is the angle of Bragg diffraction; \(\beta \) is the FWHM value, and \(D\) is the crystallite size of the samples of two main (-111) and (111) planes. The calculated parameters for the obtained thin films are presented in Table I. The crystallite size changes with Zr-doping in CuO owing to the molecular concentration at the crystal surface. Mersian et al.33 showed that Zr entrance into the CuO lattice leads to an expansion of structural parameters (lattice parameter) of the obtained films. Thus, an interchange of Cu and Zr ions should change the crystal structure of the samples because the ionic radius of Zr4+ (0.73 Å) is larger than that of Cu2+ (0.71 Å).34 \(d\) values for the films are calculated using Bragg’s equation18:
where \(n\) is the order of diffraction. Calculated and standard \(d\) values for pure and Zr-doped CuO films of (−111) and (111) are approximately 2.32 Å and 2.52 Å, respectively. The dislocation density value (\(\delta \)) to investigate the number of defects in the film is calculated using Eq. 3.18
The change in the strain value of the samples is calculated using Eq. 4.
The dislocation density values of the (−111) and (111) planes are increased from 13.4 × 1014 to 34.9 × 1014 m−2 and from 26 × 1014 to 42.7 × 1014 m−2, respectively, owing to the expansion of structural parameters with Zr dopant content in the solution. The strain values of the (−111) and (111) planes are slightly changed with an increase in Zr dopant content. Thus, the main effect of a change in crystallite size is a difference in \(\varepsilon \) value. Mageshwari and Sathyamoorthy35 demonstrated that the \(\delta \) and \(\varepsilon \) values of the film decrease with an increase in fabrication cycles. Mersian et al.33 reported that the crystallite size and the strain of the film decreased with an increase in Zr dopant content.
Structural Properties
The surface morphology of the deposited samples is important in analysing the surface properties of the thin films. Zirconium affects the surface morphology of CuO films with different doping concentrations. Figure 3 shows SEM images of CuO thin films with different Zr concentrations produced by the spin-coating method. Uniform, flat film surfaces with regular shapes are observed, indicating the existence of nanocrystallite, with no defects such as voids or cracks. The obtained thin films are comparatively smooth and well-adhered to the substrate. The densely packed topology is important for applications such as thin film solar cells to prevent photo-current leakage.36 The nanoparticle coverage rate increases with an increase in the Zr doping ratio, which changes the electrical properties (mobility and resistivity) of CuO thin film. It is understood that the CuO morphology is different; it tends to be a more quasi-spherical nanostructure with homogeneous distribution and small agglomeration. The surface properties of the obtained films indicate that the Zr molar concentration modified the CuO film. The same morphological properties have been obtained using Cu(CO2CH13)2, as reported by Jabbar.37 Anandan and Yang38 reported the synthesis of CuO crystallites using Cu(NO3)2.3H2O self-organised into a spherical shape.
Film thickness values (see supplementary Figure S1a–d) for pure and 1%, 2%, and 3% Zr-doped CuO films were 457.6 nm, 485.6 nm, 456.4 nm, and 452.5 nm, respectively. Figure 4 shows the EDX spectrum of 0%, 1%, 2%, and 3% Zr-doped CuO thin films. The doping sample of pure CuO thin film contains Cu and O, producing a deposited film made of Cu and O; 1%, 2%, and 3% Zr-doped CuO thin films contain Zr, Cu, and O, producing deposited films made of Zr, Cu, and O.
Figure 5 shows three-dimensional (3-D) AFM images of pure CuO and 1%, 2%, and 3% Zr-doped CuO thin films at different scales. The figures indicate a 1 μm × 1 μm area with rod-like particles and a grain structure. The results indicate that the films are relatively smooth in the valley region; many crystal-like structures are observed in the hill region, which shows certain orientations. It is observed in the figure that the thin film surface is not uniform. It can be inferred that the AFM images are in agreement with the film structure observed in the SEM images.
