Abstract
A series of Co3V2O8 nanoparticle precursors were successfully prepared by simple ion thermal synthesis under mild conditions. The optimized Co3V2O8 material with small and uniform spherical nanoparticles (~ 50-nm diameter) was synthesized at pH 9 and 150°C for 72 h in 1-butyl-3-methylimidazole bromide, while irregular large-block Co3V2O8 materials were obtained through the traditional hydrothermal method. After calcination, the synthesized material (CVO-IL) was used as an anode for lithium ion batteries and showed a high initial discharge capacity of 1528 mAh g−1 and a reversible capacity of ~ 600 mAh g−1 after 300 cycles, which was much higher than that of Co3V2O8 synthesized in water (CVO-W, 120 mAh g−1). Based on cyclic voltammetry tests and impedance measurements, it was found that the electrode reaction in this work was controlled by Li ion diffusion in the electrolyte and electrode materials for both CVO-IL and CVO-W. The Li ion diffusion coefficient of CVO-IL was much higher than that of CVO-W, which was likely due to its small and uniform spherical structure. These results, therefore, revealed the excellent electrochemical performance of CVO-IL as an anode material for Li-ion batteries. The Co3V2O8 materials prepared through ionothermal synthesis under mild reaction conditions have been rarely reported, but they should have significant potential usage in the field of lithium-ion batteries and other electrochemical systems.
Graphical Abstract
Co3V2O8 nanomaterial was prepared through ionothermal synthesis and exhibited excellent electrochemical performance as an anode for lithium-ion batteries.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing energy consumption has prompted researchers to conduct in-depth research into energy storage systems.1,2,3 Rechargeable lithium-ion batteries (LIBs) have many advantages, including high energy density, long cycle life, high conversion efficiency, low toxicity, and no memory effect, and they have become an area of tremendous research in recent decades.4 Although LIBs have been successfully used in portable electronic devices, electric vehicles and other applications,5 the low specific capacity of their electrode materials has limited their further development for large-scale energy storage applications.6,7 Although graphite is the main anode material for LIBs at present, its theoretical capacity is only 372 mAh g−1.8,9 Moreover, due to the safety problems caused by dendrite formation during charge and discharge cycling, graphite is not sufficient to meet the needs of large-scale battery anode materials in the future.10,11 Although other anode materials, such as tin-, silicon- and metal oxide-based materials, can provide high capacities, their large volume expansion and low lithium ion diffusion rates lead to irreversible capacity degradation.12,13
In order to solve these problems, various strategies have been proposed to improve the electrochemical performance of electrode materials, such as reducing the particle size, manufacturing mesoporous or porous structures, doping with heteroatoms and coating with high-conductivity carbon materials.14 Studies have shown that by carefully controlling the morphology and particle size of metal oxides, their limitations can be overcome and their potential for LIBs can be effectively improved. This is because nanostructured materials can be used as buffers to prevent volume expansion, which can improve the short lithium ion transport path length, as well as providing other thermodynamic advantages.15,16,17,18 Vanadium-based materials have high lithium ion storage capacity, abundance of vanadium and various oxidation states. This is where vanadium-based compounds with intriguing properties can fill the gap of the current battery technologies. Therefore, it has been widely studied as a potential anode for lithium ion batteries.19,20 In particular, transition metal vanadates have attracted significant attention, due to their multi-step reduction and enhanced electron transfer during lithium insertion compared with other compounds, such as LiCoO2.21 So far, the most widely studied vanadate materials have been nickel, cobalt, potassium and manganese vanadates.10,22,23,24,25 Of these materials, cobalt vanadates have drawn particular interest, owing to their higher electrochemical activities than those of other metal vanadates.22
Because of the multiple oxidation states of vanadium, cobalt vanadates have various phases, including Co3V2O8and Co2V2O7 . Among these phases, Co3V2O8 is the most common and stable phase reported in the literature. Recently, researchers have developed a variety of synthesis methods for nanosized subunit cobalt vanadate, including hydrothermal,26 electrospinning,27 microwave-assisted synthesis28 and coprecipitation methods.29 Park et al. synthesized one-dimensional nanofibers by electrospinning and subsequent heat treatment in air,27 and Co3V2O8 nanofibers with low and high carbon content were obtained by heat treatment at different temperatures. Chai et al. synthesized layered Co3V2O8 mesoporous microspheres via controllable coprecipitation at room temperature.1 Wu et al. successfully prepared Co3V2O8 micro-pencils coated with reduced graphene oxide via a hydrothermal synthesis and thermal reduction.30 Zhang et al. prepared a hexagonal Co3V2O8·3H2O nanosheet precursor by a hydrothermal method and obtained porous hexagonal Co3V2O8 nanosheets after heat treatment.26 Liu et al. successfully synthesized cobalt vanadate nanoplates by uniformly grinding ammonium metavanadate and cobalt nitrate hexahydrate in a microwave oven at 800°C.31 It can be seen that the traditional methods to synthesize cobalt vanadate materials mainly include hydrothermal synthesis or precipitation in aqueous solution to obtain precursors, which subsequently are calcined to obtain the final Co3V2O8 active material. The hydrothermal synthesis method needs to be carried out under high temperature and pressure reaction conditions with a potential security risk; the precipitation method often generally needs to add another template to obtain a specific morphology or reduce the particle size, which makes the reaction system more complex.
