Abstract
2-Methyl imidazole and Zn(NO3)2 were proposed as raw materials for the hemoglobin (Hb) accommodation matrix ZIF-8, and the encapsulation of Hb molecules onto ZIF-8 was achieved by hydrogen bond interaction and electrostatic attraction. The electrochemical sensing performance of H2O2 for Hb integration into metal–organic framework (MOF) material was analyzed systematically and evaluated quantitatively to identify the key factor in limiting the sensing efficiency. The influence of mutual interactions between Hb and ZIF-8 on the spectroscopic features and sensing efficiency for complexes with Hb incorporation was estimated by multiple techniques to explore the impact of those interactions on the relevant steps involved in the catalysis. The results from experiments revealed that the hydrogen bond between Hb and ZIF-8 led to non-oriented aggregation of enzyme molecules onto the MOF with reduced active surface area and inhibited catalytic activity. The relatively weak interaction between heme protein and MOF material did not result in serious distortion in the structure or chemical nature of Hb entrapment into ZIF-8. The presence of a mutual interaction between Hb and ZIF-8 did not impose a serious negative effect on heterogeneous electron shuttle (28.2–91.2 s−1) for the heme site in Hb entrapment into ZIF-8, which was classified into a typical quasi-reversible and thin-film confining mode with the involvement of four electrons and two H+ ions. The results showed that hb-induced electrocatalysis was limited by the step of substrate diffusion (1.3 × 10−3 s−1). The Hb-based electrode demonstrated excellent affinity to dissolved H2O2 (Michaelis constant: 28.0 μM) and desirable sensing performance (low detection limitation: 3.7 μM).
Graphical Abstract
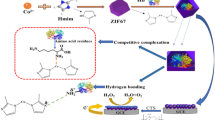
Illustration of the procedure for preparing the electrode based on MOF material (ZIF-8) with hemoglobin accommodation via physical adsorption and electrostatic attraction, which gives rise to non-oriented distribution of hemoglobin attachment onto the surface and inner cavity of ZIF-8.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, metal–organic framework (MOF) materials have emerged as a promising candidate for biomacromolecule (e.g. redox enzyme) accommodation to fabricate organic-based electrochemical devices for their enhanced chemical/thermal stability, easy modification to outer surface groups, good recyclability, tunable porosity, and favorable biocompatibility. These electrochemical devices include biofuel cells, bioelectrochemical sensors, biocatalytic reactors, heavy metal treatment, and biomedical instruments. The presence of MOF material can somewhat prevent the external interference of coexisting chemicals on the incorporated biomolecules. The combination of MOF with biomolecules makes the catalyst recovery feasible via discrete separation from the reaction system in spite of the inferior catalytic function for the entrapped biomolecules in comparison to the free biomacromolecules.
The suitable collocation of metal ions with small organic ligands and rational MOF synthetic strategy can lead to improved architecture of material with favorable porosity and surface chemistry to accommodate biomacromolecules.1,2,3,4,5,6,7,8,9 A typical MOF material, ZIF-8, with a zinc ion as the central ion and imidazole derivative as the ligand, was proposed to act as a biomolecule supporter. The biomolecule-based electrodes with ZIF-8 as the bioelectrocatalyst matrix8 demonstrate favorable affinity to specific substrate and excellent sensitivity. The distinct and competitive coordination between elements of MOF and biomolecules (e.g. redox protein) exert a notable effect on the kinetics of bioelectrocatalysis. The processes of substrate diffusion, substrate recognition-transformation mechanism and relevant efficiency of charge transport between redox sites in biomacromolecules and the conductive interface of bioelectrocatalyst supporters are included in the biomolecule-induced electrocatalysis. These mutual interactions give rise to notable influences on the nature of MOF materials and the electrocatalytic efficiency of the electrodes based on MOF with biomolecule immobilization. Previous efforts10,11,12 to date have been dedicated to evaluating the performance of electrochemical devices based on MOF materials with biomacromolecule entrapment. Unfortunately, the most relevant investigations have not focused on the relationship between the kinetics of related steps involved in the bioelectrocatalysis and the mutual interactions between MOF. The relatively weak interactions between biomolecules and elements of MOF-based nanomaterials/composites (e.g. hydrogen bond) contribute to the maintenance of the native structure, valence state and secondary structure of biomolecule attachment onto MOF. However, to the best of our knowledge, the dependence of the mutual interaction intensity between biomolecules and components of MOF on the composition of MOF and biomolecules has not been fully explored.1,2,3,4,5,6,7,8,9,10,11,12
Hemoglobin (Hb), composed of four polypeptide chains, is a classical heme protein with a Fe ion linked with porphyrin as a cofactor in the protein molecule. Previous studies have indicated that the sophisticated interactions between components of enzyme carriers and protein molecules13,14,15,16 can be regarded as efficient means of enzyme molecule immobilization. Such interactions give rise to specific changes in the structural parameters of MOF material or nanocomposites with Hb accommodation. The alteration in the structure or microenvironment of redox protein (e.g. heme protein) can result in a change in the electron transfer route and electrocatalytic mechanism of integrated redox enzyme molecules. Unfortunately, to the best of our knowledge, no studies have investigated this in depth until now.13,14,15,16,17,18,19,20,21,22,23,24 Important breakthroughs regarding the issue would promote a better understanding of the essence of biophysiological activity. Such a crucial advance in the area would be beneficial for the design and fabrication of next-generation biomimetic electrochemical devices with high performance.
Based on previous conception, discussion and analysis, ZIF-8 as a classical MOF material was used to prepare Hb-based electrodes for its advantages including high porosity for convenient diffusion of substrate, excellent mechanical stability and favorable biocompatibility with biomacromolecules. Furthermore, the geometric parameters (i.e. the porosity, the volume of the inner cavity and the diameter of the external duct) of ZIF-8 could be tuned based on the chemical composition and the synthetic procedure for ZIF-8. This article concerns the impact of mutual interactions between ZIF-8 and Hb on the kinetics of steps involved in Hb-induced catalysis on H2O2 electro-reduction which could be considered in relation to the sensing performance of the prepared electrochemical sensor. The main goal of the study is to explore the relationship between the key factor in the inhibition of catalytic efficiency and the dominant interaction between Hb and ZIF material. The conclusion from the related investigation will help to shed light on the relationship between the structural parameters of MOF materials or MOF nanocomplex and the catalytic performance of MOF or MOF complex with biomacromolecule integration.
