Abstract
(Nd, Al) co-doped ZnO was prepared via a simple sol-gel method with 1% Al and different mole fractions of Nd (0, 0.5, 1, 1.5, 2%). The microstructure, optical properties and electrical conductivity of (Nd, Al) co-doped ZnO were studied by x-Ray diffraction (XRD), field emission scanning electron microscopy (FESEM), ultraviolet-visible spectroscopy (UV-vis), photoluminescence (PL) and DC resistivity measurement. According to the XRD spectrum, the sample has a hexagonal wurtzite structure and no Nd phase. The addition of Nd inhibits the crystal growth along the c-axis and reduces the crystallite size from 24 nm to 16 nm. From the analysis of the UV-vis spectrum, it can be seen that the doping of Nd enhances the visible light absorption of the sample and reduces the band gap from 3.09 eV to 2.99 eV. The prepared samples have a negative resistance temperature coefficient (NTC). The addition of Nd hinders the electron conduction and increase the DC resistivity of samples at a certain temperature. According to the Arrhenius equation, the activation energy of (Nd, Al) co-doped ZnO increases from 0.21 eV to 0.27 eV with the addition of Nd.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ZnO is an important n-type semiconductor material1. It has been widely studied and applied because of its wide band gap, large exciton binding energy, and high electron Hall mobility.2,3 Doping can change the physical and chemical properties of semiconductor materials to produce new materials with excellent properties.4 Some literature studies have confirmed that doping an adequate amount of Cu, Al, Ni and Nd into ZnO nanocrystals can change the optical, magnetic and electrical properties.5 (Nd, Al) co-doped ZnO has been studied by many scholars because of its low cost, good thermal stability, high optical transparency and low electrical resistivity.6 Huang et al.7 prepared rod-like Al-doped ZnO by hydrothermal synthesis and obtained a minimum resistivity of 3.959×104 Ω cm when the doping ratio of Al was 2%. Hartner et al.8 found that the resistivity of ZnO doped with Al calcined in hydrogen atmosphere (1.9×102 Ω cm) is several orders of magnitude smaller than that calcined in air atmosphere (7.8×105 Ω cm). Common doping methods include vapor deposition,9 molecular beam epitaxy,10 sol-gel synthesis,11 and solvothermal processing. The sol-gel synthesis method was selected in this experiment because of its low cost, simple process and large number of nanoparticles.12 Rare earth elements possess a 4f layer electronic structure and other properties, so they are widely used in doping ZnO.13 Among rare earth elements, Nd is one of the best elements for doping because of its unique optical and magnetic properties. The ionic radius of Nd3+ (98 pm) is bigger than that of Zn2+ (74 pm). As long as the crystal structure changes slightly, Nd ions can replace Zn ions easily. Therefore, Nd and Al were chosen as co-doping elements in this paper. The known reports on (Nd, Al) co-doped ZnO mainly focus on its catalytic, optical, and magnetic effects. However, the effect of (Nd, Al) co-doping on the electrical properties of ZnO also has important research value. In particular, its magnetic properties may cause special changes in the electrical properties of ZnO. Therefore, the (Nd, Al) co-doped ZnO samples were prepared by sol-gel method, and the structure and electrical properties were investigated. In terms of electrical properties, we measured the DC resistivity and calculated the activation energy. In the research of ZnO composites, the ZnO-based ceramic is a very important application. In the ZnO-based varistor ceramics and ZnO linear ceramics, the trend of changing activation energy can roughly reflect the change of nonlinear system, in which the nonlinear coefficient can directly indicate the performance of ceramics. Therefore, the calculation of activation energy is very meaningful for the subsequent application of ZnO-based ceramics.
Experimental Procedure
Synthesis of (Nd, Al) Co-Doped ZnO
All the chemical agents used in the experiment were analytically pure and used as received. Sol-gel synthesis was the preferred preparation method for (Nd, Al) co-doped ZnO using Al(NO3)3·9H2O, Zn(NO3)2·6H2O, Nd(NO3)3·6H2O, and triethanolamine (TEA). A 10.395-g amount of Zn(NO3)2·6H2O and 0.1313 g of Al(NO3)3·9H2O were dissolved in 100 ml deionized water (Zn:Al=100:1), and the mixed solution was stirred intensely for 30 min at room temperature. Nd(NO3)3·6H2O (with different mole fractions) was put into the mixed solution and stirred intensely for 30 min. TEA of an equal mole to total metals was dropped into the mixed solution and stirred for 1 h in a water bath at 70°C. After that, the sample was died at 170°C until a dry gel was obtained. The sample was calcined at 700°C for 3 h with a heating rate of 5°C min-1 to get (Nd, Al) co-doped ZnO (Nd/Zn = 0, 0.5%, 1%, 1.5%, 2%) nanoparticle powder. In addition, the xerogel powder was milled with 2 wt% polyvinyl alcohol (PVA) as a binder for 1 h. After milling, the slurry was dried at 110°C for 2 h. The as-dried powder was then uniaxially pressed into discs of 10-mm diameter and 3-mm thickness using a universal hydraulic presser. It was calcined for 3 h under the same conditions for subsequent electrical property tests. Different samples are represented by NAZ-x (x = 0, 0.5, 1, 1.5, 2%) according to the Nd doping concentration.