Ionothermal synthesis is a method for preparing inorganic nanomaterials in recent years. Compared with traditional hydrothermal and organic solvothermal syntheses, ionothermal synthesis has many advantages, including mild conditions, simple operation, atmospheric pressure reactions and the elimination of potential safety problems.32,33,34 In general, ionic liquids have stable properties and high decomposition temperatures. Therefore, ionothermal synthesis has more advantages than hydrothermal synthesis, especially for experimental synthesis at high temperatures and atmospheric pressure. In this study, Co3V2O8 precursor was synthesized by a simple one-step ionothermal synthesis method using an ionic liquid (1-butyl-3-methylimidazole bromide, [Bmim]Br) as a green solvent and template and Co(NO3)2·6H2O and NH4VO3 as cobalt and vanadium sources, respectively. After calcination of the synthesized precursor, the active Co3V2O8 material possessed a unique morphology and crystal structure and exhibited excellent long-term cycle stability, high specific capacity and good rate performance when used as an anode material for LIBs.
Experimental
Sample Preparation
The precursor of the cobalt vanadate sample was prepared in a three-necked flask with [Bmim]Br35 as the solvent and template, Co(NO3)2·6H2O as the cobalt source and NH4VO3 as the vanadium source. Typically, 4.365 g (0.015 mol) of Co(NO3)2·6H2O and 1.17 g of (0.01 mol) NH4VO3 were dissolved in 54.8 g (0.25 mol) of [Bmim]Br. After heating at 80°C for 2 h, a clear solution was obtained and its pH was adjusted to 8 with 90 wt.% NaOH prepared in advance. The temperature of the solution was then raised to 150°C and kept under stirring for 72 h. After the reaction, the solution was cooled to room temperature. The precipitate obtained was washed with deionized water and absolute ethanol several times and then dried in a vacuum oven at 70°C for 12 h. The obtained cobalt vanadate precursor was labeled as CVO-IL-pre. The effect of pH on the reaction was studied by analyzing the samples obtained under different pH values of 7, 8, 9 and 10 at fixed 150°C for 72 h. The influence of reaction temperature was studied by analyzing the samples obtained under the different reaction temperatures of 150°C, 160°C, 170°C and 180°C at fixed pH 9 for 72 h. In addition, the effect of reaction time was also studied. Reaction times of 36 h, 48 h, 60 h and 72 h at fixed pH 9 and 150°C were set to understand the formation process of the sample.
For comparison, a cobalt vanadate sample (CVO-W-pre) was also prepared by the hydrothermal method in an autoclave using the same synthesis conditions (i.e., pH 9 and 150°C for 72 h) and post-treatment as CVO-IL-pre.
The cobalt vanadate precursors obtained (CVO-IL-pre and CVO-W-pre) were calcined in air at 500°C for 4 h to obtain the final electrode materials for the electrode tests and were denoted as CVO-IL and CVO-W, respectively.
Sample Characterization
The product morphologies were examined using a JEOL-JSM-6700F electron microscope. The content and distribution of elements were measured using energy-dispersive x-ray spectroscopy (EDX) with an X-max80 energy dispersive spectrometer. The samples were plated by sputtering gold with a plating machine. To investigate the chemical composition of the products, after-annealed products were studied by x-ray photoelectron spectroscopy (XPS) using a Thermo ESCALAB 250 spectrometer with mono-chromatic Al-Kα radiation (hv = 1486.6 eV) operating at 150 W and a pass energy of 20 eV. The binding energies for sample charging were calibrated using the C 1s peak at 284.8 eV. X-ray diffraction (XRD) patterns were obtained on a Shimadzu XRD-6000 powder diffractometer (Japan) using Cu-Kα radiation (λ = 0.1541 nm). Transmission electron microscopy (TEM) was carried out using an FEI TF20 instrument (USA). The resolution of the instrument is 0.19 nm, the maximum magnification was 1,000,000 × and the test voltage was 200 kV. N2 adsorption–desorption measurements were carried out at 77 K using a Micromeritics Gemini V 2380 autosorption analyzer.
Electrochemical Measurements
The electrochemical characterization was carried out on 2032-type coin cells, with lithium-metal foil used as a counter electrode. The active material (CVO-IL or CVO-W), acetylene black and polyvinylidene fluoride were uniformly dispersed in n-methyl-2-pyrrolidone at mass ratios of 8.0:1.0:1.0, respectively. The active material was used as the positive electrode, a metal lithium sheet was used as the negative electrode and the separator was made of a polypropylene membrane (Celgard 2400). All assembly and disassembly processes were carried out in a glove box protected by high-purity argon. Generally, the moisture and oxygen contents in the glove box were controlled below 1 ppm. The battery test system for the constant current charge discharge test of the CR2032 coin cell was LAMBO BT2018A (Wuhan, China). Cyclic voltammetry (CV) curves and electrochemical impedance were measured using a CHI660D electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd., China).
Results and Discussion
Optimization of Sample Preparation Conditions
Optimization of pH
The morphologies of the samples prepared at different pH values were observed by scanning electron microscopy (SEM). The results showed that at pH 7 or 10, the particle sizes were uneven and the particle diameters were large (Fig. 1a and d). When the pH was adjusted to 8, particles with a more uniform size were obtained and the particle diameters were reduced (Fig. 1b). When the pH was adjusted to 9, a large number of nanoparticles with a small diameter and uniform size were obtained (Fig. 1c). Therefore, the optimal pH value of the solution was pH 9, which provided a foundation for the subsequent experiments.
In addition, the morphology of the sample synthesized by the hydrothermal method with the same experimental conditions (i.e., pH 9) is shown in Fig. 2a. It can be seen that the sample prepared by the hydrothermal synthesis method had an irregular shape and larger particle diameter. Some studies have proved that nanostructures can make more ions flow through the interface, so as to accelerate the reaction of vanadium-based electrodes.20 Therefore, it was preliminarily confirmed that ionic liquid as a solvent had certain advantages over water as a solvent. In addition, the ionothermal method involved atmospheric conditions, which were more advantageous.