Experiment
Reagents and Apparatus
2-Methyl imidazole (Hmim) was provided by Shanghai Aladdin Reagent Co., Ltd. (China). Zinc nitrate hexahydrate with analytical purity was manufactured by Shanghai Titan Scientific Co., Ltd. (China). Fluorescein isothiocyanat (FITC) was obtained by Shanghai Macklin Biochemical Co., Ltd. (China). Hb from bovine blood (molecular weight: ~ 64,500 g mol−1) was purchased from Sigma-Aldrich, Inc. (USA). Chitosan (CTS, degree of deacetylation: ≥ 90%; molecular mass ~ 250,000 g mol−1) as film former was purchased from Sinopharm Chemical Reagent Co., Ltd. (China). Other routine reagents used in the preparation and characterization for the MOF and nanocomplex with enzyme integration were of analytical grade, and the chemicals were supplied by Tianjin Zhiyuan Chemical Reagent Co., Ltd. (China). Nitrogen gas with high purity was obtained from Kangdi Special Gasses Co., Ltd. (China). A B Braun 2K15 ultra-speed centrifuge was supplied by Sigma (Germany). The glassy carbon electrode (GCE, diameter: 3 mm) as the supporting electrode, Ag/AgCl in saturated KCl aqueous solution as the reference electrode, and platinum coil serving as the counter electrode were obtained from Tianjin Aida Hengsheng Technology Development Co., Ltd. (China). The pretreatment procedure of the supporting electrode and the estimation of active area for the supporting electrode capped by a thin film of ZIF-8 with Hb encapsulation were performed in compliance with the previous illustration.17 A solution of 0.2 M phosphate-buffered saline (PBS) served as the electrolyte throughout the electrochemical measurements and the electrocatalytic tests. The pH value of PBS was controlled by varying the elemental mass ratio.
Preparation and Characterization of Zeolite-Like Imidazole Ester Framework with Hb Encapsulation
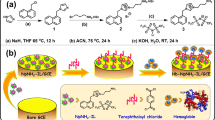
The general synthetic procedure11 for ZIF-8 was as follows: First, 5.6755 g Hmim was rapidly added into 100 mL aqueous solution containing 0.2964 g zinc nitrate hexahydrate. The mixture was subjected to vigorous magnetic stirring for 30 min. The product was separated from the system by centrifugation at a speed of 8000 rpm and washed several times with distilled water. The resulting product was dried in a vacuum oven overnight, and the zeolite-like imidazolate ZIF-8 was obtained. The procedure for preparing ZIF-8 with Hb encapsulation is summarized as follows: First, 5.0 mL Hb stock solution (enzyme concentration: 5.0 g L−1) was injected into the mixture of Hmim and zinc nitrate hexahydrate introduced earlier. The mixture was subjected to vigorous magnetic stirring for 30 min and then it was incubated in a refrigerator overnight. The sediment was collected and tagged as Hb@ZIF-8. The process for the preparation of ZIF-8 with Hb attachment is illustrated in the schematic diagram of Fig. 1. The fluorescent labeling of Hb with FITC was similar to a process described elsewhere.16 The dispersed phase of Hb@ZIF-8 was prepared for the laser confocal microscope test. Discrete phase systems (3.0 mL) of ZIF-8 and Hb@ZIF-8 in PBS were dripped onto the surface of pretreated GCEs, and those electrodes were subjected to air-drying in an inverted beaker to secure samples of electrochemical tests. Two microliters of CTS solution was pipetted onto the surface of GCEs modified by thin films of ZIF-8 or Hb@ZIF-8. These GCEs were incubated in a refrigerator overnight for further characterization and electrochemical experiments. The as-prepared electrodes were labeled ZIF-8/CTS/GCE and Hb@ZIF-8/CTS/GCE as working electrodes. The loading capacity of Hb into ZIF-8 and the ratio of Hb desorption from its supporter was determined with graphite furnace atomic absorption spectrometry (GFAAS)18 which was performed on a Hitachi Z-2000 Series atomic absorption spectrometer. The apparatus is equipped with a graphite furnace atomic absorption spectrophotometer (main frame: single-beam flame, experimental range of wavelength: 190–900 nm; Hitachi Ltd., Japan). The catalytic activity of Hb entrapment in ZIF-8 was determined by ultraviolet–visible (UV–Vis) spectroscopy. The specific activity of Hb-induced hydrogen peroxide reduction was estimated according to a previous definition.18 The combination of rhodamine 6G with I3− resulting from the reaction of OH free radicals (these radicals were produced during the process of Hb-induced reduction of hydrogen peroxide) and I− led to deterioration in the absorbance of the reaction system at 526 nm. The decrease in absorbance was proportional to the consumption of hydrogen peroxide from Hb-induced catalysis.
The geometric features of ZIF-8 and ZIF-8 with Hb encapsulation were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM) and laser confocal microscopy (LSCM), respectively. A JSM-7610F Plus scanning electron microscope operating under acceleration voltage of 1.0–15.0 kV was manufactured by JEOL. An H-800 transmission electron microscope (electronic gun: tungsten filament and operational voltage of 200.0 kV) was obtained from Hitachi Ltd. (Japan). The ZIF-8 with fluorescence-labeled Hb incorporation was evenly distributed into an aqueous buffer solution. The sample was pipetted onto the glass slide surface for further LSCM measurement, which was conducted on a C2 laser confocal microscope equipped with a standard detector (testing wavelength range: 400–750 nm, filter cube: 2 filter blocks; Nikon Corporation, Japan). All images were processed on the NIS-Elements Viewer imaging software analysis platform (Nikon Corporation). The crystal phase of the sample was characterized by x-ray diffraction (XRD) performed on a D2 PHASER diffractometer with Cu target Kα (λ = 1.5418 Å) as source of radiation (Bruker Corporation, Germany). The chemical microenvironment of Hb incorporation into ZIF-8 was explored by UV–Vis spectroscopy and Fourier transform infrared spectroscopy (FTIR), respectively. These experiments were conducted on a U-3310 UV–Vis spectrometer (thickness of color plate: 1 cm; Hitachi Ltd., Japan) and a Tensor 27 Fourier transform infrared spectrometer (KBr compacted powder; Bruker Corporation, Germany). The surface chemical characteristics were investigated by circular dichroism (CD) which was implemented on a Chirascan spectropolarimeter (Applied Photophysics Ltd., UK) equipped with a 150 W Xe lamp and air-cooling installation. The mutual interactions between ZIF-8 and the integrated Hb molecule were studied by emission fluorescence spectrometry (FRS) on a Varian Cary Eclipse spectrofluorometer (quartz cuvette thickness of 1.0 cm; Agilent, USA). FRS determinations were conducted under the following experimental protocols: Variable concentrations of ZIF-8 in well-proportioned discrete phase and uniform consistency of dissolved Hb were mixed under vigorous magnetic stirring for 10 min. Emission fluorescence spectra were recorded under the following testing parameters: excitation wavelength at 285 nm, slit width of 5 nm and wavelength sweep ranging from 285 nm to 400 nm. The electrical conductivity of the electrode based on ZIF-8 with Hb encapsulation was evaluated with electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV). EIS experiments were carried out on a Zahner Zennium electrochemical platform (Zahner-Elektrik, Kronach, Germany). EIS measurements were made under the following operational parameters: frequency ranging from 0.1 Hz to 105 Hz, open-circuit voltage at 0.42 V, K3Fe(CN)6/K4Fe(CN)6 acting as electrochemical probing species and amplitude of excitation voltage at 5.0 mV. Steady CV curves were registered on a CHI-660E electrochemical apparatus (CHI Inc., Shanghai Chenhua Co., Ltd., China). The CV curves were obtained under a potential sweep velocity of 200 mV s−1. Evaluation of leakage of Hb from the enzyme matrix was carried out by a method introduced elsewhere.17
Direct Electrochemistry and Electrocatalytic Function on Hydrogen Peroxide Reduction for Hb/ZIF-8/CTS/GCE
All electrochemical tests were executed in a conventional electrolytic cell with three electrodes immersed in oxygen-free PBS (pH = 7.0 without extra validation). The stable current–potential curves and current–time curves were measured under ambient pressure and room temperature (25.8 ± 0.4°C) without additional explanation. CV, chronoamperometry (CA) and differential pulse voltammetry (DPV) were used to investigate and evaluate the electrochemical behavior and the electrocatalytic efficiency on H2O2 reduction by Hb@ZIF-8/CTS/GCE. DPV curves were recorded under the following protocol: step potential of 4 mV and pulse spacing of 0.5 s. The steady CA curve of the Hb-based electrode was measured under a constant applied potential of –0.4 V in accordance with the previous illustration.19 The as-prepared Hb-based electrode was immersed in blank PBS with successful introduction of dissolved substrate batches at different time intervals. All potentials throughout this article are relative to a normal hydrogen electrode (NHE) without particular indication. An AFMSRCE rotating platinum ring-glassy carbon disk electrode system connected to an electrochemical workstation was furnished by Pine Research Instrumentation (USA). The rotation rate of the working electrode was varied within a range of 50–10,000 rpm.
