Abstract
The present study reports on the synthesis of Fe-doped tungsten trioxide (WO3:Fe) thin films using spray-pyrolysis technique. The films were deposited on glass substrates at 350°C and Fe-doping was achieved using granular iron in an acidic medium with \(\frac{{\left[ {{\text{Fe}}} \right]}}{\left[ W \right]}\) = 1, 3 and 5 % at. The films were heat-treated with an extended thermal treatment at 400°C and 500°C for 4 h, in order to allow both crystallization and dopant diffusion into the WO3 matrix. The effect of Fe-doping and heat-treatment on the morphological, structural and optical properties of these films was investigated. The results revealed that Fe-doping has a significant impact on the morphology of the films depending on its concentration. In addition, it was shown that the thermal treatment improves the growth of the crystallites and enhances the roughness of the surface. Grazing incidence X-ray diffraction analysis (GIXRD) confirmed the polycrystalline character of the films with a monoclinic structure (ICDD N° 89-4476, P21/c). The structural parameters were found to be both doping- and heat-treatment-dependent. Energy dispersive spectrometry (EDS) depicted homogeneous doping and confirmed the presence of W, O and Fe. The films display a good optical transmittance over the visible region sensitive to Fe-doping and thermal treatment. Moreover, the films exhibit both direct and indirect electronic transitions, where the energy of the indirect electronic allowed transition shows a redshift in all the samples due to Fe-doping. Therefore, the width of the tail states energy increases inferring the influence of disorder and the introduction of defect states within the band gap region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, transparent conducting oxide (TCO) films have attracted the attention of several researchers because of their multiple applications such as gas sensors, solar cells, biosensors and catalysts.1 Currently, various transition metal oxides, such as TiO2, SnO2, ZnO, and CdO, have been widely studied.2,3,4 Tungsten trioxide (WO3) is an interesting material due to its electrical conductivity, excellent selectivity and high sensitivity.1,5,6 Furthermore, WO3 is an n-type wide band gap semiconductor with an optical gap of 2.5–3.6 eV, which can easily be deposited with a high optical transparency in the visible range. In this respect, WO3 is a material of choice owing to its attractive physical and chemical properties for many applications such as gas sensors,7,8 batteries,9 electrochromics10,11 and photo- and/or electrocatalysis.12,13,14,15 Several methods have been used for the synthesis of WO3 films: thermal evaporation,16 magnetron sputtering,17,18,19 electron beam evaporation,20,21 laser ablation,22,23 spray-pyrolysis24 and sol-gel.25,26
Among those methods used for thin films preparation we have chosen spray-pyrolysis for the preparation of WO3 films in our study because of its simplicity, low cost with high growth rate and easy control of doping. Hence, this technique is an efficient method to deposit films with controllable properties. To improve the physical and chemical properties of WO3 thin films, several dopants have been used such as Pd, Gd, Co, In, and Zn.25,27,28,29,30 On the other hand, the dopant can be easily added to the precursor solution with a certain amount when using the sol-gel method. Commonly, we use metal salts such as hydrated chlorides, sulphates and nitrates. However, these precursors contain Cl−, NO3− and SO4− anions with an important ratio. These impurities remain in the films with small concentration (even after the post heat-treatment) and influence theirs properties. In our study, we have chosen the element (Fe) as dopant because of the closeness of the ionic radii of W6+ compared to Fe3+ and due to the remarkable bowing effect of Fe-doping on the band gap of the material. Indeed, Khan et al.31 have reported that the electronic state created by Fe3+ cations above the valence band could trap and transfer electrons and holes to inhibit the recombination of photo-excited holes and electrons. On the other hand, doping in the sol-gel process is usually achieved using mineral salts such as nitrates. The present study reports on the synthesis and the deposition of Fe-doped WO3 films with small doping concentrations (1, 3 and 5 at.%), where Fe-doping was achieved using granular iron in an acidic medium. Furthermore, an extended thermal treatment at 400°C and 500°C was used in order to allow the dopant diffusion during crystallization of WO3 films. To the best of our knowledge, this procedure was not reported previously and it is expected to permit using the dopant directly while minimizing the concentration of the undesirable anions that may influence the properties of the films when compared the procedure used commonly in the sol-gel method.