Optimization of Reaction Temperature
The morphology of the samples synthesized at different reaction temperatures (140°C, 150°C, 160°C, 170°C and 180°C, all for 72 h) was observed using SEM. The morphology of the sample obtained at 180°C in Fig. 3e shows that the product was mainly composed of ellipsoidal round particles with an uneven particle size. With decreasing temperature, the particle shape gradually became spherical. As shown in Fig. 3b, when the temperature was reduced to 150°C, uniform spherical particles with a particle size of 30–100 nm were obtained. When the reaction temperature was further decreased to 140°C, the morphology of the product was non-uniform large particles, as shown in Fig. 3a. Therefore, the optimal reaction temperature is 150°C.
Optimization of Reaction Time
In order to explore the effect of reaction time on the product, the pH of the solution and the reaction temperature were fixed at 9 and 150°C, respectively, and the reaction time was set to 36 h, 48 h, 60 h and 72 h, respectively. It can be seen from Fig. 4 that the effect of reaction time on particle size was not significant, but with increasing reaction time, the particle size seemed to be smaller and most products developed into flower ball-like structures with smooth surfaces and a uniform morphology. Therefore, 72 h was selected as the optimal reaction time.
For comparison, the sample synthesized through the hydrothermal method was also prepared under the optimized experimental conditions (i.e., pH 9 and 150°C for 72 h) and its SEM image is shown in Fig. 2b. Based on the previously reported literature,21,25,26 most of the hydrothermal synthesis operations were carried out in the range of 140–200°C, thus the optimized condition in this work is reasonable. It can be seen that the sample possessed an irregular shape and larger particle diameter, while the product synthesized in the ionic liquid had a regular morphology, smaller particle size and convex outer surface. These factors were not only beneficial for shortening the ion transport path and loading more active sites, but they also produced a larger active interface for lithium accumulation, resulting in a higher specific capacity.36 Simultaneously, the unique nanostructure of CVO-IL can be used as an “ion pool” between the electrode surface area and electrolyte to ensure ion and electron transfer.37 Therefore, it was again confirmed that the ionic liquid as a solvent had certain advantages over water as a solvent. In addition, it was dangerous to use an autoclave at high pressure for hydrothermal synthesis.
Sample Composition
The composition of the materials was studied using EDX and x-ray fluorescence spectroscopy (XRF). The EDX data for CVO-IL and CVO-W after calcination of the precursors synthesized under the optimized experimental conditions (i.e., pH 9 and 150°C for 72 h) are shown in Fig. 5. Figure 5a shows the EDX content analysis diagram of the CVO-IL sample. The contents of Co, V and O in the CVO-IL sample were 44.71 wt.%, 23.70 wt.% and 31.60 wt.%, respectively. The EDX contents of the CVO-W samples are shown in Fig. 5b. The contents of Co, V and O in the CVO-W samples determined by elemental analysis were 41.54 wt.%, 19.55 wt.% and 38.91 wt.%, respectively, which were slightly different from those of the CVO-IL sample. In addition, the XRF analysis for the CVO-IL sample was also carried out and the results were 43.5 wt.%, 25.0 wt.% and 31.5 wt.% for Co, V and O, respectively, consistent with the EDX results.
XRD Analysis
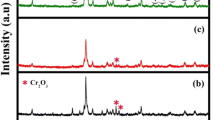
Figure 6 shows the XRD patterns of CVO-IL and CVO-W. For CVO-IL, the diffraction peaks at 15.38°, 18.72°, 27.14°, 29.52°, 31.02°, 35.26° and 43.5° corresponded to the (020), (021), (112), (200), (040), (221) and (240) crystal planes, respectively, of the Co3(VO4)2 phase (JCPDS PDF# 37-0352). In addition, the diffraction peaks at 50.96°, 53.6°, 57.96° and 63.26° corresponded to the (421), (422), (511) and (440) planes, respectively, of Co3V2O8 (JCPDS PDF# 16-0675-1). Thus, the main component of CVO-IL was concluded to be Co3V2O8 (the Co3(VO4)2 phase is also one of the crystalline forms for the Co3V2O8 component). For CVO-W, all diffraction peaks were consistent with the Co3V2O8 phase. Both CVO-IL and CVO-W possessed the Co3V2O8 phase, but there were some differences in the cell parameters, and the CVO-IL exhibited higher crystallinity than that of CVO-W, indicating the differences between the ionothermal and hydrothermal synthesis methods. High crystallinity will often lead to high hydrothermal stability,38 and the additional crystal planes (such as the (200) plane) exposed by the CVO-IL also have a positive effect on the catalytic activity.
TEM and HRTEM Analysis
In order to further determine the internal and crystal structures of the CVO-IL sample, a TEM and HRTEM analysis for the CVO-IL sample was carried out. It was difficult to determine the size of the primary nanoparticles because the images showed the aggregation of the primary nanoparticles. When the red rectangular area was enlarged, many small nanoparticles (such as the particles in the red circle) with a size of 5–10 nm were observed, which is consistent with the convex nanospheres on the surface of spherical aggregates in the above CVO-IL SEM images. The red rectangle was selected from Fig. 7a and the enlarged picture is given in Fig. 7b. The HRTEM images obtained from a nanosphere of CVO-IL (Fig. 7c) show clearly visible lattice fringes. The distances between them were 0.449 nm, 0.301 nm and 0.327 nm, which corresponded to the (221), (200) and (112) planes, respectively, indicating that Co3V2O8 was highly crystalline. Figure 7d shows the surface scanning analysis of CVO-IL (elemental mapping of Co, V and O), thereby proving the uniform distribution of Co, V and O elements among the particles.