Results and Discussion
Characterization and Exploration of Geometric Features, Structural Characteristics and Physicochemical Properties of ZIF-8 with Hb Encapsulation
The capacity for encapsulation of Hb in ZIF-8 was calculated as 80.6 mg g−1 according to a method described previously.18 This parameter can be derived from the variation in iron content of the bulk solution with dissolved Hb before and after protein integration into the zeolite-like imidazolate framework. This value was similar to another case of enzyme incorporation into a supporter via simple physical adsorption (i.e. hydrogen bond interaction, for example, the loading amount of Laccase into a magnetically separable mesoporous silica sphere is estimated to be 82.0 mg g−17). SEM and TEM images of the zeolite-like imidazole ester framework alone (a and c) and ZIF-8 with Hb encapsulation (b and d) are presented in Fig. 2, respectively. An apparently uniform appearance with clear edges and regular rhombic dodecahedron of the as-prepared ZIF-8 can be observed from Fig. 2. The average dimension of 400 nm for obtained ZIF-8 material can be identified in the SEM and TEM images. A distinct change in morphological features of ZIF-8 after Hb attachment is evident in comparison to that of ZIF-8 alone, indicating that the edge of ZIF-8 with Hb encapsulation was more obscure, and anomalous aggregation of Hb molecules encapsulated onto ZIF-8 could be discerned on the surface of the enzyme carrier. The appearance of MOF material with heme protein entrapment results from the simple physical adsorption including hydrogen bond interaction between heteroatoms within the MOF material and surface amino acid residues of Hb as well as electrostatic attraction between Hb and ZIF-8. This mutual interaction leads to a non-oriented array of Hb molecules onto ZIF-8 (i.e. the random distribution of protein molecules on the surface of the enzyme matrix). These geometric outlines were consistent with that of a similar case demonstrated previously.19 Figure 2e shows the LSCM image of the zeolite-like imidazolate framework with Hb attachment labeled with FITC. It is obvious that the partial surface of ZIF-8 was completely over-coated by some Hb molecules in a random arrangement. Moreover, some Hb molecules with apparent chartreuse label were located on the fringe of the MOF material. The color of Hb attachment onto ZIF-8 originated from the chemical coupling of the N=C=S group in FITC with the amino group on the surface of Hb to produce a unique fluorescence signal in the presence of irradiation at a specific wavelength.21 Some tiny aggregation structures could be detected on some areas of the ZIF-8 surface. The appearance of these aggregates was attributed to the casual attachment of Hb onto the interior of ZIF-8 resulting from the weak interactions between ZIF-8 and Hb.
XRD spectra of MOF material ZIF-8 and ZIF-8 with Hb encapsulation are exhibited in Fig. 3a. A series of main diffraction peaks at 10.3°, 12.8°, 14.6°, 16.5° and 18.0° can be recognized in the XRD spectrum of the zeolite-like imidazole ester framework within a sweep diffraction angle range from 6° to 80°. Five main signals are attributed to these crystalline surfaces, namely (0 0 2), (1 1 2), (0 2 2), (0 1 3) and (2 2 2), with interlayer spaces of 0.857 nm, 0.695 nm, 0.605 nm, 0.540 nm and 0.491 nm. This result is in accord with those presented previously.22,23 It confirmed the successful synthesis of pure-phase ZIF-8 with high crystallinity. The diffraction peaks in the XRD pattern of ZIF-8 with Hb encapsulation are similar to those of ZIF-8 alone, and these signals with diminished strength could be monitored in the spectrum of ZIF-8 with Hb encapsulation. Furthermore, the native diffraction peak at 19.3° for native Hb19 completely disappeared in the spectrum of ZIF-8 with Hb encapsulation. These results confirm that the aggregation of redox protein molecules on the MOF surface decreases the orderliness of pure Hb powder, and the crystalline feature of ZIF-8 with Hb encapsulation could be considered to be similar to that of ZIF-8 itself. The results also suggest that the hydrophilicity is improved after Hb attachment onto MOF material. Furthermore, Hb molecules are not intercalated into the crevice of ZIF-8 for the apparent difference between the adjacent layer gap of ZIF-8 described previously and the dimensions of the Hb molecule (~5.5–6.5 nm). The assumption could be considered consistent with the analysis in the result of LSCM.
XRD spectra of zeolite-like imidazole ester framework alone and MOF material with Hb incorporation (a); FTIR spectra of native Hb, ZIF-8 and zeolite-like imidazole ester framework with heme protein incorporation (b); UV–Vis spectra of free Hb, MOF material and zeolite-like imidazole ester framework with Hb incorporation (c); CD spectra of dissolved Hb, ZIF-8 dispersed phase and MOF material with discrete Hb incorporation (d); FRS spectra of dispersed phase systems made up of constant Hb content of 0.25 mg mL−1 and variable content of ZIF-8 (e).
Figure 3b demonstrated the FTIR spectra of free Hb, ZIF-8 and ZIF-8 with Hb encapsulation, respectively. Some diagnostic bands at 419 cm−1, 750 cm−1, 1578 cm−1 and 2932 cm−1 were found in the spectrum of the ZIF-8 MOF material. These signals were attributed to the presence of Zn-N, Zn-O, C=N (stretching vibration band), and C-H (stretching vibration band),5,22,24 respectively. The identical feature absorption peaks could be recognized in the spectrum of the ZIF-8/Hb-anchored composite. The only difference between them was the positive shift in wave number (from 1661 cm−1 to 1667 cm−1) of the amide II band after Hb integration into ZIF-8. The former showed that the combination of Hb with ZIF-8 did not lead to complete overlapping of ZIF-8 with Hb encapsulation. The latter is attributed to the impact of the hydrogen bond between the surface amino acid residues of Hb as the proton donor and N atom within ZIF-8 introduced earlier. This deduction is consistent with the previous analysis of the results for XRD and SEM measurements.