Experimental Details
Thin Film Preparation
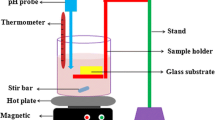
WO3 films were deposited on glass substrates (26 × 10 × 1) mm3 at constant temperature of 350°C by spray-pyrolysis using a Holmarc Spray Pyrolysis system. The glass substrates were cleaned in an ultrasonic bath with acetone and ethanol three times for 5 min then with deionized water to remove traces of microscopic impurities. All the reagents used in the synthesis of the films were purchased from Sigma-Aldrich.
The precursor solution was prepared from ammonium tungstate (NH4)10H2(W2O7)6 dissolved in 100 ml of deionized water (0.005 M). Fe-doping was achieved by adding iron powder (purity ≥ 99.99%; particle size < 10 μm) into the previous solution at the appropriate amount (\(\frac{{\left[ {{\text{Fe}}} \right]}}{\left[ W \right]}\) = 1, 3 and 5 at.%). Hydrochloric acid was added drop-wise to the solution under stirring until the powder was completely dissolved. After that, the solution was sprayed using a syringe-attached sprayer. An air-directed spray stream was used as a carrier gas. The substrate-jet distance and the spraying speed were 12 cm and 200 μl min−1, respectively. The deposition time was 8 min with an air pressure of 1.5 bar. The contact of the solution with the heated substrates at 350°C allows the thermal decomposition of the precursor solution, thus producing WO3 films according to the following chemical reaction:
After cooling to room temperature, the films were heat-treated at 400°C and 500°C for 4 h using a MAGMA THERM programmable tubular oven with a heating rate of 10°C min−1. The purpose of this heat treatment is to allow densification and crystallization of the films. Table I depicts the deposition parameters of the films. In addition, the extended thermal treatment is meant to allow dopant diffusion into WO3 during crystallization.
Characterization Methods
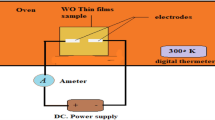
The thickness of the films was measured using a Dektak XT profilometer, which also gives the roughness parameters of the surface. The data processing was carried out by Vision 64 software. The structural properties were obtained by grazing incidence X-ray diffraction (GIXRD) using an INEL Equinox 3000 diffractometer operating in the range of 0–120° with a filtered Cu-Kα radiation (λ = 1.54056 Å). The data processing was performed by Match 3 software. The surface morphology and roughness were examined by atomic force microscopy (AFM, Nanosurf, Flex-Axiom C 3000) in tapping mode. The optical transmittance and absorbance spectra were recorded at room temperature by a dual beam UV–Vis spectrophotometer (Specord 210 Plus) in the range of 300–1100 nm. The surface morphology of the films was analyzed by scanning electron microscopy (SEM, VEGA3-TESCAN) equipped with an energy dispersive spectrometer (EDS).
Results and Discussion
Film Thickness and Profile Analysis
The surface profilometry is a non-destructive technique and allows studying the film profile. Several parameters are reliable to the roughness of the surface. Table II shows the primary profile (Pp), total profile (Pt) and thickness (e) of WO3:Fe films heat-treated at 400°C and 500°C for 4 h. It is observed that the surface primary and total profiles decrease with Fe-doping when compared to the undoped film and increase with increasing the temperature of heat treatment.
In addition, the other parameters such as the root mean square deviation of the assessed profile (Pq), the skewness of the assessed profile (Psk), the kurtosis of the assessed profile (Pku), which are gathered in Table III. These parameters are often used to get insights on the surface quality. The assessed profile is characterized by three parameters: its maximum height (Pz), its maximum profile peak height (Pp), and its maximum profile valley depth (Pv).
Figure 1a and b shows the variation of the surface profile parameters with Fe-doping of the films heat-treated at 400°C and 500°C. It is observed that the parameters Pq, Pz, Pp and Pv decrease with the increase of the dopant concentration for both temperature of thermal treatment. Hence, Fe-doping improves the quality of the surface. In addition, the values of these parameters are relatively lower in the films heat-treated at 500°C. The values of the skewness (Psk) and the kurtosis (Pku) of the assessed profile increases monotonically for the films heat-treated at 500°C, while they seem to be somewhat scattered for the films heat-treated at 400°C. It is evident that thermal treatment at higher temperature improves the growth of the crystallites and enhances the roughness of the surface.