XPS Analysis
The surface chemical information and valence of the CVO-IL sample were measured by XPS. Figure 8a shows the XPS full spectrum of CVO-IL. All the spectra were checked based on the aliphatic carbon at a binding energy of 284.6 eV. Four characteristic peaks were observed at 284.6 eV, 516.4 eV, 529.9 eV and 780.4 eV, representing the chemical characteristics of the C 1s, V 2p, O 1s and Co 2p orbitals, respectively. Figure 8b shows the fitting peak of the V 2p map of CVO-IL. Four peaks were obtained from the V 2p spectrum. Among them, the two peaks at 517.02 eV and 524.45 eV corresponded to V5+ 2p3/2 and V5+ 2p1/2, respectively.39,40 The other two peaks at 516.31 eV and 523.42 eV typically belonged to V4+.41 Figure 8c shows the fitting peak of the Co 2p map of CVO-IL. Two peaks were observed at 780.33 eV and 782.58 eV for Co 2p3/2, corresponding to Co2+ and Co3+, respectively. The peak at 786.79 eV was the satellite of Co 2p3/2. Similarly, the three peaks at 796.06 eV, 797.64 eV and 803.02 eV for the Co 2p1/2 level were attributed to Co2+, Co3+ and the satellite, respectively.42,43 The analysis data are summarized in Table I. The surface Co/V atomic ratio was higher than that for the Co3V2O8 as the main phase obtained from the XRD patterns and the surface Co/V mass contents ratio was also slightly higher than that obtained from the EDX and XRF results, indicating that there are some differences between the bulk and surface components of CVO-IL sample. For the valence state of elements, in addition to the Co2+ and V5+, there are a small amount of oxidized Co3+ and a large amount of reduced V4+ in the CVO-IL sample, probably due to the reduction of ammonium salt from [Bmim]Br and autoredox reaction between the Co2+ and V5+ species.
N2 Adsorption–Desorption Analysis
The nitrogen adsorption isotherms of the prepared CVO-IL and CVO-W are shown in Fig. 9 A third kind of isotherm with hysteresis loop can be observed when the relative pressure P/P0 is 0.85–1.0, indicating that the two samples have the characteristics of hierarchical porous structure.14,44 The specific surface areas of CVO-IL and CVO-W were analyzed by the Brunauer–Emmett–Teller (BET) method. The specific surface area of CVO-IL material is 25.2 m2 g−1 and that of CVO-W material is about 15.1 m2 g−1. It can be summarized that the specific surface product of CVO-IL synthesized by ionic liquid method is slightly higher than that of CVO-W synthesized hydrothermally. The superiority of the ionic liquid thermal synthesis method is further proved.
Electrochemical Performance
CVO-IL is a promising anode material because it has the advantages of internal voids and small particle sizes. Thus, the prepared material was used as an anode for LIBs and a button cell (CR2032) was assembled for electrochemical testing. Figure 10a shows the first four CV curves for the CVO-IL electrode in the potential window of 0.01–3.00 V (relative to Li+/Li) at a scan rate of 0.3 mV s−1. During the first discharge process, two sharp reduction peaks were observed at 0.64 V and 0.20 V. According to a previous study,45 the peak of 0.64 V was attributed to the destruction of a crystal structure of Co3V2O8, the appearance of CoO, the formation of Lix + yV2O5 and the partial appearance of a solid electrolyte interfacial (SEI) film. The strong cathode peak at 0.20 V corresponded to the conversion of CoO to metallic Co and the further reduction of LiX + yV2O5. These two reduction peaks disappeared after the first cycle, probably due to the irreversible phase transition, the formation of the SEI film and the decomposition of the electrolyte in the first cycle.45,46,54,55 During the first anodic cycle, an oxidation peak near 1.29 V was observed, corresponding to the oxidation of cobalt and the dissociation of lithium from LiX + yV2O5.45 In the discharge process after the first cycle, three reduction peaks at 1.26 V, 0.90 V and 0.54 V appeared, probably caused by Li+ intercalation into the CVO-IL. They almost overlapped from the second cycle to the subsequent cycle, indicating that the reversibility was good.
(a) CV curves of the initial four cycles scanned at 0.3 mV s−1 for CVO-IL electrode; (b) cycling capacities for CVO-IL and CVO-W electrodes at a current density of 300 mA g−1; (c) galvanostatic discharge and charge profiles between 0.01 V and 3.00 V at a current density of 300 mA g−1 for CVO-IL electrode; (d) rate performance at various current densities.
Figure 10b shows a performance comparison of CVO-IL and CVO-W during 300 charge-discharge cycles at 0.3 A g−1. The first discharge capacities of CVO-IL and CVO-W were 1528 mAh g−1 and 1217 mAh g−1, respectively, indicating the high discharge capacity for CoVO electrodes. However, in the first 30 cycles, the discharge capacity for both electrodes decreased sharply. After the ~ 50th cycle, the discharge capacity of the CVO-IL electrode increased gradually and was maintained at 600 mAh g−1 for 300 cycles. The phenomenon of capacity increase has also been observed for other transition-metal oxide composite anode materials,47,48 which might be due to the reversible formation of a polymer gel film from decomposition of the electrolyte49 and a possible activation process in the electrode.50,51 The discharge capacity of the CVO-W electrode still decreased gradually after the 50th cycle and only provided 120 mAh g−1 at the 300th cycle, which was much lower than that of the CVO-IL electrode. The Coulombic efficiencies of CVO-IL and CVO-W were 77.64% and 64.78%, respectively, in the first cycle and they gradually increased to ~ 98% after about five cycles, similar to previous studies,52,53 probably due to the formation of the SEI film and the decomposition of the electrolyte.