Characterization
The morphology of the powder sample was analyzed by using SEM (JEOL JSM-7500F microscope). The crystalline phases of (Nd, Al) co-doped ZnO were analyzed by x-ray powder diffraction (XRD) using a Rigaku D/max-2550 diffractometer (operated at 40 kV per 200 mA) with Cu Kα radiation (λ = 1.5406 Å). The optical properties of the sample were studied by emission spectroscopy (Perkin Elmer LS-55 fluorescence spectrometer) and UV-vis spectroscopy (Hitachi U-3900 UV-vis spectrophotometer). The change of DC resistivity with temperature was measured on an LCR meter (Applent AT821).
Results and Discussion
Morphology and Structure
The SEM pictures of NAZ-x (x = 0-2%) are shown in Fig. 1a-e. With the increase of Nd doping concentration, the morphology of ZnO grains has no obvious change. It can be observed from the figure that the (Nd, Al) co-doped ZnO sample is composed of irregular small particles with an average diameter around 40 nm. Figure 1f shows the EDS analysis spectrum of the NAZ-2% sample at point 01. EDS analysis indicates the co-existence of Zn, Al, Nd, and O, which further confirms the formation of NAZ-x powders.
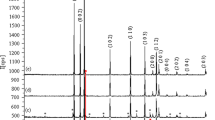
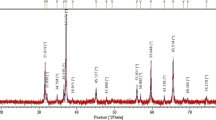
Figure 2 shows the XRD result of (Nd, Al) co-doped ZnO powder. All samples conform to a hexagonal wurtzite structure of ZnO (JCPDS. No: 36-1451, space group: p63mc). The XRD patterns of (Nd, Al) co-doped ZnO show nine diffraction peaks corresponding to (100), (110), (101), (200), (102), (103), (112), (002) and (201) crystal planes of ZnO hexagonal structure, respectively. There is no second phase in the results, which indicates that the doped Nd3+ successfully enters the ZnO lattice, and the Nd3+ is substituted at the Zn site. It can be observed from the picture that with the increase of Nd molar ratio, the diffraction peak intensity decreases obviously and the crystallinity becomes worse. This may be caused by the lattice distortion of ZnO doped with an Nd ion.14
The average crystallite size of the (Nd, Al) co-doped ZnO sample was calculated by using Scherrer’s formula:
where β is full-width at half maximum of a distinctive peak (rad), θ is the Bragg angle of diffraction and λ is the x-ray wavelength (1.5406 Å for Cu Kα).
The lattice parameters (a, c) of the (Nd, Al) co-doped ZnO samples were calculated using Bragg's equation:
where n is the diffraction order, λ is the x-ray wavelength, d is the spacing between crystal planes, θ is the angle between the incident x-ray and the corresponding crystal plane.
The lattice parameters of (Nd, Al) co-doped ZnO are listed in Table I. The value of c decreases with the increase of Nd mole fraction because the growth of ZnO lattice along the c-axis is inhibited by Nd ions. In addition, the peak width of the (101) crystal plane increases gradually, which indicates that the crystallite size of ZnO decreases.15 The average crystallite size decreases with the increase of Nd mole fraction. When the Nd doping ratio is 1.5%, the average crystallite size reaches the minimum value of 14.97 nm. When the mole fraction of Nd ions is 2%, the c-axis and unit cell volume increase, which may be caused by the excessive doping of Nd ions. The reason for the decrease of crystallite size may be due to the larger radius of the Nd ion, which also proves that Nd was successfully doped into ZnO.