Optical Properties
The optical properties of CuO thin films with Zr doping were comprehensively analysed to investigate the effect of metal doping of SLG substrate using UV–Vis data from 300 to 1100 nm at 300 K. The transmittance spectra of samples fabricated with different doping concentrations are shown in Fig. 6. To express the changes in data, standard deviation (SD) is used to visualise error bars, calculated using Eq. 5:
where \(X\) is the data point, \(M\) is the mean, and \(\Sigma \) (sigma) refers to the sum of all data points. The SD is typically the difference between the data points and their \(M\) value. In Fig. 6, the CuO film transparency increases with Zr dopant content in the all-region within the range of ± 0.02 error bars.
The increase in transparency depends on the structural properties of the samples, as it is known that changes in transmittance are related to the material structure properties. The increase in the transmittance of the samples can be attributed to the Moss–Burstein (M–B) effect.39
Thus, it is understood that the optical properties of pure CuO films can be altered by increasing Zr doping in the visible spectrum. The increase in transmittance suggests that Zr-doped CuO films can be used in optical applications such as optical windows.40
The shift in transmittance is associated with an increase in the energy band gap related to valence and conduction electrons. The sample band gap energy is determined using Tauc's relation.41
where \(h\upsilon \) is the incident photon energy of radiation; \({E}_{g}\) is the energy band gap of the materials; \(A\) is a constant; \(\alpha \) is a constant that indicates the absorption coefficient in cm−1. The absorption coefficient is expressed as \(\alpha =2.303(A/T)\). The energy band gap was determined by constructing Tauc plots for \({\left(\alpha h\upsilon \right)}^{2}\) versus incident photon energy \(\left(h\upsilon \right)\). Figure 7 shows the plot of \({\left(\alpha h\upsilon \right)}^{2}\) versus incident photon energy \(\left(h\upsilon \right)\) for pure and Zr-doped CuO films. The energy band gap values for pure and 1%, 2%, and 3% Zr-doped CuO thin films are 1.67 eV, 1.80 eV, 1.97 eV, and 2.03 eV; the error bars in the calculation of the energy band gap are small, approximately ± 0.04 eV. The \({E}_{g}\) value increased from 1.67 eV to 2.03 eV with an increase in Zr content in CuO from 0 to 3%. The energy band gap increases with a change in Zr doping concentration, because the energy band gap of ZrO2 is greater than that of CuO,42,43 the basis of the M–B shift.44 It is thought that increasing Zr concentration in solution affects oxygen evaporation; the thin film becomes more Cu-rich because the O-rich samples generally have a smaller band gap value than Cu-rich samples.44 The increase in energy band gap with Zr doping may be attributed to the band-sustaining effect. The surge in the band gap energy in conjunction with Zr doping may be due to the band-filling effect. The widening of the energy band gap is explained by a slight variation in the crystallite size. Consequently, band gap widening with changes in Zr doping is attributed to the change in charge-carrier density calculated using the Hall effect measurement. According to the Burstein–Moss shift, the band gap widens with increasing charge-carrier concentration, as observed in the electrical results. Thus, it is understood that Zr can be used as a regulator of optical band gap energy in CuO films for potential electronic equipment and as an absorber layer in a solar cell.
Electrical Properties
In this study, we examined electrical properties including resistivity (\(\rho \)), mobility, and carrier concentration using measurements of the Hall effect system with a magnetic field of 0.58 T at 300 K. The Zr doping effect on the investigated electrical properties of thin films with different doping concentrations is presented in Table II. Positive values of the Hall coefficient indicate that the undoped CuO film annealed at 500°C exhibits p-type conductivity; CuO thin films with 2% and 3% Zr doping exhibit n-type conductivity, which may be attributed to oxygen vacancies.45 Hu et al.46 reported that CuO thin films fabricated at 3–5 Pa exhibit p-type conductivity and those fabricated from 8–12 Pa exhibit n-type conductivity, attributed to a break in the Cu–O interaction and creation of O vacancies using Hall effect and gas-sensing measurements. Figueiredo et al.15 demonstrated that samples with a Cu2O phase exhibited p-type conductivity; the conductivity type of the films changed to n-type. Thus, our findings are in agreement with the literature (Fig. 8).