Figure 10c shows the five charge-discharge curves (i.e., 1st, 2nd, 100th, 200th and 300th) of CVO-IL at 0.3 A g−1. From the first charge-discharge curve, it can be seen that the initial charge-discharge capacities of CVO-L were 1186 mAh g−1 and 1528 mAh g−1, respectively, and the Coulombic efficiency was 77.64%. At the 100th cycle, the charge-discharge capacities of CVO-IL were 511 mAh g−1 and 527 mAh g−1, respectively, and the Coulombic efficiency was 96.9%. The capacity loss in the initial cycle was likely because of the irreversible phase transition, the formation of the SEI film and the decomposition of the electrolyte in the first cycle.45,46,54,55 The subsequent slight increase in capacity from the 100th to 300th cycle may due to the interaction between lithium ions in the electrolyte solution and CVO-IL from the surface to interior, the activation process in the electrode.56,57,58
Figure 10d shows the rate capability of the CVO-IL and CVO-W electrodes with increasing current (from 100 mA g−1 to 200 mA g−1, 500 mA g−1, 1000 mA g−1 and then back to 100 mA g−1). The rate performance of the CVO-IL electrode was much higher than that of the CVO-W electrode. In addition, the CVO-IL electrode delivered an outstanding capacity of 568 mA h g−1 when the current density was restored to 100 mA g−1, which was ascribed to its small hierarchical architecture and inner void structure. Based on a previous study,42 smaller hierarchical size and more space among loosely connected nanoparticles increased the contact between the electrolyte and electrode materials, thus providing more facile Li-ion migration.
In order to understand the source of the superior electrochemical performance of the CVO-IL electrode, CV tests at scan rates of 0.4, 0.5, 0.6, 0.7 and 0.8 mV/s were carried out at 0.01–3.00 V, as shown in Fig. 11a1 and a2. Based on the previous literature,59 the arithmetic square root of different scanning rates in the CV curves (Fig. 11a1 and a2) is extracted as abscissa and the peak current (Ip) of CV curves as ordinate, then the fitting diagrams (Fig. 11b1 and b2) can be obtained. There was a linear relationship between the peak current and the square root of the scanning rate at various peak potentials, indicating that the electrode reaction under different potential plateaus was controlled by the diffusion of Li ions in the electrolyte and both electrodes.26,59 Compared with the CVO-W electrode, CVO-IL nanoparticles had a smaller hierarchical size. The loose connection of the CVO-IL nanoparticles leads to more space between the particles, which increased the contact between the electrolyte and electrode materials and thereby provided more facile Li-ion migration. This may be one of the reasons why the rate capacity of the CVO-IL electrode was much larger than that of the CVO-W electrode.
In order to further understand the internal reason for the difference in the electrochemical performance between CVO-IL and CVO-W, AC impedance measurements for the two electrodes were carried out. As shown in Fig. 12a, CVO-IL and CVO-W showed similar Nyquist curves composed of semicircles in the high and medium frequency regions and oblique lines in the low frequency region. The charge transfer resistances of CVO-IL and CVO-W were 329.7 Ω and 669.3 Ω, respectively, and the electrolyte resistances of CVO-IL and CVO-W were 3.90 Ω and 4.82 Ω, respectively. The prepared CVO-IL electrode had lower Rs, Rct and Zw, which indicated that it was more easily activated by the lithium insertion/removal process. This was mainly because the regular smaller spherical nanoparticles shortened the ion transport path and increased the density of electroactive centers. The Li ion diffusion coefficient (DLi) was calculated according to Eqs. 1 and 2. As shown in Fig. 12b and c, the fitted CVO-IL parameter is 299.15, which is far lower than that of CVO-W (401.44). For the same active substance, DLi was proportional to the reciprocal of the square root of σw. Therefore, we can infer that the DLi for CVO-IL was much larger than that for CVO-W, indicating that the Li-ion diffusion between the two electrodes in the active material of CVO-IL was much faster.
Based on the above discussion, it can be concluded that the reasons for the excellent electrochemical performance of CVO-IL were as follows: (1) The small and uniform spherical morphology had more space, which promoted the diffusion of lithium ions between the electrolyte and electrode. (2) The more uniform and continuous structure was more easily adapted to the volume change caused by battery charging and discharging, and the electron transmission was smoother, thus reducing the resistance. (3) The smaller particle size was beneficial to increasing the contact surface area between the electrolyte and electrode and shortening the Li-ion diffusion length in order to obtain high rate and long-life battery capacity.
Conclusions
A series of cobalt vanadate nanoparticles were successfully prepared via an ionothermal synthesis method under atmospheric pressure in a [Bmim]Br solvent. The ionic liquid solvent, the pH of the reaction system and the reaction temperature each had a significant influence on the properties of the prepared cobalt vanadate materials. Small and uniform spherical nanoparticles with a particle diameter of 30–100 nm were synthesized at pH 9 and 150°C for 72 h in [Bmim]Br, while irregular large block cobalt vanadate materials were obtained through the traditional hydrothermal method in water under the same reaction conditions.
The typical CVO-IL sample exhibited excellent electrochemical performance as an anode material for Li-ion batteries. At a current density of 0.3 A g−1, the capacity of the CVO-IL anode was maintained at ~ 600 mAh g−1 after 300 cycles, while that of the cobalt vanadate material prepared by the hydrothermal method (CVO-W) was only 120 mAh g−1. The reasons for the excellent electrochemical performance of CVO-IL were also assessed in detail. It was found that the electrode reaction was controlled by the diffusion of Li ions in the electrolyte and both electrodes (CVO-IL and CVO-W). The Li-ion diffusion coefficient of CVO-IL was much higher than that of CVO-W, probably due to its small and uniform spherical structure. Thus, cobalt vanadates synthesized in ionic liquids under mild reaction conditions with high electrochemical performance can be used as potential electrode materials for lithium-ion batteries and other energy storage systems.