Optical Characterization
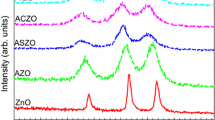
The UV-vis spectrum of samples is shown in Fig. 3a. First, it can be observed from the figure that all five samples have an intense absorption initiation at ~380 nm, which matches the 1Sh→1Se exciton transition of ZnO and is similar to the absorption initiation of Eu3+ -doped ZnO.16 With the addition of Nd, a weak absorption peak appears at 585 nm and 746 nm, respectively, which is related to the unique near-infrared optical performance of Nd. Then the absorption band edge of the (Nd, Al) co-doped ZnO is about 400 nm. With the increase of Nd mole fraction, the absorption band edge shows a redshift which means the absorption range of visible light increases. The band gap width of (Nd, Al) co-doped ZnO is found by this formula:
where α is optical absorption coefficient, A is a constant, hν is the photon energy and Eg is the optical band gap. We drew the relation curve between (α hν)1/2 and hν and then extrapolatd the linear portion of the absorption edge. The band gap of (Nd, Al) co-doped ZnO can be obtained by counting the intercept with the energy axis.
It is observed from Fig. 3b that the band gap width of (Nd, Al) co-doped ZnO is 3.09 eV, which gradually decreases to 2.99 eV with the increase of Nd mole fraction. The decrease of band gap width corresponds to the change of visible light absorption range. These results can be attributed to the surface plasma resonance of Nd,17 which further confirms that Nd has been successfully doped into (Nd, Al) co-doped ZnO. However, when the mole fraction of Nd exceeds 1.5%, the band gap width increases again which could be related to the intense interaction between ZnO and Nd surface oxide.18
The PL spectra of (Nd, Al) co-doped ZnO are shown in Fig. 4. There are ultraviolet excitation peaks, ultraviolet emission peaks and red emission peaks at 335 nm, 375 nm and 672 nm, respectively. The UV excitation and emission peaks of ZnO with a band gap of 3.37 eV are located at 325 nm and 369 nm. The position of the two peaks in the spectrum show a redshift, which is caused by the decrease of the band gap of ZnO after (Nd, Al) co-doping. When the Nd mole fraction is 1.5%, the UV excitation intensity of (Nd, Al) co-doped ZnO is obviously enhanced. Since the UV excitation is caused by exciton recombination, the intensity of UV excitation is bound up with the concentration of excitons in (Nd, Al) co-doped ZnO.19 Nd doping improves the crystal quality and free exciton concentration of the (Nd, Al) co-doped ZnO, resulting in the increase of electron-hole recombination rate. In addition, it was found that when the Nd doping concentration is 1.5%, the ratio of the UV excitation intensity of the (Nd, Al) co-doped ZnO to the visible light emission intensity is the largest, which means 1.5% Nd doping reduced the defects in the (Nd, Al) co-doped ZnO.18 However, the intensity of UV emission weakens when the Nd mole fraction is greater than 1.5%. The reason may be that excessive Nd doping reduces the crystal quality of (Nd, Al) co-doped ZnO, resulting in the decrease of free exciton concentration and electron-hole recombination rate.
DC Resistivity
The relationship between DC resistivity and temperature of (Nd, Al) co-doped ZnO has been analysed and is shown in Fig. 5. The DC resistivity decreases with the increase of temperature and shows a typical NTC effect. The increase in temperature of the semiconductor material provides enough energy for the electrons trapped in the atom to pull out the VB. This triggers the change of effective density of electrons and holes, which improves the conductivity.20
The DC resistivity of (Nd, Al) co-doped ZnO increases with the increase of Nd mole fraction at a certain temperature. which can be explained by the larger concentration of Nd ion intensifying the defects in the crystal materials. These defects cause electron scattering and hinder the electron conduction process, thus reducing the conductivity.21 In addition, the decrease of crystallite size results in the increase of crystallite boundary in the (Nd, Al) co-doped ZnO. The electron trapping near the grain boundary hinders the motion of electron and reduces the conductivity of the sample.22
The relationship between the change of DC resistivity and temperature is found by the Arrhenius formula:
where ρ is the DC resistivity, ρ0 is a constant with the dimensions of resistivity, Ea is activation energy for the material and KB is Boltzmann's constant.
Figure 6 shows the relationship between ln (ρ) and 1000/T for (Nd, Al) co-doped ZnO. The activation energy of the (Nd, Al) co-doped ZnO was found according to the slope of the fitting line and the Arrhenius formula. Ea of samples showed an overall increasing trend with the increase of Nd mole fraction, which was 0.21 eV, 0.25 eV, 0.26 eV, 0.27 eV and 0.22 eV, respectively. When the mole fraction of Nd increases from 0 to 1.5%, Ea of the sample increases from 0.21 eV to 0.27 eV. The main reason may be that the defects produced by dopants hinder ion transport because the Ea is closely related to ion mobility.20 When the Nd doping concentration increases from 1.5% to 2%, the activation energy of the sample decreases from 0.27 eV to 0.22 eV. The decrease of Ea of the NAZ-2% may be related to the increase of gap width and the improvement of electron mobility.