Surface profile parameters of WO3:Fe films as a function of doping concentration: (a) the parameters Pq, Pz, Pp and PV for the films heat-treated at 400°C, (b) the parameters PSK and PKu for the films heat-treated at 400°C, (c) the parameters Pq, Pz, Pp and PV for the films heat-treated at 500°C and (d) the parameters PSK and PKu for the films heat-treated at 500°C.
GIXRD Analysis
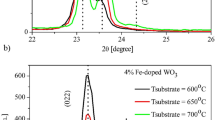
Grazing incidence X-ray diffraction (GIXRD) was used to determine the structure, crystallographic growth directions, mesh parameters, and average grain size. Figure 2a, b and c shows the X-ray diffraction patterns of WO3:Fe films before and after the heat treatment at 400°C and 500°C. The data show that before heat treatment, the films are amorphous irrespective of the doping concentration (Fig. 2a).
It is observed that the films have a polycrystalline character after heat treatment for 4 h (Fig. 2b and c). All the observed peaks are assigned to the monoclinic structure of WO3 tungsten trioxide according to the ICDD card #89-4476. In addition, the patterns confirm the absence of secondary phase related to Fe-doping or crystallization. Table IV shows the Bragg angles (θ), Miller indices (hkl), lattice parameters (a, b, c and \(\beta\)), full width at half maximum (FWHM) of the major intense peak and the average crystallite size (D). All the patterns of the films show at least three well-resolved peaks: (002), (020), (200) and/or (\(\overline{1 }\) 12). The FWHM of the peaks were calculated using Warren's formula32:
where B is the experimental widening, which is given directly by the data processing software of the diffractometer, and b is instrumental broadening which is equal to 0.08° in our case. Table IV shows the structural parameters of the films heat-treated at 400°C and 500°C for 4 h. The values of the unit cell parameters are very close to the common values reported in the literature.33 Particularly, the data reported by Chang et al.24 using spray coating and Mathankumar et al.27 using simple acid precipitation.
It is observed that the crystallinity of the films was enhanced with the heat treatment. The average crystallite size (D) was determined using Scherrer's formula34:
where λ is the X-ray wavelength and FWHM is the real widening of the diffraction peak. The average crystallite size of WO3:Fe films varies in the range of 13.0—24.7 nm, depending on the temperature of heat treatment and the dopant concentration. It is observed that the crystallite size decreases when compared to that of the undoped film and then increases with increasing concentration of the dopant (3 and 5 at.%). In addition, it is evident that the thermal treatment at 500°C improves the crystal growth,35 and we have observed that the crystallite sizes are bigger when compared to those of the films heat-treated at 400°C. This variation of the crystallite sizes agrees well with the results of both the profile and AFM analysis. Furthermore, substitution of W+6 cations (ionic radii: 74 pm) with Fe3+ cations (ionic radii: 69 pm) induces a distortion in the crystal lattice and oxygen vacancies (OV) have a tendency to form neighboring these impurities. The increase in OV concentration is considered very advantageous for gas detection.36 The inter-planar distance (dhkl) in the monoclinic system is given by:
Table IV shows that the lattice parameters change with Fe-doping when compared to the undoped film. The unit cell volume decreases and then increases, inferring that the insertion of the dopant occurs dominantly with Fe3+ cations occupying the octahedral sites (Scheme 1) for low Fe-doping (1 and 3%) because of the proximity of the ionic radii. However, it is presumed that the dopant cations are inserted in the interstitial sites for a higher amount of dopant. This behavior is further correlated with the non-stoichiometry of the films for which WO3 crystal consist of a mixture of W4+/5+ and W6+ cations.37,38,39 Therefore, the heterovalent substitution also supports the increase of the unit cell volume for Fe-doping at a higher amount (ca. 5 at.%).