References
H. Chai, Y.C. Wang, Y.C. Fang, Y. Lv, H. Dong, D.Z. Jia, and W.Y. Zhou, Low-cost synthesis of hierarchical Co3V2O8 microspheres as high-performance anode materials for lithium-ion batteries. Chem. Eng. J. 326, 587 (2017).
Y.S. Ji, D. Fang, C. Wang, Z. Zhou, Z.P. Luo, J. Huang, and J.H. Yi, Cobalt-doped V2O5 nanowire arrays on Ti foil for enhanced lithium-ion storage. J. Mater. Chem. A 742, 567 (2018).
Q.F. Li, X.H. Rui, D. Chen, Y.Z. Feng, N. Xiao, L.Y. Gan, Q. Zhang, Y. Yu, and S.M. Huang, A high-capacity ammonium vanadate cathode for zinc-ion battery. Nano-Micro Lett. 12, 157 (2020).
H.L. Chen, D. Yang, X.Y. Zhuang, D. Chen, W.L. Liu, Q. Zhang, H.H. Hng, X.H. Rui, Q.Y. Yan, and S.M. Huang, Superior wide-temperature lithium storage in a porous cobalt vanadate. Nano Res. 13, 1867 (2020).
B. Sambandam, V. Soundharrajan, V. Mathew, J.J. Song, S. Kim, J. Jo, D.P. Tung, S. Kim, and J. Kim, Metal–organic framework-combustion: a new, cost-effective and one-pot technique to produce a porous Co3V2O8 microsphere anode for high energy lithium ion batteries. J. Mater. Chem. A 4, 14605 (2016).
V. Soundharrajan, B. Sambandam, J.J. Song, S. Kim, J. Jo, D.P. Dung, S. Kim, V. Mathew, and J. Kim, Facile green synthesis of a Co3V2O8 nanoparticle electrode for high energy lithium-ion battery applications. J. Colloid Interface Sci. 501, 133 (2017).
H. Hu, L. Yu, X.H. Gao, Z. Lin, and X.W. Lou, Hierarchical tubular structures constructed from ultrathin TiO2(B) nanosheets for highly reversible lithium storage. Energy Environ. Sci. 8, 1480 (2015).
J. Chen, N. Zhao, Y.G. Zhao, J. Hu, Y.N. Wang, Y.J. Wu, and Z.Y. Zhang, Morphology-controlled synthesis of the porous Co3O4 with rugby-shaped and spherical structures and theirs electrochemical properties as negative materials for Li-ion batteries. J. Alloys Compd. 844, 156058 (2020).
B. Wu, Y. Xie, Y.Q. Meng, C. Qian, Y.Y. Chen, A.H. Yuan, X.M. Guo, H.X. Yang, S.J. Wan, and S.L. Lin, Construction of unique heterogeneous cobalt–manganese oxide porous microspheres for the assembly of long-cycle and high-rate lithium ion battery anodes. J. Mater. Chem. A 7, 6149 (2019).
C.L. Liu, S.H. Luo, H.B. Huang, Z.Y. Wang, Q. Wang, Y.H. Zhang, Y.G. Liu, Y.C. Zhai, and Z.W. Wang, Potassium vanadate K0.23V2O5 as anode materials for lithium-ion and potassium-ion batteries. J. Power Sources 389, 77 (2018).
P. Prahasini, R. Subadevi, F.M. Wang, W.R. Liu, and M. Sivakumar, A brannerite type cobalt vanadate conversion anode for lithium batteries. Ionics 22, 347 (2016).
P. Guan, W. Zhang, C.Y. Li, N. Han, X.C. Wang, Q.F. Li, G.J. Song, Z. Peng, J.J. Li, L. Zhang, and X.Y. Zhu, Low-cost urchin-like silicon-based anode with superior conductivity for lithium storage applications. J. Colloid Interface Sci. 575, 150 (2020).
Y. Yan, B. Li, W. Guo, H. Pang, and H.G. Xue, Vanadium based materials as electrode materials for high performance supercapacitors. J. Power Sources 329, 148 (2016).
Z.G. Yin, J.W. Qin, W. Wang, and M.H. Cao, Rationally designed hollow precursor-derived Zn3V2O8 nanocages as a high-performance anode material for lithium-ion batteries. Nano Energy 31, 367 (2017).
H. Kim, N. Venugopal, J. Yoon, and W.S. Yoon, A facile and surfactant-free synthesis of porous hollow λ-MnO2 3D nanoarchitectures for lithium ion batteries with superior performance. J. Alloys Compd. 778, 37 (2019).
T.R. Hendricks, J. Lu, L.T. Drzal, and I. Lee, Intact pattern transfer of conductive exfoliated graphite nanoplatelet composite films to polyelectrolyte multilayer platforms. Adv. Mater. 20, 2008 (2008).
Y.R. Lim, Y. Ko, J. Park, W.I. Cho, S.A. Lim, and E.H. Cha, Morphology-controlled WO3 and WS2 nanocrystals for improved cycling performance of lithium-ion batteries. J. Electrochem. Sci. Technol. 10, 89 (2019).
X.H. Rui, X.H. Zhang, S.T. Xu, H.T. Tan, Y. Jiang, L.Y. Can, Y.Z. Feng, C.C. Li, and Y. Yu, A low-temperature sodium-ion full battery: superb kinetics and cycling stability. Adv. Funct. Mater. 31, 2009458 (2021).
J.C. Zhang, B.Q. Yuan, S.F. Cui, N.N. Zhang, J.J. Wei, X. Wang, D.J. Zhang, R.C. Zhang, and Q.S. Huo, Facile synthesis of 3D porous Co3V2O8 nanoroses and 2D NiCo2V2O8 nanoplates for high performance supercapacitors and their electrocatalytic oxygen evolution reaction properties. Dalton Trans. 46, 3295 (2017).