UV–Vis spectra of free Hb, ZIF-8, and ZIF-8 with heme protein encapsulation are presented in Fig. 3c. A common sharp peak in a more negative location than 250 nm was identified in the spectra of three cases: native heme protein, MOF material alone and ZIF-8 with Hb complex. This signal is related to the π–π* electron transition within the aromatic ring of these systems. The same peaks at 277 nm and 405 nm in both free Hb and ZIF-8 with Hb encapsulation are ascribed to the π–π* electron transition within the porphyrin unit of Hb and d-d electron transition for the Fe-porphyrin complex.25,26 These results imply that the successful attachment of Hb molecules onto MOF material and intrinsic structure of the heme site in Hb was maintained essentially in spite of the presence of hydrogen bond interaction between Hb and ZIF-8. This conclusion is consistent with the analysis in FTIR experiments. Moreover, the catalytic activity determination results demonstrated that the efficiency of native Hb-induced catalysis on H2O2 reduction is attenuated in comparison to that of native heme protein. The specific activity of free Hb and Hb entrapment into ZIF-8 was measured as 0.85 and 0.66 U mg−1, respectively. The apparent reduction in catalytic efficiency of Hb attachment onto ZIF-8 is attributed to the non-directed adsorption of protein leading to the formation of large conglomeration clusters with decreased active area. UV–Vis and GFAAS measurements confirmed that the mechanical strength of Hb attachment onto ZIF-8 is not considered to be favorable. The considerable detachment of Hb molecules from ZIF-8 was identified in the presence of an external shear force from mechanical agitation. The leakage ratio of Hb in the total mass of protein encapsulation into ZIF-8 was estimated as 41.9%. The inferior robustness of Hb attachment onto ZIF-8 is attributed to the weak interactions between Hb and ZIF-8 referred to previously.
Figure 3d shows the CD spectra of native Hb, MOF material ZIF-8 and ZIF-8 with Hb encapsulation. Two sharp bands at 209 nm and 221 nm with negative absorption were observed in the spectrum of native Hb. The presence of these bands correlates with л→л* as well as n→л* electron transitions for the porphyrin ring near the heme site within the free Hb molecule. A single negative absorption band with weak intensity was observed at lower than 210 nm in the spectrum of ZIF-8. The emergence of this featured peak is attributed to the л→л* electron transition in the conjugation system of ZIF-8. Distinct characteristics were recognized in the spectrum of ZIF-8 with Hb encapsulation, meaning that both decreased negative absorption peaks were identified in the spectrum of ZIF-8 with Hb encapsulation. Moreover, the negative absorption peak at lower wavelength exhibited a slight blueshift resulting from the hydrogen bond between ZIF-8 and surface amino acid residues of Hb. Another one at higher wavelength remained unchanged in comparison to the case of the native Hb molecule. This result was different from that of other cases in the presence of adjacent ligation between cofactors in the redox enzyme and element of the protein matrix (i.e. a prominent negative shift of diagnostic band(s) for immobilized redox enzyme in comparison to the native one could be detected).19 This also indicated the absence of abutting complexation between Hb and ZIF-8. The attenuation in absorption strength confirmed the improved hydrophilicity of ZIF-8 with Hb encapsulation, which resulted from the formation of Hb aggregations with a micelle-like structure. This structure has a hydrophilic outer surface and hydrophobic inner core. The integration of Hb into ZIF-8 did not exert a considerable effect on the inherent conformation of the heme site in Hb. This conclusion is consistent with the analysis of the results for the UV–Vis spectrum for ZIF-8 with Hb encapsulation. This deduction was validated by the experiments of dispersivity for the testing systems of ZIF-8 alone and ZIF-8 with Hb encapsulation. The dispersed phase of ZIF-8 began to coalesce and subside after storage for 3 days. The discrete Hb@ZIF-8 remained steady without any conglomeration or sedimentation for approximate 7 days. The improved discrete stability of ZIF-8 with Hb encapsulation is ascribed to the amphiphilic structure for Hb aggregation onto ZIF-8.
The FRS curves for a series of dispersed phases consisting of constant Hb concentration at 0.25 mg mL−1 and varying content of ZIF-8 are shown in Fig. 3e. No prominent attenuation in the intensity of the featured band for native Hb can be discerned from Fig. 3e after the addition of ZIF-8 into the aqueous solution with dissolved Hb. The strong emission peak at ~ 335 nm is ascribed to the presence of amino acid residues such as tryptophan and tyrosine.26 This result indicates that the fluorescence quenching phenomenon is not remarkable. It also suggests that the interaction between ZIF-8 and surface amino acid residues of Hb cannot be regarded as the strong mode (i.e. this mode originates from the covalent coupling and the adjacent ligation26,27,28,29) to form intermediate species with distinct spectroscopic features. This conclusion is supported by the fact that no obvious shift in the characteristic band of native Hb is observed and no other peaks can be recognized in the spectrum of Hb encapsulated in ZIF-8. The weak interactions between Hb and ZIF-8 also suggest that the native microenvironment of Hb is maintained to a great extent. This conclusion is in line with the analysis of the UV–Vis spectra of ZIF-8 with Hb encapsulation. The result of FRS measurement for ZIF-8 with Hb encapsulation was completely different from previous cases described elsewhere.19,26
The EIS spectra and the relevant CVs of naked GCE, basal electrode capped by MOF materia: ZIF-8 and GCE over-coated by a thin film of ZIF-8 with heme protein encapsulation in PBS solution containing K3Fe(CN)6/K4Fe(CN)6+KCl are shown in Fig. 4a and b, respectively. The impedance in charge transfer of bare GCE was remarkably lower in comparison with that of a basal electrode with ZIF-8 coverage. This result was attributed to the presence of MOF material ZIF-8 with inferior electrical conductivity. The result of CV measurements was consistent with that of EIS experiments. The potential difference between oxidation and reduction peaks for electrochemical species for GCE with ZIF-8 over-coating were enlarged and the redox peak current responses of K3Fe(CN)6/K4Fe(CN)6 couple for GCE with ZIF-8 modification were suppressed. This shows that the GCE with ZIF-8 coverage hinders the redox process of K3Fe(CN)6/K4Fe(CN)6 resulting from the shrinkage in the active area of the electrode surface. The conclusion was confirmed by the measurement in active areas of as-prepared electrodes (active surface area of the ZIF-8-based electrode is approximately 1/22 of that for bare GCE) which was carried out according to the illustration elsewhere.17 UV–Vis experiment also confirmed that no apparent interaction between K3Fe(CN)6/K4Fe(CN)6 and ZIF-8 could be identified within the testing wavelength range (i.e. no prominent attenuation in the intensity of diagnostic signal could be detected at the maximal absorption wavelength, data not provided). However, the magnitude of the charge transport resistance increase for the case of ZIF-8 with Hb encapsulation relative to GCE over-coated by ZIF-8 was large. The gap between oxidation and reduction peak potential for the Hb-based electrode in PBS with electroactive species was further widened, and the current response was decreased sharply. This result is attributed to the low electrical conductivity of protein backbone and the loss in electrochemical activity from the combination of electrochemical probing species with Hb integration into ZIF-8. The latter deduction was supported by the similar result of electrochemical reference test which was conducted in electrolyte with dissolved Hb and electrochemical probing species (data not provided). Furthermore, FRS measurement also maintained the previous assumption in the deterioration of the featured peak for native Hb in the presence of electrochemical probing species (data not shown).