The dislocation density (δ) in the film defines the length of the dislocation lines per crystal volume unit. This density was calculated using the following relation40:
where D is the average crystallite size evaluated from the XRD data. The deformation varies proportionally with the stress (Ɛ), which is the result of the internal forces in matter. The stress (Ɛ) in the studied films was evaluated using the following formula41:
where β represents the real widening of the diffraction line and θ the diffraction angle. Table IV shows the results of intrinsic deformations and dislocation density of the films for both series of samples. It is observed that the stress and the dislocation density increase with increasing dopant concentration from 1% to 3% when compared to the undoped film and then decrease for Fe-doping at 5% regardless of the temperature of thermal treatment, thus indicating the formation of high crystal quality and the reduction of imperfections.39,42 In addition, the average crystallite size values are small when compared to the data reported by Luxmi and Kumar43 for the same dopant concentration. On the other hand, they are much greater when compared to those values reported by Ngigi et al.44 for the same dopant concentration. By contrast, the values of the stress in our samples are smaller by three orders of magnitude when compared to the data reported by Nguyen et al.45 established for nanobrick hybrid WO3 , and they are compared to the values obtained by Thwala and Dlamini46 for Mg-doped WO3 films prepared with a hydrothermal method.
AFM Analysis
Atomic force microscopy (AFM) is an effective technique used to determine the surface morphology and roughness of films. Figure 3 shows the three-dimensional (3D) surface morphology of Fe-doped WO3 films heat-treated at 500°C for 4 h.
The average height of the roughness profile (Rc) and the mean square deviation of the roughness profile (Rrms) of the surface, were determined using Nanosurf C3000 software on a surface of (2 × 2) μm2. The morphological parameters of the films are gathered in Table V.
For all the films, the 3D images show the presence of well-developed grains. The Rrms values are 16.9 nm (film doped at 1%), 29.1 nm (film doped at 3%) and 51.0 nm (film doped at 5%), which confirms the enhancement of the roughness of the films. As the doping concentration increases, the average height of the roughness profile (Rc) of the films also increases. The average particle size increases from 104.3 nm (film doped at 1%) to 327.1 nm (film doped at 3%) and 528.4 nm (film doped at 5%) with Fe-doping.
SEM Analysis
Figure 4a, b and c shows the SEM images of Fe-doped WO3 films heat-treated at 500°C. It is observed that the surface morphology of the films is significantly modified by doping. The film doped at 1% (Fig. 4a) exhibits a porous morphology with spherically shaped small grains on the surface. This particular morphology is highly attractive for gas sensing application.47 The regular distribution of these spherical grains is due to the homogeneous substrate temperature and uniform deposition.
On the contrary, the microstructure of film doped at 3% (Fig. 4b) shows differently shaped aggregations, i.e. nanowires of 762 nm length and Ø = 192 nm, oriented differently and homogeneously dispersed. The surface morphology of the film doped at 5% (Fig. 4c) displays a homogeneous crack-free appearance in all scanned areas with numerous crystallites shaped differently. EDS analysis of the film doped at 5% (Fig. 5) confirmed the presence of W and O and shows that the dopant is homogeneously dispersed in the film. It is evident that Fe-doping has a significant impact on the morphology of the films depending on the concentration.
Finally, the analysis of the structural and morphological data can be summarized as follows:
-
We have analyzed the structural properties of three types of samples: amorphous, samples heat-treated at 400°C, and those heat-treated at 500°C. The data showed that the films heat-treated at 500°C have a good crystallinity. Therefore, we have carried out additional analysis on this latter (heat-treated at 500°C) to examine the effect of Fe-doping on their properties.
-
The effect of Fe-doping on the structural properties was evidenced by the observed change of the structural parameters (Table V)
-
The effect of Fe-doping on the morphology of the sample was evidenced first by observed changes of the profiles parameters (Tables II and III) and then by the observed changes on the SEM micrographs (Fig 4). The results show that the samples exhibits different morphologies depend on the Fe-concentration. Samples doped at both 1% and 3% (Fig. 4a and b) present spherically shaped grains. However, the surface of the film doped at 5% appears more homogenous and dense with an average radius relatively greater when compared to that of the film doped at 1 at.%. In addition, the film doped at 3% present a completely different morphology with nanowires of 762 nm length and Ø = 192 nm, oriented differently and homogeneously dispersed.
