Abstract
The measurement of electrical resistance, capacitance and impedance is central to the electrical characterization of materials, whether the materials are in bulk, thick film or thin film forms. However, there are numerous common pitfalls in the measurement. The pitfalls mainly relate to the electrode design, electrode configuration and measurement instrument utilization. This commentary provides an overview of these pitfalls, along with the methods of measurement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrical resistance and capacitance are two of the most basic attributes in the electrical characterization of materials. The relevant materials include bulk materials, thick films and thin films, whether they are metals, ceramics, polymers or semiconductors. They also include conductors, insulators and materials with intermediate levels of conductivity. Furthermore, they include electronic materials and multifunctional structural materials. Multifunctional structural materials attain certain nonstructural functions, such as the sensing function, by exploiting the electrical behavior of the structural materials, such as the effect of damage on the electrical behavior of the structural materials. Examples of multifunctional structural materials include carbon fiber polymer-matrix composites and cement-based materials.
The resistance, which relates to the resistivity (a material property), pertains to the conduction behavior. Conduction is at the heart of numerous applications, including electrical interconnections, electrodes, conductive joints, electric cables, resistance heating, thermoelectrics, solenoids and transformers. The capacitance, which relates to the electric permittivity (a material property), pertains to the dielectric behavior. The dielectric behavior does not mean insulation behavior, but it means polarization behavior, which pertains to numerous applications, including insulation, conduction, ferroelectrics, piezoelectrics, pyroelectrics, and electrets.
Insulating materials with low values of the relative permittivity (known as low-k dielectrics) are valuable for reducing the capacitance between conduction lines, thereby reducing the signal propagation delay associated with the RC time constant. On the other hand, insulating materials with high values of the relative permittivity (known as high-k dielectrics) are valuable for capacitors.
The resistivity and permittivity are not independent of one another. They are related through the Kramers-Kronig relation, which involves consideration of the behavior over a wide frequency range.
The resistance can be DC or AC, whereas the capacitance, as measured using an LCR meter or an impedance meter, is AC. Multimeters are widely used for such measurements. However, there are serious common pitfalls in how the measurements are made, as discussed in this commentary. Resistance, capacitance and impedance measurements are addressed in Sects. 2, 3 and 4, respectively.
This commentary concerns DC and low-frequency AC (frequencies much below the radio wave, microwave and optical regimes). The low-frequency regime is pertinent to most electrical applications and includes the utility frequency (e.g., 60 Hz in the U.S.). Measurement techniques differ between the low-frequency regime and the high-frequency regime. For example, in the low-frequency regime, the measurement of permittivity involves capacitance measurement using instruments such as an LCR meter, whereas, in the high-frequency regime, the measurement of permittivity involves assessing the absorption of the electromagnetic radiation incident on the specimen through radiation propagation.
This commentary is aimed at addressing the pitfalls in resistance and capacitance measurements and clarifying the associated methods of measurement. The relevant materials broadly include bulk, thick film and thin film forms, whether they are conductive or not.
Resistance Measurement
This section addresses two main pitfalls in resistance measurement. They are the pitfall due to the neglect of the contact resistance (“Pitfall Due to the Neglect of the Contact Resistance” Section) and the pitfall due to the electric polarization of the material (“Pitfall Due to the Electric Polarization of the Material” Section).
Pitfall due to the Neglect of the Contact Resistance
Four-Probe Method versus Two-Probe Method
There are in general two methods for measuring the electrical resistance of a material. They are the four-probe method and the two-probe method. The four-probe method involves four electrical contacts (electrodes), such that the outer two contacts are for passing a current, while the inner two contacts are for measuring the voltage. By separating the current and voltage contacts, the contact resistance is essentially eliminated from the measured resistance. On the other hand, the two-probe method involves only two electrical contacts, each of them being used for both current and voltage. As a consequence, the measured resistance using the two-probe method includes the contact resistance. Unless the contact resistance is negligible compared to the specimen resistance, the result obtained using the two-probe method is not reliable. A related pitfall is the assumption that the contact resistance is negligible when the contact material (e.g., silver) is highly conductive. Although the resistance may be small or negligible within the contact material, the resistance of the interface between the contact material and specimen can be substantial.