The resistivity of the obtained films was 141.10 Ω cm, 41.99 Ω cm, 17.06 Ω cm, and 37.89 Ω cm for 0%, 1%, 2%, and 3% Zr concentrations, respectively. This change may be attributed to stoichiometric changes due to more copper ion vacancies and neutral defects in the samples.47 Impurities and oxygen centres stem from increased resistivity; the number of oxygen cations decreases with Zr content in the CuO lattice. The resistivity of the thin film decreases compared to pure CuO film. Shokr et al.48 indicated that relatively low electrical resistivity observed in conjunction with Sb content in the SnO2 lattice was attributed to deviation from the variation in stoichiometric changes induced by oxygen vacancies, which changes the free carrier concentration; relatively high resistivity was observed with further addition of Sb to the SnO2 lattice.
The charge-carrier concentration of samples varied from 1.08 × 1016 to 5.06 × 1018 cm−3 with Zr doping. The carrier concentration of samples increased considerably with Zr; the decrease in resistivity is attributed to an increase in carrier concentration attributed to the exchange of Zr4+ ions with Cu2+ ions in the CuO lattice, creating two free electrons in the conduction band. Figueiredo et al.15 reported that the carrier concentration of CuO films decreased from 3.75 × 1022 to 3.69 × 1016 cm−3 with increasing annealing temperature; the carrier concentration increased to 7.19 × 1017 cm−3 at an annealing temperature of 450°C using a thermal oxidation method.
The maximum charge-carrier concentration was 5.06 × 1018 cm–3 and the minimum electrical resistivity was 17.06 Ω cm for 2% Zr-doped CuO film owing to incorporation of Zr4+ ions in the lattice and an increase in free carrier concentration. The steadily decreasing resistivity with increased Zr doping is attributed to increasing carrier concentration. Zr doping of CuO film provides good electrical properties. Shinho49 reported that the charge-carrier concentration increased from 1.6 × 1015 to 8.5 × 1018 cm−3 when the annealing temperature increased from 100°C to 200 °C.
The mobility decreased from 4.10 × 10−2 to 7.23 × 10−2 cm2V−1 s−1; the greatest mobility was 4.10 cm2 V−1 s−1 for pure CuO film, attributed to smaller carrier scattering. Jundale et al.50 reported that the mobility of CuO films was 3.7–5.4 × 10−5 cm2 V−1 s−1, annealed at 300–700°C using a sol–gel method. The greater carrier mobility of the hole corresponds to lower carrier concentration attributed to reduced scattering of holes at native defects; positive and negative oxygen content deviation from the ideal stoichiometry was observed with changing hole mobility in CuO.51
Measurement of \(C-V\) is known as the Mott–Schottky method, an important non-destructive method for obtaining detailed impurity concentration information and Fermi energy level position \(\left({E}_{F }\right)\) for p-Si and n-Si Schottky-type contacts. In accordance with this theory, the metal/semiconductor contact depletion layer capacitance can be analysed, retaining the \(C-V\) measurements using Eq. 7:52
where \(A\) is a constant that indicates the area of the p-Si diode; \(k\) is the Boltzmann constant; \(q\) is the electron charge; \(T\) is the temperature in Kelvin; \(V\) is the reverse bias voltage; \({V}_{bi}\) is the zero bias diffusion potential attained from extrapolation of the \({C}^{-2}-V\) graph to the \(\mathrm{V}\) axis, in which \({\varepsilon }_{s}\) is the dielectric constant of the semiconductor (\({11.8 \varepsilon }_{0}\) for p-Si) and \({N}_{A}\) is the acceptor concentration of the p-type semiconductor. To calculate these characteristics, the \({C}^{-2}-V\) plot of a Zr:CuO/p-Si diode at a constant frequency of 500 kHz at 300 K is shown in Fig. 8. The change in polarity is verified by variation in heterojunction behaviour owing to conductivity type conversion.