Conclusion
The structure, optical and electrical conductivity of (Nd, Al) co-doped ZnO powders synthesized by sol-gel method were systematically studied. The doping of Nd ions into the ZnO lattice induces lattice distortion and suppresses the growth along the c-axis direction so that the average crystallite size is reduced from 30 nm to about 20 nm. The increase of the Nd mole fraction widens the visible light absorption range of the (Nd, Al) co-doped ZnO and the band gap width also reduces from 3.085 eV to 2.994 eV. The DC resistivity of the sample decreases with increasing temperature and shows the NTC effect. With the increase of the concentration of Nd ions, the resistivity of the samples at a certain temperature increases. The doping of Nd produces defects, reduces the ion migration ability and increases the activation energy.
References
A. Henni, A. Merrouche, and L. Telli, J. Electroanal. Chem. 763, 149 (2016).
I. Khan, S. Khan, and R. Nongjai, Opt. Mater. 35, 1189 (2013).
S.J. Pearton, D.P. Norton, and K. Ip, J. Vac. Sci. Technol. B 22, 932 (2004).
L. Duan, X. Zhao, and Z. Zheng, J. Phys. Chem. Solids 76, 88 (2015).
F.Z. Nouasria, D. Selloum, A. Henni, D. Zerrouki, and S. Tingry, Ceram. Int. 47, 11 (2021).
N. Neves, R. Barros, and E. Antunes, J. Eur. Ceram. Soc. 32, 4381 (2012).
S. Huang, Q. Xiao, and H. Zhou, J. Alloy. Compd. 486, L24 (2009).
S. Hartner, M. Ali, and C. Schulz, Nanotechnology 20, 445701 (2009).
A.S.H. Hussein, Z. Hassan, and S.M. Thahab, Appl. Surf. Sci. 257, 4159 (2011).
B.K. Sonawane, M.P. Bhole, and D.S. Patil, Mater. Sci. Semicond. Process. 12, 212 (2009).
K. Tang, S. Gu, S. Li et al., Influence of thermally diffused aluminum atoms from sapphire substrate on the properties of ZnO epilayers grown by metal-organic chemical vapor deposition. J. Vac. Sci. Technol. A 29, 03A106 (2011).
A.A. Manoharan, R. Chandramohan, and K.D.A. Kumar, Transition metal (Mn) and rare earth (Nd) di-doped novel ZnO nanoparticles: a facile sol–gel synthesis and characterization. J. Mater. Sci. Mater. Electr. 29, 13077 (2018).
A.G. El Hachimi, H. Zaari, and M. Hamedoun, Experimental and theoretical investigation of Nd doped ZnO. J. Magn. Magn. Mater. S0304–8853, 33271–33281 (2017).
J. Chen, D. Chen, and J. He, Appl. Surf. Sci. 255, 9413 (2009).
P. Bindu, and S. Thomas, Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J Theor Appl Phys 8, 123 (2014).
Y. Liu, W. Luo, and R. Li, J. Nanosci. Nanotechnol. 10, 1871 (2010).
H. Damm, P. Adriaensens, and C. De Dobbelaere, Chem. Mater. 26, 5839 (2014).
F. Xian, and X. Li, Opt. Laser Technol. 45, 508 (2013).
W.E. Mahmoud, J. Cryst. Growth 312, 3075 (2010).
N. Ahmad, and S. Khan, J. Alloy. Compd. 720, 502 (2017).
S. Liu, J. Liu, and W. Jiang, Appl. Phys. A 122, 878 (2016).
F. Chen, X. Li, and J. Wu, Scripta Mater. 68, 297 (2013).
Funding
This research was partially supported by a grant from Changzhou University with KYCX20-2566 and Postgraduate Research & Practice Innovation Program of Jiangsu Province with SJCX21-1235.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, QG., Shi, Y., Chen, Y. et al. Investigation of the Effect of (Nd, Al) Co-doping on the Microstructure and Electrical Conductivity of ZnO. J. Electron. Mater. 51, 1505–1511 (2022). https://doi.org/10.1007/s11664-021-09418-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-021-09418-1