S.P. Zhang, H.T. Tan, X.H. Rui, and Y. Yu, Vanadium-based materials: next generation electrodes powering the battery revolution? Acc. Chem. Res. 53, 1660 (2020).
F. Gong, D.W. Xia, Q. Zhou, J.X. Liao, and M.Q. Wu, Novel spherical cobalt/nickel mixed-vanadates as high-capacity anodes in lithium ion batteries. J. Alloys Compd. 766, 442 (2018).
M. Gyanprakash, P.K. Gupta, G.P. Sharma, and R.G.S. Pala, Surface-enhanced OER activity in Co3V2O8 using cyclic charge-discharge to balance electrocatalytic active site generation and degradation. Electrochim. Acta 367, 137538 (2021).
D.R. Deng, Y.J. Zhang, G. Li, X.Y. Wang, L.H. Gan, L. Jiang, and C.R. Wang, 2 D manganese vanadate nanoflakes as high-performance anode for lithium-ion batteries. Chem. Asian J. 9, 1265 (2014).
Z. Zulkifli, B. Sambandam, S. Lslam, S. Kim, and J. Kim, A green route for preparation of nickel-vanadate-nanoparticles as negative electrode for rechargeable lithium-ion batteries. ECS Meet. Abstr. 2, 3430 (2020).
F. Gong, D.W. Xia, C. Bi, J. Yang, W. Zeng, C. Chen, Y.L. Ding, Z.Q. Xu, J.X. Liao, and M.Q. Wu, Systematic comparison of hollow and solid Co3V2O8 micro-pencils as advanced anode materials for lithium ion batteries. Electrochim. Acta 264, 358 (2018).
Q. Zhang, J. Pei, G. Chen, C.F. Bie, D.H. Chen, Y. Jiao, and J.C. Rao, Co3V2O8 hexagonal pyramid with tunable inner structure as high performance anode materials for lithium ion battery. Electrochim. Acta 238, 227 (2017).
J.S. Park, J.H. Kim, and Y.C. Kang, Synthesis of carbonaceous/carbon-free nanofibers consisted of Co3V2O8 nanocrystals for lithium-ion battery anode with ultralong cycle life. Electrochim. Acta 313, 48 (2019).
H. Sun, Y.Y. Li, H. Chai, Y.L. Cao, and W.Y. Zhou, Facile synthesis of cobalt doped nickel vanadate microspheres as battery-type electrode material for hybrid supercapacitor. J. Alloys Compd. 805, 388 (2019).
M.C. Liu, L.B. Kong, L. Kang, X.H. Li, F.C. Walsh, M. Xing, C. Lu, X.J. Ma, and Y.C. Luo, Synthesis and characterization of M3V2O8 (M = Ni or Co) based nanostructures: a new family of high performance pseudocapacitive materials. J. Mater. Chem. A 2, 4919 (2014).
F.F. Wu, S.L. Xiong, Y.T. Qian, and S.H. Yu, Hydrothermal synthesis of unique hollow hexagonal prismatic pencils of Co3V2O8⋅n H2O: a new anode material for lithium-ion batteries. Angew. Chem. Int. Ed. 54, 10787 (2015).
S.X. Liu, S. Sarwar, H.P. Zhang, Q.P. Guo, J.J. Luo, and X.Y. Zhang, One-step microwave-controlled synthesis of CoV2O6·2H2O nanosheet for super long cycle-life battery-type supercapacitor. Electrochim. Acta 364, 137320 (2020).
G.M. Wang, M. Valldor, K.V. Dorn, M. Wlik-Kozubek, V. Smetana, and A.V. Mudring, Ionothermal synthesis enables access to 3D open framework manganese phosphates containing extra-large 18-ring channels. Chem. Mater. 31, 7329 (2019).
E.R. Cooper, C.D. Andrews, P.S. Wheatley, P.B. Webb, P. Wormald, and R.E. Morris, Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 430, 1012 (2004).
R.N. Li, Y.S. Meng, Q.R. Hu, F.L. Zhu, and Y. Zhang, Ionic liquid-derivatized hierarchical N-doped carbon-coated Co3O4 nanosheet array as high-performance anode materials for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 31, 4997 (2020).
E.R. Parnham and R.E. Morris, 1-Alkyl-3-methyl imidazolium bromide ionic liquids in the ionothermal synthesis of aluminium phosphate molecular sieves. J. Mater. Chem. 18, 4882 (2006).
J. Yoon, W. Choi, H. Kim, Y.S. Choi, J.M. Kim, and W.S. Yoon, The effects of nanostructures on lithium storage behavior in Mn2O3 anodes for next-generation lithium-ion batteries. J. Power Sources 493, 229682 (2021).
W.B. Zhang, L.B. Kong, X.J. Ma, Y.C. Luo, and L. Kang, Design, synthesis and evaluation of three-dimensional Co3O4/Co3(VO4)2 hybrid nanorods on nickel foam as self-supported electrodes for asymmetric supercapacitors. J. Power Sources 269, 61 (2014).
G.Z. Fang, J. Zhou, Y. Hu, X.X. Cao, Y. Tang, and S.Q. Liang, Facile synthesis of potassium vanadate cathode material with superior cycling stability for lithium ion batteries. J. Power Sources 275, 694 (2015).
X.F. Chu, H. Wang, Y.D. Chi, C. Wang, L. Lei, W.T. Zhang, and X.T. Yang, Hard-template-engaged formation of Co2V2O7 hollow prisms for lithium ion batteries. RSC Adv. 8, 2072 (2018).
A.K. Shah, T.K. Sahu, D. Gogoi, N.R. Peela, and M. Qureshi, Surface-engineering of decahedron shaped bismuth vanadate for improved photoelectrochemical water oxidation by indium doping coupled with graphitic carbon nitride quantum dots. J. Power Sources 477, 229024 (2020).