To investigate the change, the valence band edge position and p-type semiconductor Fermi energy level can be calculated as53
where \({N}_{V}\) is the state density at the valence band. The band gap energy diagram of CuO:Zr thin films was calculated using these results to show the conversion from p-type to n-type conductivity. The position of the Fermi energy level from the edge of the top valence band was obtained from the acceptor concentration from the \({C}^{-2}-V\) plot extrapolation to the \(V\) axis. The significant shift in the valence band edge position and Fermi energy levels from the top valence band edge with Zr in the CuO structure can be attributed to new energy levels near the conduction band edge. Amplification of n-type conductivity in the films indicates the replacement of Cu2+ atoms with Zr4+ in the CuO structure because the ionic radius of Zr4+ is larger than that of Cu2+. Asl and Rozati54 reported that a Co-doped CuO structure was converted from p-type to n-type conductivity, a direct consequence of the increase in free electron carrier concentration in the thin films. The barrier \(({\phi }_{b})\) value can be calculated using the \(C-V\) data as
The Zr:CuO diode barrier height was calculated as 1.17 eV, 0.73 eV, 1.14 eV, and 1.13 eV within ± 0.03 error bars for 0%, 1%, 2%, and 3% Zr-doped CuO thin films, respectively. Baturay et al.55 reported that the barrier height of Cr-doped CuO film was 0.765 eV using a sol–gel method.
Conclusion
Pure and Zr-doped CuO films were grown on SLG substrates using a spin-coating method with annealing at 500°C for 1 h in a furnace. The effect of metal doping on the structural, optical, topological, and electrical properties of the thin film was investigated. CuO film with Zr dopant had a monoclinic polycrystalline structure with two main peaks near 35.6° and 38.5°, with \({d}_{hkl}\) of approximately 2.52 Å and 2.32 Å related to the (-111) and (111) planes, respectively. XRD parameters including \(D, \delta , \mathrm{and }\varepsilon \) value changed with increasing Zr concentration. Uniform, flat film surfaces with regular shapes were observed in SEM images of samples grown using the spin-coating method. The thickness of CuO films with Zr doping was approximately 460 nm. AFM images indicate that the films are relatively smooth in the valley region; many crystal-like structures are observed in the hill region. The film transmittance was improved in the visible range, indicating a decrease in CuO absorbance. The energy band gap was 1.67 eV, 1.80 eV, 1.94 eV, and 2.03 eV, respectively, with increasing Zr concentration; error bars in the energy band gap calculation were approximately ± 0.04 eV. Room-temperature Hall effect measurements indicated that the electrical properties of the obtained thin films were significantly changed by Zr dopant concentration. Negative Hall coefficient values indicate that Zr-doped CuO thin film has n-type conductivity; 2% and 3% Zr-doped CuO structures converted from p-type to n-type conductivity, indicating a significant increase in free electron carrier concentration in the obtained films. A maximum free charge-carrier concentration of 5.06 × 1018 cm–3 and a minimum electrical resistivity of 17.06 Ω cm were observed for the 2% Zr-doped CuO film. The conductivity type of the obtained thin films changed at 2 and 3% Zr doping ratios. The conversion mechanism from p-type to n-type conductivity was confirmed by \(C-V\) measurements. Thus, the structural and electrical properties of a CuO thin film can be modified using a transition metal dopant. The results indicate that pure and Zr-doped CuO thin films can be used as an absorber layer in a solar cell.
References
I. Singh and R.K. Bedi, Studies and Correlation Among the Structural, Electrical and Gas Response Properties of Aerosol Spray Deposited Self Assembled Nanocrystalline CuO. Appl. Surf. Sci. 257, 7592 (2011).
L. Zheng and X. Liu, Solution-Phase Synthesis of CuO Hierarchical Nanosheets at Near-Neutral pH and Near-Room Temperature. Mater. Lett. 61, 2222 (2007).
S.C. Ray, Preparation of Copper Oxide Thin Film by the sol–gel-Like Dip Technique and Study of Their Structural and Optical Properties. Sol. Energy Mater. Sol. Cells. 68, 307 (2001).
J. Ghijsen, L.H. Tjeng, J. van Elp, H. Eskes, J. Westerink, G.A. Sawatzky, and M.T. Czyzyk, Electronic Structure of Cu2O and CuO. Phys. Rev. B. 38, 11322 (1988).
L.O. Grondahl, Copper-Cuprous-Oxide Rectifier and Photoelectric Cell. Rev. Mod. Phys. 5, 141 (1933).