Direct Electrochemistry and Electrocatalysis on Reduction of Hydrogen Peroxide for the Electrode Based on ZIF-8 with Hb Incorporation
The CV curves of the GCE with ZIF-8 modification and a supporting electrode capped by a thin film of ZIF-8 with Hb encapsulation in deaerated and neutral PBS are illustrated in Fig. 5a. Only one pair of weak and broad redox bands with mean potential at ~ 265 mV can be discerned from the CV curve of the GCE with ZIF-8 over-coating. The asymmetric shape in the CV curve of the GCE with MOF material coverage is ascribed to the irreversibility in the redox process of the electroactive sites in the ZIF-8: complex of Zn with heteroatoms of the imidazole ring.28 A special case with complicated electrochemical signals was identified from the CV curve of the Hb-based electrode, implying that the symmetric redox peaks (ratio of anodic current against cathodic current, ip,a/ip,c at 1.1) with formal potential at ~ −154.5 mV was detected within the same range of potential sweep except for the native redox waves of the electroactive site within ZIF-8. This electrochemical signal is ascribed to the process of electron shuttle between the heme site within Hb and conductive supporter for its formal potential near that of the cofactor in native Hb as introduced elsewhere.19 All these confirmed that partial Hb molecule integration into ZIF-8 achieved the direct charge transfer between the cofactor in Hb and the conductive interface of the enzyme carrier. Finally, the surface coverage of electrical-wired Hb molecules were calculated as 7.8 × 10−10 mol cm−2 according to similar means shown previously.30 This means that this parameter can be derived from the normalization of the mean value in integration areas of redox peaks to the product of Faraday constant, surface active area and potential sweep rate.
Direct electrochemistry of Hb@ZIF-8/CTS/GCE in deaerated PBS at pH = 7.0 in the absence of any external electron relay which was characterized by CV (a); CV curves a of static Hb-based electrode in neutral and oxygen-free PBS registered at varying rates of potential scanning (b) and its inset: linear-fitting plots of redox peak currents versus square root of potential sweep rate (sweep rates from inner plot to outer one indicating 50, 100, 150, 200, 250, 300, 350, 400, 450, 500 mV s−1); DPV curves of Hb@ZIF-8/CTS/GCE in nitrogen-bubbling PBS at neutral pH recorded at different pulse widths for negative potential scanning (c) and positive potential scanning (d); CV curves of a quiescent Hb-based electrode in oxygen-free PBS with regulated pH values recorded at a scanning rate of 200.0 mV s−1 (e) and relevant linear-fitting dependence plot of reduction peak potential for Hb@ZIF-8/CTS/GCE on pH value of buffer solution (f).
Steady i-E curves of Hb/ZIF-8/CTS/GCE in oxygen-free PBS recorded under varying velocities of potential scanning are shown in Fig. 5b, and linear-fitting plots of oxidation peak current and reduction current versus square root of sweep rate are presented in the inset of Fig. 5b. The oxidation peak potential and the reduction in peak potential for the Hb-based electrode does not shift prominently with the increase in potential sweep rate. The slight deviation in the formal potential of the Hb-based electrode from that of the heme site within immobilized Hb with the native structure31 (~ −150 mV) suggests that the original structure of the cofactor in Hb attachment onto ZIF-8 was largely maintained intact. This conclusion is consistent with the analysis results of FRS spectra and UV–Vis measurements. Moreover, the value of ip,a/ip,c increased from 1.07 to 1.4 with the increase in potential scanning rate, indicating that the reversibility is impeded at higher sweep rates. The good linear relationship between redox current responses and square root of potential scanning velocity was retained within the testing range of the potential sweep rate. All these results revealed that the quasi-reversible redox process of the Hb-based electrode is attributed to a typical thin-film controlling mode for redox enzyme molecules incorporated in MOF material via relatively weak interactions instead of covalent bonding and competitive ligation.19,32 Theoretically apparent diffusion coefficient (Da) for electrically wired Hb molecules was deduced from the slope of linear-fitting plot in the inset of Fig. 5b to be 9.1 × 10−5 cm2 s−1 from the definition introduced elsewhere.33,34 This value was much higher than that of a similar case demonstrated previously.33 This is ascribed to the hydrogen bond between Hb and ZIF-8 which could not avoid the efficient diffusion of electroactive sites in Hb attachment onto MOF material.
DPVs of Hb/ZIF-8/CTS/GCE in deaerated and neutral electrolyte registered at variable pulse widths (i.e. 10 mV, 20 mV, 30 mV, 50 mV, 100 mV, 150 mV, 200 mV, 250 mV, 300 mV and 350 mV) for both potential sweep directions: negative scanning (i.e. from 1.0 V to −1.0 V) and positive scanning (i.e. from −1.0 to 1.0 V) are exhibited in Fig. 5c and d, respectively. The strength of the reduction wave at ~ −215 mV is enhanced and another negative reduction band at lower potential (i.e. negative than −0.5 V) is observed with the decrease in pulse width in the negative sweep. The former is attributed to the reduction reaction of the heme site in Hb attachment onto ZIF-8. The latter is ascribed to the electro-reduction of the oxygen molecule entrapment into ZIF-8. The reduction peak potential causes a negative shift, and the strength of the reduction band is enhanced with the decrease in pulse width. Similar results have been observed in previous reports.19 The reduction in consistency of dissolved O2 in buffer solution after incubation of the Hb-based electrode into air-saturated electrolyte for 6 h was determined by a Clark oxygen electrode. The loss ratio in total amount of dissolved O2 was estimated as ~ 10.8% according to an illustration elsewhere.35 The consecutive oxidation waves including main oxidation peak of heme site in immobilized Hb and another side peak for the redox process of the Hb-ZIF-8 complex resulting from the hydrogen bond interaction could be identified in the DPV curve of positive scanning. The intensity of the oxidation bands increased with the decrease in pulse width. At the same time, an electrochemical signal at negative potential of entrapped O2 reduction was suppressed with the reduction in pulse width to a great extent. The signal of the inherent oxidation band for ZIF-8 could be regarded as minor in comparison to that of the main oxidation band of the heme site in incorporated Hb at low pulse widths. All these results indicate that the hydrogen bond interaction between Hb and ZIF-8 imposes a considerable effect on the electron transfer between electroactive sites within ZIF-8 and the conductive supporter. The consecutive redox processes with the heme site in Hb as the primary electroactive site and other electroactive species redox site in ZIF-8 as inner charge relay were achieved when the pulse strength was relatively high. This result also confirms that the electrochemical activity of Hb attachment onto MOF material was better than that of the redox group within ZIF-8.