The two-probe method works if the distance l between the two electrical contacts is varied and the resistance is measured for each distance. The measured resistance Rm is given by
where R is the specimen volume resistance and Ri is the contact resistance for each of the two contacts. R is related to the resistivity ρ by the equation
where A is the cross-sectional area perpendicular to the resistance direction. The combination of Eqs. 1 and 2 gives
Ri is inversely related to A. Thus, a large value of A helps to reduce Ri. However, a large area cannot be used to support the assumption that Ri is negligible.
By plotting Rm versus l, a straight line is obtained, if all the electrodes used to provide the various values of l are similar in quality. The slope of the line equals ρ/A, according to Eq. 3. The intercept of this line with the vertical axis at l = 0 gives 2Ri. Thus, Ri is decoupled from R. At least three values of l are needed for this method, which is known as the transmission line method. In contrast, in the four-probe method, only one value of l (the distance between the two inner contacts) is needed.
Resistance Response to a Stimulus
For both the science and applications, the response of the resistance to a certain stimulus is commonly studied. Examples of the stimulus include the voltage/current, strain/stress, temperature and damage. The response enables the sensing of the stimulus through the resistance measurement. In this situation, the material being tested serves as the sensor, as it senses itself without any sensor incorporation (i.e., self-sensing). Self-sensing is particularly valuable when the material is a structural material, so that the integrity of the structure may be monitored. For such studies, the two-probe method is not reliable, even if the contact resistance is small. This is because both the contact resistance and specimen volume resistance can change as the degree of the stimulus is varied. As a consequence, the observed change in resistance upon variation in the stimulus degree may be due to a combination of a change in contact resistance and a change in the volume resistance. In general, the contact resistance and volume fraction may change in the same direction or in opposite directions in response to the same stimulus. Even if the two contacts are embedded in the specimen, the contact resistance can change upon stimulus application. This is because the quality of the interface between the embedded contact and the material being studied can change upon experiencing the stimulus.
For example, upon the change in temperature, the thermal expansion of the contact material (e.g., silver paint after drying) and/or the change in the degree of conformability of the contact material with the specimen surface may occur, thus affecting the contact resistance. An increase in the fluidity and hence the conformability may occur upon heating and this tends to decrease the amount of air voids at the interface between the contact and specimen surface, thereby decreasing the contact resistance. As another example, upon compression, the interface between each of the two contacts and the specimen volume is tightened and hence improved. Thus, the contact resistance decreases with increasing compressive stress. This problem is particularly significant in case of contacts that are attached to the surface of the specimen. To alleviate this problem, the top contact (i.e., the contact on the surface receiving the load) may be limited to only a part of the area of the specimen, such that the contact does not experience the stress while the remaining area of the specimen does. For example, the loaded area is in the center part of the area, while the remaining area is large enough to allow the current used in the testing to go through essentially the entire area of the specimen. In case that the specimen is stiff enough, the force applied to a part of the specimen area is spread to the entire area. However, if the specimen is not stiff enough, the spreading of the force would not be adequate. A better solution, which does not require the material to be stiff, is to avoid using contacts on the surface, but to use embedded contacts.
Types of Electrical Contacts
The quality of attached contacts can vary greatly. Contacts (known as pressure contacts) in the form of a metal contact material (e.g., a conductor foil or tip) that touches the specimen surface through the application of pressure tend to be relatively low in quality, because the proximate surfaces are never perfectly smooth in the microscopic level and, as a consequence, microscopic air pockets exist at the interface. Since air is an insulator, the contact quality is low. Furthermore, the contact resistance decreases with increasing pressure, which blunts or deforms the hillocks in the surface topography. Pressure contacts involving conductor tips (such as pointed metallic electrical probes) are particularly problematic, as the small area of the tip makes the contact resistance particularly large.