H.C. Lu, C.L. Chu, C.Y. Lai, and Y.H. Wang, Property Variations of Direct-Current Reactive Magnetron Sputtered Copper Oxide Thin Films Deposited at Different Oxygen Partial Pressures. Thin Solid Films 517, 4408 (2009).
C. Zuo and L. Ding, Solution-Processed Cu2O and CuO as Hole Transport Materials for Efficient Perovskite Solar Cells. Small 11, 5528 (2015).
H. Wang, P. Qinmin, Z. Jianwei, Y. Geping, and Z. Pengjian, Fabrication of CuO Film with Network-like Architectures Through Solution-Immersion and Their Application in Lithium Ion Batteries. J. Power Sources. 167, 206 (2007).
B.S. Kang, S.E. Ahn, M.J. Lee, G. Stefanovich, K.H. Kim, W.X. Xianyu, C.B. Lee, Y. Park, I.G. Baek, and B.H. Park, High-Current-Density CuOx/InZnOx Thin-Film Diodes for Cross-Point Memory Applications. Adv. Mater. 20, 3066 (2008).
T. Ishihara, M. Higuchi, T. Takagi, M. Ito, H. Nishiguchi, and Y. Takita, Preparation of CuO Thin Films on Porous BaTiO3 by Self-Assembled Multibilayer Film Formation and Application as a CO2 Sensor. J. Mater. Chem. 8, 2037 (1998).
N.D. Hoa, N.V. Quy, H. Jung, D. Kim, H. Kim, and S.K. Hong, Synthesis of Porous CuO Nanowires and its Application to Hydrogen Detection. Sens. Actuat B 146, 266 (2010).
D.P. Dubal, D.S. Dhawale, R.R. Salunkhe, V.S. Jamdade, and C.D. Lokhande, Fabrication of Copper Oxide Multilayer Nanosheets for Supercapacitor Application. J. Alloys Compd. 492, 26 (2010).
M. Al-Kuhaili, Characterization of Copper Oxide Thin Films Deposited by the Thermal Evaporation of Cuprous Oxide (Cu2O). Vacuum 82, 623 (2008).
S.H. Wee, P.S. Huang, J.K. Lee, and A. Goyal, Heteroepitaxial Cu2O thin film solar cell on metallic substrates. Sci. Rep. 5, 1 (2015).
V. Figueiredo, E. Elangovan, G. Gonçalves, P. Barquinha, L. Pereira, N. Franco, E. Alves, R. Martins, and E. Fortunato, Effect of Post-Annealing on the Properties of Copper Oxide Thin Films Obtained from the Oxidation of Evaporated Metallic Copper. Appl Surf Sci 254, 3949 (2008).
Y. Gülen, F. Bayansal, B. Şahin, H.A. Çetinkara, and H.S. Güder, Fabrication and Characterization of Mn-Doped CuO Thin Films by the SILAR Method. Ceram. Int. 39, 6475 (2013).
C.Y. Chiang, Y. Shin, and S. Ehrman, Li Doped CuO Film Electrodes for Photoelectrochemical Cells. J. Electrochem. Soc. 159, 227 (2011).
J.S. Shaikh, R.C. Pawar, R.S. Devan, Y.R. Ma, P.P. Salvi, S.S. Kolekar, and P.S. Patil, Synthesis and Characterization of Ru Doped CuO Thin Films for Supercapacitor Based on Bronsted Acidic Ionic Liquid. Electrochim Acta. 56, 2127 (2011).
J. Huang, H. Wu, D. Cao, and G. Wang, Influence of Ag Doped CuO Nanosheet Arrays on Electrochemical Behaviors for Supercapacitors. Electrochim Acta. 75, 212 (2012).
J. Morales, L. Sánchez, F. Martín, J.R. Ramos-Barrado, and M. Sánchez, Nanostructured CuO Thin Film Electrodes Prepared by Spray Pyrolysis: A Simple Method for Enhancing the Electrochemical Performance of CuO in Lithium Cells. Electrochim Acta. 49, 4589 (2004).
H.Y. Bae and G.M. Choi, Electrical and Reducing Gas Sensing Properties of ZnO and ZnO–CuO Thin Films Fabricated by Spin Coating Method. Sens. Actuators. B. 55, 47 (1999).