-
The AFM analysis revealed that the roughness parameters increase by 170% after doping at 3% Fe (compared to the film doped at 1% Fe) and increase by more than 300% after doping at 5% Fe. On the other hand, The average grain size increases by more than 300% after doping at 3% Fe (compared to the film doped at 1% Fe) and by more than 500% after doping at 5% Fe.
We have considered the above results to be strong evidence on the changes of the structural and morphological properties induced by Fe-doping.
Optical Analysis
The spectrophotometry performed at room temperature is a non-destructive technique used to determine the absorbance and the transmittance (T) as a function of the wavelength (λ). Figure 6 shows a sharp decrease of the ultraviolet absorption with increasing wavelength (from 1.4 to 0.17 and 0.12 a.u. for the films heat-treated at 400°C and 500°C, respectively). The average absorbance relatively decreases in the visible range (from 0.17 a.u. to 0.13 a.u. and from 0.12 a.u. to 0.09 a.u. for the films heat-treated at 400°C and 500°C, respectively). The transmittance/absorbance of a film is generally influenced by some factors such as the surface roughness, grain boundary scattering and optical inhomogeneity.48
Figure 7 shows that the films have a good optical transmittance in the visible region sensitive to Fe-doping and thermal treatment. Table VI reports the values of the average optical transmittance of the films in the visible range.
Indeed, this variation can be correlated with the thickness, porosity and enhanced scattering effect due to the roughness of the surface.49 Particularly, the decrease in the optical transmission is attributed to the propagation losses at the grain boundaries and electronic defects.39
The absorption coefficient (α) was calculated from the transmission spectra using the following formula50:
where e represents the thickness (cm) and T is the optical transmittance (%). Figure 8 shows the variation of the optical absorption coefficient of WO3:Fe films. The optical absorption coefficient values were reached at low energies 1.27×104 cm−1 (0.84 × 104 cm−1), 1.9 × 104 cm−1 (1.45 × 104 cm−1) and 2 × 104 cm−1 (1.72 × 104 cm−1) for 1%, 3% and 5% Fe-doped WO3 films for the films heat-treated at 400°C (at 500°C, respectively). Furthermore, it is observed that the absorption coefficient increases with Fe-doping for both series of the films.
To determine the energy of the band gap, we have used Tauc's formula, which is given as follows51:
where A is a constant, h is the Planck constant, ν is the frequency of the incident light. The constant \(n = 1/2\) in the case of a direct optical transition and \(n = 2\) in the case of an indirect optical transition. \(E_{g}\) is deduced from the plot (αhv)2 versus \(h\nu\) by linear extrapolation to the abscissa axis (zero absorption).52,53 Figure 9a and b shows the plots for indirect and direct allowed optical transitions in the films, respectively. The extrapolation of the linear parts of these curves yields the value of the optical band gap (EgI: Direct optical band gap; EgII: Indirect optical band gap). Table VI shows the obtained values of WO3:Fe films heat-treated at 400°C and 500°C for 4 h. It is observed that EgII decreases for both series (from 3.83 eV to 3.76 eV for the films heat-treated at 400°C, and from 3.75 eV to 3.65 eV for the films heat-treated at 500°C), while EgI decreases only in the films heat-treated at 500°C (from 4.22 eV to 4.13 eV). The decrease of the optical band gap with doping is consistent with the introduction of the impurity levels below the conduction band.54 The obtained values of indirect optical band gap (EgII) are relatively greater when compared to ~ 3.48 eV of WO3 obtained by inorganic and sol-gel methods.55 On the other hand, Eglitis et al. reported using ab initio calculations that the optical transition \(\Gamma - \Gamma\) is of 4.95 eV, which is overestimated when compared to our data.56 The obtained values of EgI for both amorphous and polycrystalline films are close to 3.8 eV reported by Acosta et al.57 for Ti doped WO3 thin films deposited by spray-pyrolysis.
Optical transitions of WO3:Fe films: (a) Indirect allowed transitions for the films heat-treated at 400°C, (b) Indirect allowed transitions for the films heat-treated at 500°C, (c) Direct allowed transitions for the films heat-treated at 500°C and (d) Direct allowed transitions for the films heat-treated at 500°C.