CV curves of the quiescent Hb-based electrode in blank and electron relay-free PBS with different pH values recorded at 200.0 mV s−1 and corresponding linear-fitting plot of reduction peak potential versus pH value of buffer solution are displayed in Fig. 5e, f, respectively. The potential of reduction waves shifts towards negative potential with the increase in the pH value of electrolyte according to the Nernst equation. The ratio of n (electrons participating in redox process) versus x (numbers of H+ involved in the redox process of Hb attachment onto MOF material) was found to be 2:1 from the slope for the linear-fitting plot in Fig. 5f (−34.5 mV/pH). Usually, four electrons were involved in the electrochemical process of native Hb (single electron participated in the redox reaction of Fe3+/Fe2+ of each heme site in Hb, and four heme sites are included in one Hb molecule).36 This result revealed that the electrochemical reaction of the Hb-based electrode is attributable to a typical quasi-reversible redox process with involvement of four electrons and two H+ ions. Similarly, two electrons were involved in the redox process of MOF material ZIF-811 (redox transformation of Zn2+ and reduced Zn). The slope in the linear-fitting plot of the oxidation peak potential versus logarithm of potential scanning rate was estimated at approximately 62.2 mV/pH (data not shown here). This result indicated the participation of two protons and two electrons in the redox process of ZIF-8 MOF material, meaning that the hydrogen bond interaction between Hb and ZIF-8 is related to the redox process of the Hb-based electrode. Furthermore, the heterogeneous charge transfer constant was estimated as 3.1 × 10−2 cm s−1 according to the definition introduced elsewhere.37 The thickness of the diffusion layer (δ) was determined using the same method as previously illustrated,37,38 and the parameter ranged from 3.4 × 102 μm to 1.1 × 103 μm, which implies that this parameter can be deduced from the square root of the product for the apparent diffusion coefficient of electrical-wired redox enzyme, π, and duration of potential scanning. Hence, the normalized rate of heterogeneous electron transport could be determined, and this parameter is in the range of 28.2–91.2 s−1.
CV curves of quiescent Hb/ZIF-8/CTS/GCE in neutral PBS with variable hydrogen peroxide concentrations recorded at 200 mV s−1 are shown in Fig. 6a. A prominent improvement in the onset potential of substrate electro-reduction for the Hb-based electrode was observed in comparison to that of the reference electrode (i.e. electrode based on MOF material alone, data not provided), which indicated that an approximate positive shift with 400 mV at onset potential of the substrate electro-reduction could be detected (the parameters for Hb-based electrode and MOF modified one are ~ 126 mV and −280 mV, respectively). The intensity of the substrate electro-reduction band for the Hb-based electrode increased sharply with the increase in the consistency of H2O2 in the electrolyte. This result revealed the good sensitivity of the Hb-based electrode to dissolved hydrogen peroxide. The limited catalytic current for the Hb-based electrode (ilim) was derived from Fig. 6a as 1.14 × 102 μA according to the Michaelis–Menten equation, which implies that this parameter could be obtained from the intercept in the linear-fitting double-reciprocal plot of steady reduction current against the H2O2 concentration. The turnover frequency of substrate transformation (kTF) was calculated as 6.4 × 101 s−1 via the equation ilim = nFNEkTF as shown earlier.39 n is the number of electrons involved in the catalytic reaction of substrate electro-reduction, and NE is the total amount of electrically wired enzyme molecules as previous discussion.
CV curves of static Hb-based electrode in neutral PBS with different concentrations of dissolved hydrogen peroxide recorded at a potential sweep rate of 200 mV s−1 (a); electrochemical sensing performance to dissolved H2O2 for Hb@ZIF-8/CTS/GCE in magnetically stirred PBS registered at −10.4 V (b) and its inset: Lineweaver–Burk plot of steady catalytic current response of the Hb-based electrode against concentration of dissolved substrate; dependence plot of the steady catalytic current at −0.4 V for Hb@ZIF-8/CTS/GCE in PBS with 0.45 mM H2O2 recorded at variable rates of rotation of the enzyme-based electrode on the square root of the electrode rotation velocity (c); the relationship curve of Hb incorporation capacity versus operation temperature for composite of ZIF-8 with Hb encapsulation (d); the relationship curve between Hb loading mass onto ZIF-8 and the pH of electrolyte (e).
CA curves of the static Hb-based electrode in electron mediator-free PBS containing variable substrate concentration levels at different time intervals were observed under magnetic stirring and constant applied potential as illustrated in Fig. 6b. The corresponding linear-fitting plot of stable current response against H2O2 concentration is shown in the inset graph of Fig. 6b. A large peak in the front segment of the current–time relationship curve and the rapid drop in current response with elapsed time can be observed in Fig. 6b. This result suggests that the kinetics of the Hb-induced electrocatalysis on H2O2 are dominated by the process of substrate mass transfer. This assumption was verified by the experimental result for LSV in combination with rotating disk electrodes (see the later description concerning the relevant issue). Moreover, the steady current response is achieved in a short period after the aliquot of PBS with dissolved hydrogen peroxide was injected into the testing system. This showed that the as-prepared Hb-based electrode was sensitive to the content of dissolved substrate, suggesting the relatively favorable conversion efficiency of substrate attachment onto the Hb-based electrode. The result was consistent with the previous analysis in CV curves of the Hb-based electrode in PBS with different H2O2 concentrations. The desirable linear relationship between steady catalytic current response and substrate consistency was maintained within the testing concentration range. The sensitivity and the detection limit of the as-prepared Hb/ZIF-8/CTS/GCE to hydrogen peroxide was found to be 1.3 × 10−2 A L mol−1 and 3.7 μmol L−1, in agreement with the definition introduced elsewhere.40 The Michaelis constant KM was found to be 28.0 μM from the slope and intercept of the reciprocal linear-fitting plot based on the data shown in the inset graph of Fig. 6b. The estimation of this parameter was made according to the same means depicted elsewhere.30 The low detection limit and the high affinity of Hb/ZIF-8/CTS/GCE in the current system to dissolved H2O2 in comparison to other cases (KM = 2.87 mM for Hb encapsulation into ordered mesoporous silicas36 and 200.0 μmol L−1 for the heme protein integration into conductive polymer41) can be attributed to the efficient maintenance of the inherent activity of electrocatalysis and excellent substrate accessibility of the binding pocket within Hb for the existence of a hydrogen bond between Hb and ZIF-8. Previous UV–Vis spectra support the former conclusion. The latter is confirmed by the excellent kinetics of enzyme-induced substrate recognition and subsequent transformation as described previously. Moreover, the average consumption rate of hydrogen peroxide was found to be 1.94 × 10−7 mol L−1 s−1 according to the same method introduced earlier.42 The normalized rate constant of substrate attachment onto ZIF-8 with Hb encapsulation was calculated as 2.9 × 10−2 s−1 according to the previous method.42
The relationship plot of steady substrate electro-reduction current for the Hb-based electrode in neutral and electron mediator-free PBS recorded at −0.4 V against the square root of the electrode rotation rate is exhibited in Fig. 6c. The favorable linear relationship could be kept within the testing range of electrode rotation velocity as demonstrated in Fig. 6c. The apparent turnover frequency (kTF) and the substrate diffusion coefficient was estimated as 68.7 s−1 and 1.8 × 10−7 cm2 s−1 according to the same equations and similar theory described previously.39,43 The standard substrate diffusion rate was deduced from the normalization of the calculated H2O2 diffusion coefficient to surface active area of the testing electrode. This physical quantity was found to be 1.3 × 10−3 s−1. The mass transfer processes of the substrate and electroactive species, the chemical attachment of substrate onto ZIF-8 with Hb incorporation, and the heterogeneous charge transfer between redox sites in the composite and conductive interface were included in the steps involved in enzymatic H2O2 electro-reduction. On the basis of quantitative analysis in the derived dynamic parameters of the steps involved in the enzyme-induced H2O2 electro-reduction, the process of substrate diffusion was attributed to the key step in restraining the Hb-involved electrocatalytic efficiency under the same dimension.