K. Santra, C.K. Sarkar, M.K. Mukherjee, and B. Ghosh, Copper Oxide Thin Films Grown by Plasma Evaporation Method. Thin Solid Films 213, 226 (1992).
S. Sohrabnezhad, M.M. Moghaddam, and T. Salavatiyan, Synthesis and Characterization of CuO–Montmorillonite Nanocomposite by Thermal Decomposition Method and Antibacterial Activity of Nanocomposite. Spectrochim. Acta, Part A. 125, 73 (2014).
T. Serin, Ş Horzum, and Y. Celik, Annealing Effects on the Properties of Copper Oxide Thin Films Prepared by Chemical Deposition. Semicond. Sci. Technol. 20, 398 (2005).
D.P. Joseph, C. Venkateswaran, S. Sambasivam, and B.C. Choi, Effect of Fe Alloying on the Structural, Optical, Electrical and Magnetic Properties of Spray-Deposited CuO Thin Films. J. Korean Phys. Soc. 61, 449 (2012).
Y. Wang, T. Jiang, D. Meng, D. Wang, and M. Yu, Synthesis and Enhanced Photocatalytic Property of Feather-Like Cd-Doped CuO Nanostructures by Hydrothermal Method. Appl. Surf. Sci. 355, 196 (2015).
J. Tamaki, K. Shimanoe, Y. Yamada, Y. Yamamoto, N. Miura, and N. Yamazoe, Dilute Hydrogen Sulfide Sensing Properties of CuO–SnO2 Thin Film Prepared by Low-Pressure Evaporation Method. Sens. Actuat B 49, 121 (1998).
J. Morales, L. Sánchez, F. Martín, J.R. Ramos-Barrado, and M. Sánchez, Use of Low-temperature Nanostructured CuO Thin Films Deposited by Spray-Pyrolysis in Lithium Cells. Thin Solid Films 474, 133 (2005).
L. Obulapathi, A.G. Kumar, T.S. Sarmas, and T.S. Rao, Room Temperature Studies of Cr Doped Copper Oxide Thin Films by Reactive Dc Magnetron Sputtering. Int J Nanotechnol Appl. 4, 29 (2014).
C. Tamuly, I. Saikia, M. Hazarika, and M.R. Das, Reduction of Aromatic Nitro Compounds Catalyzed by Biogenic CuO Nanoparticles. RSC Adv. 4, 53229 (2014).
D. Gopalakrishna, K. Vijayalakshmi, and C. Ravidhas, Effect of Annealing on the Properties of Nanostructured CuO Thin Films for Enhanced Ethanol Sensitivity. Ceram. Int. 39, 7685 (2013).
B.D. Cullity, Elements of X-ray Diffraction, 1st ed., (Boston: Addison-Wesley Publishing, 1956).
H. Mersian, M. Alizadeh, and N. Hadi, Synthesis of Zirconium Doped Copper Oxide (CuO) Nanoparticles by the Pechini Route and Investigation of their Structural and Antibacterial Properties. Ceram. Int. 44, 20399 (2018).
M. Barsoum, Fundamentals of Ceramics, 2nd ed., (Boca Raton: CRC Press, 2019).
K. Mageshwari and R. Sathyamoorthy, Physical Properties of Nanocrystalline CuO Thin Films Prepared by the SILAR Method. Mater. Sci. Semicond. Process. 16, 337 (2013).
C. Gao, H. Shen, L. Sun, and Z. Shen, Chemical Bath Deposition of Bi2S3 Films by a Novel Deposition System. Appl. Surf. Sci. 257, 7529 (2011).
S.M. Jabbar, Synthesis of CuO Nano Structure Via Sol-gel and Precipitation Chemical Methods. Al-Khawarizmi Eng. J. 12, 126 (2016).
S. Anandan and S. Yang, Emergent Methods to Synthesize and Characterize Semiconductor CuO Nanoparticles with Various Morphologies–An Overview. J. Exp. Nanosci. 2, 23 (2007).