Figure 10a and b shows the semi-logarithmic plots of the absorption coefficient as a function of photon energy. This region is called the Urbach region. The following relation gives the absorption coefficient58:
Therefore:
where α0 is a constant and \(E_{{\text{u}}}\) is the Urbach energy.
To measure the width of tail states, the Urbach energy (\(E_{{\text{u}}}\)) was calculated from the formula59:
The Urbach energy values of WO3: Fe films heat-treated for 4 h are gathered in Table VI. Figure 11 shows the variation of the Urbach energy with Fe-doping. It is observed that \({E}_{u}\) increases indicating the widening of the tail states.58,59 Moreover \({E}_{\mathrm{u}}\) is sensitive to disorder and the increase of the crystallinity in the films,60 which is believed to explain the variation observed for the films heat-treated at 400°C.
Conclusion
Fe-doped WO3 films were deposited using the reactive chemical spray technique in liquid phase (spray pyrolysis) from an aqueous solution based on ammonium tungstate. From the profile and the morphology analysis, the surface profiles were found to decrease with Fe-doping and increase with increasing temperature of heat treatment. The thermal treatment promotes the growth of the crystallites and enhances the roughness of the surface. All of the WO3:Fe films obtained before thermal treatment are amorphous, whereas those obtained after heat treatment at 400°C and 500°C for 4 h are of polycrystalline nature with a monoclinic structure. Both the stress and the dislocation density increase with Fe-doping in the films heat-treated at 400°C. By contrast, a decrease in the films heat-treated at 500°C is observed. The average crystallite size decreases with Fe-doping while the average particle size increases from 104.3 nm (film doped at 1%) to 327.1 nm (film doped at 3%) and 528.4 nm (film doped at 5%) with Fe-doping.
On the other hand, the films display a good optical transmittance in the visible region sensitive to Fe-doping and thermal treatment. Furthermore, it is observed that EgII values decrease for both series, while those of EgI decrease only in the films heat-treated at 500°C. The decrease of the optical band gap with doping is consistent with the introduction of the electronic levels below the conduction band of WO3. The width of the tail states in the forbidden region and the Urbach energy (\(E_{{\text{u}}}\)) is sensitive to electronic and structural disorder increases in the films. The above results show that the films prepared in the present study are suitable for application as efficient window material in solar cells, owing to their wider optical transparency.
References
P.S. Kolhe, P. Mutadak, N. Maiti, and K.M. Sonawane, Sens. Actuators A 304, 111877 (2020).
D. Komaraiah, E. Radha, J. Sivakumar, M.R. Reddy, and R. Sayanna, Opt. Mater. 108, 110401 (2020).
S.K. Muhammad, E.S. Hassan, K.Y. Qader, K.H. Abass, S.S. Chiad, and N.F. Habubi, Nano Biomed. Eng 12, 67 (2020).
A.M. Mostafa, and E.A. Mwafy, J. Mol. Struct. 1221, 128872 (2020).
Q. Wang, H. Fu, J. Ding, C. Yang, and S. Wang, Opt. Laser Technol. 125, 106036 (2020).
A.K. Mohamedkhair, Q.A. Drmosh, M. Qamar, and Z.H. Yamani, Catalysts 11, 381 (2021).
Y. Kimura, K. Ibano, K. Uehata, I. Hirai, H.T. Lee, and Y. Ueda, Appl. Surf. Sci. 532, 147274 (2020).
S. Hajirnis, P. Chavan, V. Manapure, A. Patil, A. Khan, B.S. Nadekar, P.S. More, and A.V. Kadam, Mater. Res. Express 8, 095503 (2021).
F.J. García-García, J. Mosa, A.R. Gonzalez-Elipe, and M. Aparicio, Electrochim. Acta 321, 134669 (2019).
Y. Zhao, X. Zhang, X. Chen, W. Li, L. Wang, F. Ren, J. Zhao, F. Endres, Y. Li, and A.C.S. Sustain, Chem. Eng. 8, 11658 (2020).
A. Abareshi, and H. Haratizadeh, Iran. J. Phys. Res. 16, 47 (2019).
D. Nunes, A.R. Fragoso, T. Freire, M. Matias, A.C. Marques, R.F.D.P. Martins, E. Fortunato, and A. Pimentel, Phys. Status Solidi - Rapid Res. Lett. 15, 2100196 (2021).