In fact, the linear relationship between the steady catalytic current of the Hb-based electrode and the square root of the disk electrode rotation rate did not remain stable when the rotation velocity of the Hb-based electrode was higher than 1600 rpm. The deviation from the linear-fitting plot (i.e. increased current more than expected in the linear-fitting plot) was ascribed to the disruption of Hb molecule clusters under the acute impact from external vigorous agitation which increased the active area of Hb attachment onto ZIF-8. The attached Hb molecules detached from ZIF-8 in the presence of the shear force on the surface of the electrode when the rotation rate of the disk electrode was relatively high (> 1600 rpm). The results of GFAAS measurements implied that the ratio of detached Hb in the total amount of Hb attachment onto ZIF-8 was enhanced with the increase in the rotation of the disk electrode (parameters were 16.2%, 24.4% and 41.9% when the rotation velocity of the disk electrode was 1600 rpm, 2500 rpm and 3600 rpm, respectively). This result is ascribed to the unstable anchoring of Hb onto ZIF-8 through simple physical adsorption alone. This assumption is supported by the result of the relationship plot between Hb encapsulation capacity on the operational temperature as illustrated in Fig. 6d, which shows that the Hb loading amount decreased with the increase in the temperature of incubation. A bell shape in the relationship curve between the loading capacity of Hb into ZIF-8 and the pH of the electrolyte can be seen in Fig. 6e. The optimal pH for Hb entrapment (pH = 7.2) is close to that for the maximal efficiency of Hb-induced catalysis on H2O2 electro-reduction (pH = 7.4). This result is related to the surface electrical property of ZIF-8 and Hb molecules under the optimal pH value.11,36
The repeatability in catalytic current response, long-term stability in electrocatalysis, relationship between catalytic activity and pH value of the electrolyte and the dependence of catalytic efficiency on operational temperature for Hb-induced electro-reduction of hydrogen peroxide are displayed in Fig. 7a, b, c and d, respectively. Results from Fig. 7a imply that the difference in steady catalytic current among different Hb-based electrodes could be regarded as insignificant. The RSD in steady H2O2 electro-reduction currents for five Hb/ZIF-8/CTS/GCE registered in the same operational condition was ~ 3.9%. This indicates that desirable repeatability in catalytic efficiency of Hb-involved H2O2 electro-reduction was obtained, which is related to the minor impact on the original structure of free Hb from the mild hydrogen bond interaction between Hb and MOF material. It is obvious from Fig. 7b that the residual activity in Hb-induced electro-reduction of hydrogen peroxide decreases gradually with elapsed time. Approximately 83.8% of initial catalytic efficiency for Hb/ZIF-8/CTS/GCE is retained after storage at 4°C for 17 days. This result is ascribed to the slow attenuation in catalytic function of Hb incorporation into ZIF-8. The advantage in long-term usability is ascribed to the MOF material protection of the inherent structure of native Hb from external interference. The relationship curve of the catalytic effect on the electro-reduction of H2O2 against the pH value of buffer solution as shown in Fig. 7c displays a bell shape, which implies that the highest catalytic current response in the testing pH range of PBS was achieved at pH = 7.4. This optimal pH was similar to that of free heme protein and the case of redox enzyme entrapment into conductive polymer with intact structure and desirable catalytic performance as elucidated elsewhere.41 This confirmed that the hydrogen bond interaction between ZIF-8 and heme protein molecule does not have a serious impact on the structure and inherent catalytic mechanism of integrated Hb in comparison to the heme site of native Hb. The relationship curve of the catalytic function on H2O2 electro-reduction for the Hb-based electrode against operational temperature is presented in Fig. 7d, which shows that the catalytic current for H2O2 electro-reduction decreases linearly with the increase in the experimental temperature. This result indicates the occurrence of Hb desorption from ZIF-8 under higher temperature. Naturally, the process of Hb attachment onto ZIF-8 is attributed to a typical physical adsorption. The strength of the diagnostic peak at 405 nm for native Hb after Hb-based electrode immersion in blank PBS was enhanced when the system temperature was gradually increased. This result can be regarded as direct proof to support the previous deduction.
Reproducibility (a), long-term usability (b), acid-base endurance (c) and thermal stability (d) in catalytic efficacy on electro-reduction of hydrogen peroxide for the Hb-based electrode evaluated by CV. The steady catalytic currents in CV curves were obtained under the following experimental conditions: consistency of substrate at 164.0 μM and potential sweep rate of 200 mV s−1.
Conclusion
Zn(NO3)2 as metal source reacted with 2-methyl imidazole as ligand to give rise to a typical MOF material ZIF-8 for Hb incorporation. The attachment of Hb onto ZIF-8 was achieved via the synergy of simple hydrogen bond interaction between the surface amino acid of Hb and N atoms within ZIF-8 and electrostatic attraction. Multiple techniques including microscopy, spectrometry and electrochemical means were deployed to investigate the influence of the mutual interactions between the ZIF-8 and integrated Hb on the structural features and electrocatalytic efficiency of the H2O2 reduction of ZIF-8 with Hb integration. The synergy of hydrogen bond and electrostatic attraction between the element of ZIF-8 and heme protein did not reduce the inherent catalytic activity of Hb for the intact structure of Hb after integration into ZIF-8. This mutual interaction did not hinder the direct electron shuttle between the heme site in Hb and conductive supporter. The redox process on the surface of the Hb-based electrode is regarded as quasi-reversible, and the thin-film controlling the electrochemical reaction involves four electrons and two H+ ions. This redox reaction occurs with moderate dynamics (102–103 s−1). The step of substrate diffusion is considered the principal factor limiting the catalytic efficiency of the Hb-based electrode and it should be considered independently of the mutual interaction between Hb and ZIF-8. The relatively weak interactions between Hb and ZIF-8 do not give rise to a harmful influence on the substrate affinity (KM: 28.0 μM), detection limit (3.7 μM) and target sensitivity (1.3 × 10−2 A L mol−1) of the as-prepared Hb-based electrochemical sensor.
References
Q.M. Qiu, H.Y. Chen, Y.X. Wang, and Y.B. Ying, Coord. Chem. Rev. 387, 60 (2019).