Contacts involving a conductive paint or paste (e.g., silver paint, nickel paint and graphite paint) present between a metal contact material (e.g., a metal foil) and the specimen surface tend to be superior to the pressure contacts, because the paint or paste displaces the air and is more conductive than air. For the purpose of displacing the air, the fluidity of the paint or paste is important. Between paints and pastes, paints tend to give superior contacts, due to their greater fluidity and hence stronger ability to enter the microscopic valleys in the surface topography of the specimen surface. For both paints and pastes, the fluidity decreases with increasing content of the conductive filler, while the conductivity increases. Therefore, for maximizing the effectiveness of the paints or pastes, the filler content should be kept sufficiently low. A paint or paste with high conductivity but low fluidity is not attractive. Obviously, the paints or pastes should be applied in the fluid (wet) state. As long as the paint or paste is more conductive than air, its presence helps the contact quality, unless the thickness of the paint or paste is excessive. The thickness should ideally be just enough for the paint or paste to fill the valleys in the topography of the proximate surfaces. The greater is the thickness, the higher is the contact resistance. In case that the paint or paste cannot fill the valleys completely, air pockets remain at the interface, thereby reducing the contact quality.
Contacts in the form of vacuum-deposited thin films should not be assumed to be associated with negligible contact resistance. The interface between the film and the specimen surface may still have substantial contact resistance, due to the contamination that may be present at the interface between the contact and specimen surface during the deposition. The amount of contamination depends on the quality of the vacuum used in the deposition. Furthermore, the typically small area of such contacts (e.g., in the form of dots) contributes to causing the contact resistance to be substantial. The possible non-ohmic nature of the contacts is an additional concern.
Similar interface considerations apply to embedded contacts, though conductive paints or pastes are typically absent at the interface between an embedded contact and the material being tested. The application of pressure to the material containing embedded contacts tends to improve the quality of these contacts, as the pressure tends to tighten the interface. The processing of the material may induce pressure, as in the case that the material precursor shrinks as it is converted to the material during the process. However, the process-induced pressure is usually low compared to the pressure that is applied to the material.
Other Remarks
Since the purpose of the testing is to study how the volume resistance of the specimen changes with the stimulus, the inclusion of the contact resistance in the measured resistance defeats the purpose. Numerous papers have been published in various journals based on the measurement of the two-probe resistance during stimulus application, particularly during stress/strain variation. The fact that this pitfall is common does not make this practice scientifically acceptable.
The use of a Wheatstone Bridge circuit does not remove the contact resistance. This is because the volume resistance and contact resistance are included in each leg of the bridge.
Current-voltage characteristic (curve) measurement is valuable, especially when the curve is not linear, as the curve gives more information than resistance measurement at a particular value of the current or voltage. However, the effect of the contact potential drop on the voltage in the curve remains a concern, which can be resolved by using the four-probe method.
There can be application-related material structures that make the implementation of the four-probe method difficult. Under this situation, the use of a non-ideal four-probe method in which the current and voltage electrodes that are not in electrical contact are in the same plane (rather than in distinct planes) may be used. This non-ideal four-probe method is more reliable than the two-probe method. In the case where even the use of the non-ideal four-probe method is not feasible, the two-probe method may be used, provided the conclusions drawn from the study do not involve the assumption that the contact resistance is zero or negligible.
Pitfall due to the Electric Polarization of the Material
An electrically conductive material can exhibit a degree of dielectric behavior. This means that the conductive material is associated with both resistance and capacitance, although the capacitance tends to be small.
The dielectric behavior refers to electric polarization behavior. The polarization means the separation between the positive charge center and negative charge center in the material. The polarization results in an electric dipole and hence an electric field that opposes the electric field applied to cause the conduction. As a consequence, the polarization increases the apparent DC resistance beyond the true resistance. The build-up of the DC polarization takes time, so the increase of the apparent DC resistance occurs over a period of time. Typically, the higher the conductivity, the faster the polarization. For the purpose of measuring the true resistance, the resistance should be measured before the polarization becomes substantial. Since resistance measurement involves the meter putting out a small current to the specimen, polarization occurs as the measurement continues. Therefore, the resistance obtained at the very start of the resistance measurement is the value that is close to the true resistance. A pitfall is to wait until the resistance has become stable, i.e., essentially not changing with time. When the resistance becomes stable, the polarization becomes saturated and the resistance corresponding to this state is higher than the true resistance.