S. Manjunatha, R.H. Krishna, T. Thomas, B.S. Panigrahi, and M.S. Dharmaprakash, Moss-Burstein Effect in Stable, Cubic ZrO2: Eu+3 Nanophosphors Derived from Rapid Microwave-Assisted Solution-Combustion Technique. Mater. Res. Bull. 98, 139 (2018).
C. Muiva, T. Sathiaraj, and K. Maabong, Effect of Doping Concentration on the Properties of Aluminium Doped Zinc Oxide Thin Films Prepared by Spray Pyrolysis for Transparent Electrode Applications. Ceram. Int. 37, 555 (2011).
J. Tauc, Amorphous and Liquid Semiconductors, 1st ed., (Berlin: Springer, 2012).
M. Balog, M. Schieber, M. Michman, and S. Patai, The Chemical Vapour Deposition and Characterization of ZrO2 Films from Organometallic Compounds. Thin Solid Films 47, 109 (1977).
S. Jena, R.B. Tokas, S. Thakur, and N.K. Sahoo, Optical Properties of Electron Beam Evaporated ZrO2: 10% SiO2 thin films: Dependence on Structure. Indian J. Phys. 90, 951 (2016).
G.Y. Naser, W.N. Raja, A.S. Faris, Z.J. Rahem, M.A. Salih, and A.H. Ahmed, Some Optical Properties of CdO Thin Films. Energy Procedia. 36, 42 (2013).
M. Wang, J. Liu, J. Hu, and F. Liu, O2–CO2 Mixed Gas Production Using a Zr-Doped Cu-Based Oxygen Carrier. Ind. Eng. Chem. Res. 54, 9805 (2015).
H. Xihong, F. Gao, Y. Xiang, H. Wu, X. Zheng, J. Jiang, J. Li, H. Yang, and S. Liu, Influence of Oxygen Pressure on the Structural and Electrical Properties of CuO Thin Films Prepared by Pulsed Laser Deposition. Mater. Lett. 176, 282 (2016).
A. Ogwu, T. Darma, and E. Bouquerel, Electrical Resistivity of Copper Oxide Thin Films Prepared by Reactive Magnetron Sputtering. J. Achiev. Mater. Manuf. Eng. 24, 172 (2007).
E.K. Shokr, M.M. Wakkad, H.A. Abd El-Ghanny, and H.M. Ali, Sb-Doping Effects on Optical and Electrical Parameters of SnO2 Films. J. Phys. Chem. Solids. 61, 75 (2000).
S. Cho, Optical and Electrical Properties of CuO Thin Films Deposited at Several Growth Temperatures by Reactive RF Magnetron Sputtering. Met. Mater. Int. 19, 1327 (2013).
D.M. Jundale, P.B. Joshi, S. Sen, and V.B. Patil, Nanocrystalline CuO Thin Films: Synthesis, Microstructural and Optoelectronic properTies. J. Mater. Sci.: Mater. Electron. 23, 1492 (2012).
Y. Shen, M. Guo, X. Xia, and G. Shao, Role of Materials Chemistry on the electrical/electronic properties of CuO thin films. Acta Mater. 85, 122 (2015).
S.M. Sze, Y. Li, and K.K. Ng, Physics of Semiconductor Devices, 4th ed., (Hoboken: Wiley, 2021).
D.A. Neamen, Semiconductor Physics and Devices Basic Principles, 3rd ed., (New York: McGraw-hill, 2003).
H.Z. Asl and S.M. Rozati, Spray Deposition of n-Type Cobalt-Doped CuO Thin Films: Influence of Cobalt Doping on Structural, Morphological, Electrical and Optical Properties. J. Electron. Mater. 49, 1534 (2020).
S. Baturay, I. Candan, and C. Ozaydın, Structural, Optical and Electrical Characterizations of Cr-Doped CuO Thin Films. J. Mater. Sci. Mater. Electron. 33, 7275 (2022).
Funding
This study was funded by the Scientific Research Projects Coordination Unit of Dicle University for the support with the number FEN.18.007.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baturay, Ş. Conversion from p- to n-Type Conductivity in CuO Thin Films Through Zr Doping. J. Electron. Mater. 51, 5644–5654 (2022). https://doi.org/10.1007/s11664-022-09836-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09836-9