M. Younas, M. Rezakazemi, M. Daud, M.B. Wazir, S. Ahmad, N. Ullah, Inamuddin, and S. Ramakrishna, Prog. Energy Combust. Sci. 80, 100849 (2020).
J.X. Chen, K.L. Liu, M.H. Jiang, J. Han, M.L. Liu, C.B. Wang, and C.L. Li, Colloid Surf. A 568, 461 (2019).
J.Y. Song, W.T. He, H. Shen, Z.X. Zhou, M.Q. Li, P. Su, and Y. Yang, Chem. Eng. J. 363, 174 (2019).
J.D. Cui, S.Z. Ren, B.T. Sun, and S.R. Jia, Coord. Chem. Rev. 370, 22 (2018).
N. Gu, H. Li, and Y. Zhao, Mater. China 36, 833 (2018).
Y.F. Zhu, S. Kaskel, J.L. Shi, T. Wage, and K.-H.V. Pée, Chem. Mater. 19, 6408 (2007).
C. Zhang, X.R. Wang, M. Hou, X.Y. Li, X.L. Wu, and J. Ge, ACS Appl. Mater. Int. 9, 13831 (2017).
R. Perveen, Inamuddin, S.U. Haque, A. Nasar, A.M. Asiri, and G. M. Ashraf, Sci. Rep. 7, 13353 (2017).
K.T.N.A. Tuan, M.F. Ismail, R.M.B. Abdul, K.E. Cordova, and L.M.A. Mohammad, J. Phys. Chem. B. 124, 3678 (2020).
B.K. Ma, L.Z. Cheong, X.C. Weng, C.P. Tan, and C. Shen, Electrochim. Acta 283, 509 (2018).
S.U. Haque, Inamuddin, and M. Naushad, Enzyme Microb. Technol. 87, 29 (2016).
H. Mitta, V. Perupogu, R. Boddula, S.R. Ginjupalli, Inamuddin, and A.M. Asiri, Int. J. Hydrogen Energy 45(50), 26445 (2019).
H. Zhang, J.Q. Luo, S.S. Li, J.M. Woodley, and Y.H. Wan, Chem. Eng. J. 359, 982 (2018).
C.K. Wang, Q.Q. Wang, and R. Tan, Analyst 143, 4118 (2018).
C. Zhang, Master's Thesis. Zhejiang University, China (2008).
Y. Yang, H. Zeng, Q. Zhang, X. Bai, C. Liu, and Y.H. Zhang, Chem. Phys. Lett. 658, 259 (2016).
J. Huang, J.Y. Zhou, H.Y. Xiao, S.Y. Long, and J.T. Wang, Acta. Chim. Sin. 63, 1343 (2005). (in Chinese).
J.W. Xu, T.M. Ma, M. Zhang, and H. Zeng, J. Mater. Sci. Mater. Electron. 32, 6064 (2021).
P.L. Gao, J.W. Wang, M. Zheng, and Z.G. Xie, Chem. Eng. J. 381, 122665 (2019).
J.F. Shi, X.L. Wang, S.H. Zhang, L. Tang, and Z.Y. Jiang, J. Mater. Chem. B 4, 2654 (2016).
F.J. Lyu, Y.F. Zhang, R.N. Zare, J. Ge, and Z. Liu, Nano Lett. 14, 5761 (2014).
S.S. Nadar and V.K. Rathod, Enzyme Microb. Technol. 108, 11 (2018).
L.N. Zhao, H. Zhang, J. Zhang, W.S. Zong, and R.T. Liu, Luminescence 34, 290 (2019).
X.J. Liu, W.W. Chen, M.L. Lian, X. Chen, Y.L. Lu, and W.S. Yang, J. Electroanal. Chem. 833, 505 (2019).
H. Mao, B.F. Cai, B. Zhao, and Z.W. Wang, Chin. J. Appl. Chem. 26, 1332 (2009). (in Chinese).
C.H. Liu, J. Xu, and Z.F. Wu, Bioprocess Biosyst. Eng. 34, 931 (2011).
M.M. Liu, Master's Thesis, Taiyuan University of Technology, China (2014).
J.J. Zhou, P. Wang, C.X. Wang, Y.T. Goh, Z. Fang, P.B. Messersmith, and H.W. Duan, ACS Nano 9, 6951 (2015).
H.J. Qiu, C.X. Xu, X.R. Huang, Y. Ding, Y.B. Qu, and P.J. Gao, J. Phys. Chem. C. 112, 14781 (2008).
S.F. Wang, T. Chen, Z.L. Zhang, X.C. Shen, Z.X. Lu, D.W. Pang, and K.Y. Wong, Langmuir 21, 9260 (2005).
K. Karnicka, K. Miecznikowski, B. Kowalewska, M. Skunik, M. Opallo, J. Rogalski, W. Schuhmann, and P.J. Kulesza, Anal. Chem. 80, 7643 (2008).
C. Krishnananda and M. Shyamalava, Bioelectrochemistry 53, 17 (2001).
H.Y. Zhao, H.M. Zhou, J.X. Zhang, W. Zheng, and Y.F. Zheng, Biosens. Bioelectron. 25, 463 (2009).
W.E. Farneth, B.A. Diner, T.D. Gierke, and M.B. D’Amore, J. Electroanal. Chem. 581, 190 (2005).
Y.Z. Xian, Y. Xian, L.H. Zhou, F.H. Wu, Y. Ling, and L.T. Jin, Electrochem. Commun. 9, 142 (2006).
S. Shleev, A. Christenson, V. Serezhenkov, D. Burbaev, A. Yaropolov, L. Gorton, and T. Ruzgas, Biochem. J. 385, 745 (2005).
W.E. Farneth and M.B. D’Amore, J. Electroanal. Chem. 581, 197 (2005).
S. Tsujimura, Y. Kamitaka, and K. Kano, Fuel Cells 7, 463 (2007).
Y. Zhang, G.M. Zeng, L. Tang, D.L. Huang, X.Y. Jiang, and Y.N. Chen, Biosens. Bioelectron. 22, 2121 (2007).
K. Young-Tae, B. Mannan, and S. Yoon-Bo, Biosens. Bioelectron. 19, 227 (2003).
Y. Yang, W.S. Huo, Z. Zhou, Q. Zhang, and H. Zeng, Chin. J. Inorg. Chem. 32, 2117 (2016). (in Chinese).
H. Zeng, Z.Q. Tang, L.W. Liao, J. Kang, and Y.X. Chen, Chin. J. Chem. Phys. 24, 653 (2011).
Acknowledgments
This project was financially supported by the Xinjiang Autonomous Region Colleges and Universities Scientific Research Plan Key Projects of Natural Science (XJEDU2021I020) and the “13th five-year” Plan for Key Discipline Chemistry, Xinjiang Normal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest in the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y.T., Wang, F., Zhang, M. et al. The Catalytic Effect on H2O2 Electro-Reduction of an Electrode Based on MOF Material ZIF-8 as Hemoglobin Supporter via Hydrogen Bond Interaction. J. Electron. Mater. 51, 4493–4508 (2022). https://doi.org/10.1007/s11664-022-09654-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09654-z