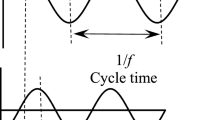
An alternate method for obtaining the true resistance for a material that can be polarized is not to measure at the start of the polarization, but to reverse the polarity and take the average of the resistance immediately before the reversal (R1) and that immediately after the reversal (R2), as illustrated in Fig. 1. The average of R1 and R2 equals the true resistance in case of electrical symmetry. However, in general, R1 and R2 can be off from the true resistance by different amounts. The four-probe method should be used for the resistance measurement in accordance with this method. If the two-probe method is used, polarization that also occurs at the electrical contacts (i.e., at the interface between the contact and specimen surface) causes asymmetry upon polarity reversal, so that R1 and R2 are not equally far from the true resistance and the average of R1 and R2 does not equal the true resistance.
DC resistance measurement for a material that exhibits dielectric behavior. Electric polarization (indicated by the change in the apparent resistance) occurs during DC electric field application (which occurs during DC resistance measurement) and the polarity reversal that occurs after a period of time of polarization. R1 is the apparent resistance immediately before polarity reversal and R2 is the apparent resistance immediately after polarity reversal. The average of R1 and R2 equals the true resistance in case of electrical symmetry. However, in general, R1 and R2 can be off from the true resistance by different amounts.
The phenomenon of dielectric relaxation differs greatly between conductors and nonconductors. For conductors, the polarization is due to the interaction of a fraction of the carriers (typically electrons) with the atoms. For nonconductors, the polarization is due to the asymmetry in the position of the ions or charged species in the material, e.g., asymmetry in the crystal structural unit cell. Because of the difference in polarization mechanism and thermodynamics, the dynamics of dielectric relaxation differ between conductors and nonconductors. In this regard, the case of conductors is still an emerging area of science.
Capacitance Measurement
This section addresses two main pitfalls in capacitance measurement. They are the pitfall due to the assumption that the electrical contacts are perfect (“Pitfall Due to the Assumption that the Electrical Contacts are Perfect” Section) and the pitfall due to the assumption that an LCR meter works similarly for both conductors and nonconductors (“Pitfall Due to the Assumption that an LCR Meter Works Similarly for Both Conductors and Nonconductors” Section).
Pitfall due to the Assumption that the Electrical Contacts are Perfect
Decoupling the Volumetric Capacitance and the Electrode Interface Capacitance
The capacitance is commonly performed using the parallel-plate capacitor geometry, in which the specimen is sandwich by two electrical contacts (electrodes). This configuration corresponds to three capacitors in series electrically. The three capacitors are the specimen volume capacitance and the two interfacial capacitances associated with the two electrical contacts. According to the well-known equation for capacitors in series, the measured capacitance Cm is given by
where C is the volumetric capacitance of the specimen and Ci is the capacitance for each of the two specimen-contact interfaces. The volumetric capacitance C in Eq. 4 is given by
where εo = the permittivity of free space (8.85 × 10−12 F/m), l = the inter-electrode distance, A = the cross-sectional area of the specimen perpendicular to the capacitance direction, and κ denotes the relative permittivity of the specimen. Eqs. 4 and 5 together give
As indicated by Eq. 6, the plot of 1/Cm versus l is linear with slope 1/(εoκA), as illustrated in Fig. 2. Hence, from the slope, κ is obtained. At least three values of l are needed for this method. However, the material structure should not depend on l. If it does, the l range should be kept within a small enough range, so that the material structure is independent of l within this range.
Schematic plot of 1/Cm versus l , for the determination of Ci and κ based on Eq. 6, where Ci is the capacitance of a specimen-contact interface and κ is the relative permittivity of the specimen. The slope equals 1/(εoκA). The l is the thickness of the specimen and A is the area of the specimen. The intercept on the vertical axis equals 2/Ci.
An alternate method of determining κ involves using different values of A (with l fixed) instead of the abovementioned use of different values of l (with A fixed). Rearrangement of Eq. 6 gives
In the case when Ci >> 2εoκA/l (i.e., Ci is sufficiently large due to the adequate quality of the electrical contact), Eq. 7 becomes
and a plot of Cm versus A is a straight line with slope εoκ/l. Hence, from the slope, κ is obtained. Although decoupling by varying A is less clean than decoupling by varying l, it may be experimentally more convenient to vary A instead of l.