Y.C. Liang, and C.W. Chang, Coatings 9, 90 (2019).
M. Feng, Y. Liu, Z. Zhao, H. Huang, and Z. Peng, Mater. Res. Bull. 109, 168 (2019).
J. Juodkazytė, M. Petrulevičienė, M. Parvin, B. Šebeka, I. Savickaja, V. Pakštas, A. Naujokaitis, J. Virkutis, and A. Gegeckas, J. Electroanal. Chem. 871, 114277 (2020).
X. San, Y. Lu, G. Wang, D. Meng, X. Gong, and Q. Jin, Mater. Lett. 271, 127716 (2020).
A. Rydosz, K. Dyndał, K. Kollbek, W. Andrysiewicz, M. Sitarz, and K. Marszałek, Vacuum 177, 109378 (2020).
N. Matsunami, M. Sataka, and S. Okayasu, Nucl. Instrum. Methods Phys. Res. B 460, 185 (2019).
L. Pan, Q. Han, Z. Dong, M. Wan, H. Zhu, Y. Li, and Y. Mai, Electrochim. Acta 328, 135107 (2019).
R. Shakoury, A. Arman, S. Rezaee, A.G. Korpi, S. Kulesza, C. Luna, and M. Mardani, J. Mater. Sci. Mater. Electron. 32, 798 (2021).
W. Li, X. Zhang, X. Chen, Y. Zhao, L. Wang, M. Chen, Z. Li, J. Zhao, and Y. Li, Electrochim. Acta 355, 136817 (2020).
Y. Nishijima, K. Enomonoto, S. Okazaki, T. Arakawa, A. Balčytis, and S. Juodkazis, Appl. Surf. Sci. 534, 147568 (2020).
L. S. Parshina, O. A. Novodvorsky, O. D. Khramova, A. A. Lotin and P. A. Shchur, in Journal of Physics: Conference Series 1164, 012003 (2019)
C.M. Chang, Y.C. Chiang, M.H. Cheng, S.H. Lin, W.B. Jian, J.T. Chen, and K. Tsukagoshi, Sol. Energy Mater. Sol. Cells 223, 110960 (2021).
Z. Han, J. Ren, J. Zhou, S. Zhang, Z. Zhang, L. Yang, and C. Yin, Int. J. Hydrog. Energy. 45, 7223 (2020).
X. Li, Z. Li, W. He, H. Chen, X. Tang, Y. Chen, and Y. Chen, Coatings 11, 959 (2021).
G. Mathankumar, P. Bharathi, M.K. Mohan, S. Harish, M. Navaneethan, J. Archana, and C. Muthamizhchelvan, Mater. Sci. Semicond. Process. 105, 104732 (2020).
V. Saasa, T. Malwela, Y. Lemmer, M. Beukes, and B. Mwakikunga, Mater. Sci. Semicond. Process. 117, 105157 (2020).
S. Buathet, K. Simalaotao, P. Reunchan, V. Vailikhit, P. Teesetsopon, D. Raknual, and A. Tubtimtae, Electrochim. Acta 341, 136049 (2020).
M. Arshad, S. Ehtisham-ul-Haque, M. Bilal, N. Ahmad, A. Ahmad, M. Abbas, and M. Iqbal, Mater. Res. Express 7, 015407 (2020).
H. Khan, M. Habib, A. Khan, and D.C. Boffito, J. Environ. Chem. Eng. 8, 104282 (2020).
S.S. Hossain, K. Praveena, and P.K. Roy, J. Mater. Sci. Mater. Electron. 31, 15097 (2020).
M. Kumar Mohanta, T. Kanta Sahu, S. Alam, and M. Qureshi, Chem. Asian J. 15, 3886 (2020).
C. Qiu, Q. Meng, M. Panchal, C. Li, and B. Wu, Catal. Commun 147, 106149 (2020).
M. Farooqi, M. Hasan, R. K. Srivastava, in Proc. Natl. Acad. Sci. India - Phys. Sci. 90 (2020), pp. 845–859
Z. Li, X. Liu, M. Zhou, S. Zhang, S. Cao, G. Lei, C. Lou, and J. Zhang, J. Hazard. Mater. 415, 125757 (2021).
N. Bashirom, and Q.L. Lee, Mater. Sci. Forum 1010, 405 (2020).
D. Dong, J. Robichaud, and Y. Djaoued, Can. J. Chem. 99, 549 (2021).
Y.S. Haiduk, A.A. Khort, M.A. Makhavikou, and A.A. Savitsky, Mod. Electron. Mater. 5, 115 (2019).