L.W. Dong, R.G. Xu, P.P. Wang, S.C. Sun, Y. Li, L. Zhen, and C.Y. Xu, Layered potassium vanadate K2V6O16 nanowires: a stable and high capacity cathode material for calcium-ion batteries. J. Power Sources 479, 228793 (2020).
M. Xing, L.B. Kong, M.C. Liu, L.Y. Liu, L. Kang, and Y.C. Lou, Cobalt vanadate as highly active, stable, noble metal-free oxygen evolution electrocatalyst. J. Mater. Chem. A 2, 18435 (2014).
Y.K. Zhao, Y.D. Liu, X.Q. Du, R.X. Han, and Y. Ding, Hexagonal assembly of Co3V2O8 nanoparticles acting as an efficient catalyst for visible light-driven water oxidation. J. Mater. Chem. A 2, 19308 (2014).
C. Choi, K.J. Hwang, Y.J. Kim, G. Kim, J.Y. Park, and S. Jin, Rice-straw-derived hybrid TiO2-SiO2 structures with enhanced photocatalytic properties for removal of hazardous dye in aqueous solutions. Nano Energy 20, 76 (2016).
G.Z. Yang, H. Cui, G.W. Yang, and C.X. Wang, Self-assembly of Co3V2O8 multilayered nanosheets: controllable synthesis, excellent Li-storage properties, and investigation of electrochemical mechanism. ACS Nano 8, 4474 (2014).
C.X. Peng, B.D. Chen, Y. Qin, S.H. Yang, C.Z. Li, Y.H. Zou, S.Y. Liu, and J.H. Yang, Facile ultrasonic synthesis of CoO quantum dot/graphene nanosheet composites with high lithium storage capacity. ACS Nano 6, 1074 (2012).
T. Morishita, K. Nomura, T. Inamasu, and M. Inagaki, Synthesis of anhydrous manganese vanadate powder by coprecipitation and its anodic performance for lithium secondary battery. Solid State Ionics 176, 2235 (2005).
X.H. Wang, Z.B. Yang, X.L. Sun, X.W. Li, D.S. Wang, P. Wang, and D.Y. He, NiO nanocone array electrode with high capacity and rate capability for Li-ion batteries. J. Mater. Chem. 21, 9988 (2011).
S. Grugeon, S. Laruelle, L. Dupont, and J.M. Tarascon, An update on the reactivity of nanoparticles Co-based compounds towards Li. Solid State Sci. 5, 895 (2003).
S.B. Ni, X.H. Lv, J.J. Ma, X.L. Yang, and L.L. Zhang, A novel electrochemical reconstruction in nickel oxide nanowalls on Ni foam and the fine electrochemical performance as anode for lithium-ion batteries. J. Power Sources 270, 564 (2014).
S.B. Ni, X.H. Lv, T. Li, X.L. Yang, L.L. Zhang, and Y. Ren, A novel electrochemical activation effect induced morphology variation from massif-like CuxO to forest-like Cu2O nanostructure and the excellent electrochemical performance as anode for Li-ion battery. Electrochim. Acta 96, 253 (2013).
S.Y. Zhang, H.L. Yun, and H.W. Yu, Fabrication of Co3V2O7(OH)2·2H2O hexagonal microplatelets and its application in lithium-ion batteries. J. Alloys Compd. 735, 700 (2018).
D. Zhang, S.B. Xi, G.S. Li, B.Y. Li, J.M. Fan, X.Q. Liu, D.D. Chen, and L.P. Li, Facile synthesis of Mn2.1V0.9O4/rGO: a novel high-rate anode material for lithium-ion batteries. J. Power Sources 426, 197 (2019).
S.B. Ni, J.J. Ma, J.C. Zhang, X.L. Yang, and L.L. Zhang, Electrochemical performance of cobalt vanadium oxide/natural graphite as anode for lithium-ion batteries. J. Power Sources 282, 65 (2015).
C.N. He, S. Wu, N.Q. Zhao, C.S. Shi, E.Z. Liu, and J.J. Li, Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium-ion battery anode material. ACS Nano 5, 4459 (2013).
C.D. Lv, J.X. Sun, G. Chen, C.S. Yan, and D.H. Chen, Achieving Ni3V2O8 amorphous wire encapsulated in crystalline tube nanostructure as anode materials for lithium-ion batteries. Nano Energy 33, 138 (2017).
L.H. Gan, D.R. Deng, Y.J. Zhang, G. Li, X.Y. Wang, L. Jiang, and C.R. Wang, Zn3V2O8 hexagon nanosheets: a high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2, 2461 (2014).
V. Soundharrajan, B. Sambandam, J.J. Song, S. Kim, J. Jo, S. Kim, S. Lee, V. Mathew, and J. Kim, Co3V2O8 sponge network morphology derived from metal–organic framework as an excellent lithium storage anode material. ACS Appl. Mater. Interfaces 8, 8546 (2016).
J. Lin, H. Peng, J. Kim, B.R. Wygant, M.L. Meyerson, R. Rodriguez, Y. Liu, K. Kawashima, D.D. Gu, D.L. Peng, H. Guo, A. Heller, and C.B. Mullins, Lithium fluoride coated silicon nanocolumns as anodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 12, 18465 (2020).
Acknowledgments
Financial support from NSFC (21763016) and the University Industry Support Program for University in Gansu Province (2020C-06) and the Youth Natural Science Foundation of Gansu Province (20JR10RA189) are acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Guan, R., Hou, Z. et al. Ionothermal Synthesis of Cobalt Vanadate Nanoparticles As High-Performance Anode Materials for Lithium-Ion Batteries. J. Electron. Mater. 51, 3260–3275 (2022). https://doi.org/10.1007/s11664-022-09586-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09586-8