S. M. Abd Al Hussan, N. A. Bakr, A. N. Abd, in IOP Conference Series: Materials Science and Engineering, vol. 928 (2020), pp. 072142
S. Sivakumar, E. Manikandan, and B. Mahalakshmi, Vacuum 173, 109116 (2020).
A.A. Akl, I.M. El Radaf, and A.S. Hassanien, Optik 227, 165837 (2021).
V. Luxmi, and A. Kumar, Mater. Sci. Semicond. Process. 104, 104690 (2019).
E.M. Ngigi, P.N. Nomngongo, and J.C. Ngila, Catal. Lett. 149, 49 (2019).
C.T. Nguyen, T.P. Pham, T.L.A. Luu, X.S. Nguyen, T.T. Nguyen, H.L. Nguyen, and D.C. Nguyen, Ceram. Int. 46, 8711 (2020).
M.M. Thwala, and L.N. Dlamini, Environ. Technol. 41, 2277 (2020).
S.S. Shendage, V.L. Patil, S.A. Vanalakar, S.P. Patil, J.L. Bhosale, J.H. Kim, P.S. Patil, and Z. Phys, Chem. 234, 1819 (2020).
I.A. Rodionov, A.S. Baburin, A.R. Gabidullin, S.S. Maklakov, S. Peters, I.A. Ryzhikov, and A.V. Andriyash, Sci. Rep. 9, 1 (2019).
L. Xu, G. Zheng, F. Xian, and J. Su, Mater. Chem. Phys. 229, 215 (2019).
A. Haichour, and N. Hamdadou, J. Nano- Electron. Phys. 11, 06020 (2019).
L.K. Sharma, M. Kar, R.K. Choubey, and S. Mukherjee, Chem. Phys. Lett. 780, 138902 (2021).
G. Soman, A. Shajan, J. Jassi, N. K. Vijay, in AIP Conference Proceedings, vol. 2263 (2020), p. 050006.
P.K. Pothuganti, A. Bhogi, M.R. Kalimi, and P. Reniguntla, Glass Phys. Chem. 46, 146–154 (2020).
A. Ammari, and M. Trari, Colloids Surf. A 61, 178–186 (2019).
J. Liu, G. Zhang, K. Guo, D. Guo, M. Shi, H. Ning, T. Qiu, J. Chen, X. Fu, R. Yao, and J. Peng, Micromachines 11, 311 (2020).
R.I. Eglitis, J. Purans, and R. Jia, Curr. Comput.-Aided Drug Des. 11, 455 (2021).
D. Acosta, F. Hernández, A. López-Suárez, and C. Magaña, Solid State Phenom. 286, 49–63 (2019).
S. Kumari, K. Singh, P. Singh, S. Kumar, and A. Thakur, SN Appl. Sci. 2, 1 (2020).
T. Hamai, S. Inoue, S. Arai, and T. Hasegawa, Phys. Rev. Mater. 4, 074601 (2020).
A. Ammari, M. Trari, and N. Zebbar, Mater. Sci. Semicond. Process. 89, 97–104 (2019).
Acknowledgments
The authors which to thank Professor Bouslama M’Hammed, the Director of the Materials Laboratory (ENPO-Oran), for providing access to AFM measurements, and Dr. Mhamed Guezzoul from the same laboratory for his help in these measurements.
Funding
This work was funded by the Directorate-General for Scientific Research and Technological Development, Ministry of Higher Education and Scientific Research (Algeria).
Author information
Authors and Affiliations
Contributions
All authors contributed to this research work equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ouadah, E., Hamdadou, NE. & Ammari, A. Morphological, Structural and Optical Properties of Fe-Doped WO3 Films Deposited by Spray-Pyrolysis. J. Electron. Mater. 51, 356–369 (2022). https://doi.org/10.1007/s11664-021-09300-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-021-09300-0