The above discussion is for a parallel-plate capacitor configuration. In case of coplanar electrodes (i.e., the electrodes being on the same plane, such as being on the same surface of the specimen), varying the electrode area is relatively simple. For example, the two coplanar electrodes for capacitance measurement are in the form of two parallel strips of the same length that are separated by a fixed small distance. In order to vary the electrode area, different lengths of the parallel strips are used.
In this configuration, the electrode area is not the same as the cross-sectional area A of the capacitor. Since the capacitance is in the plane of the coplanar electrodes, in the direction from one electrode to the other, the cross-sectional area is perpendicular to this plane. Nevertheless, the greater is the electrode area (i.e., the longer is each electrode strip), the larger is the cross-sectional area. Therefore, Eqs. 7 and 8 are still applicable, with the understanding that A is proportional to the length of the electrode strip. It should be noted that the depth of penetration of the electric field below the coplanar electrodes is not uniform in the direction from one electrode to the other, since the depth increases nonlinearly with the distance away from either electrode. However, provided that the electrode strips are sufficiently long, the electric field lines are essentially entirely in the direction perpendicular to the electrode strips and the electric field non-uniformity in this perpendicular direction is the same for the different lengths of the electrodes. As a consequence, under this condition, the proportionality mentioned above is reliable.
The vast majority of reported work concerns the parallel-plate capacitor configuration rather than the coplanar electrodes configuration. As a result, the vast majority of reported work was performed on specimens with small thicknesses, with the thickness direction being the direction of the capacitance measurement. However, the material behavior may differ between the through-thickness direction and in-plane direction, particularly if the material is inherently anisotropic. An example of an anisotropic material is a thick film with preferred orientation of the functional particles of the film in the plane of the film. Another example of an anisotropic material is a thin film with preferred crystallographic orientation. Furthermore, the material dimension is much larger in an in-plane direction than the through-thickness direction and the dimension affects the charged species excursion distance under an AC electric field, thereby affecting the permittivity and capacitance. In other words, the permittivity can be larger in the in-plane longitudinal direction than the through-thickness direction because of the dimensional difference between these directions. For measuring the permittivity or capacitance in an in-plane direction, the coplanar electrode configuration is suitable, whereas the parallel-plate capacitor configuration is not suitable.
For a nonconductive material, the inter-electrode distance l (whether in the parallel-plate capacitor configuration or the coplanar electrodes configuration) must be relatively small, in order to alleviate the issue related to the fringing electric field. The parallel-plate configuration is more amenable to a small value of l than the coplanar electrodes geometry. Thus, the parallel-plate capacitor geometry involving sandwiching electrodes is commonly used.
For a conductive material, the fringing field is negligible, so l can be quite large and coplanar electrodes can be suitable. Because of the feasibility of a large value of l, the coplanar electrodes configuration is more suitable for implementation to multifunctional structures (such as a bridge) than the parallel-plate capacitor configuration. The coplanar electrodes can be applied, such that they are spaced along the length of the material or structure.
In the case where the material is not conductive, the fringing field causes the capacitance measured using the coplanar electrodes to be inaccurate. However, the change of the measured capacitance in response to a certain stimulus (e.g., stress) can still be measured, thus providing a means to sense the stimulus.
The providing of multiple values of l is typically feasible in a materials research setting. However, in an application-related material system, it may be infeasible to provide multiple thicknesses of the material for testing using the parallel-plate capacitor configuration. Under this situation, the electrode-specimen interface quality needs to be as high as possible, and the conclusions drawn from the study should not involve the assumption that the interface is perfect or nearly perfect.
The linearity in Fig. 2 would not occur adequately if the specimen is not uniform in quality or dimensions, or the electrical contacts are not uniform in quality. In the case of linearity, the slope gives κ, which is an important material property. The intercept of the linear plot with the vertical axis at l = 0 is equal to 2/Ci Eq. 6, thus allowing the determination of Ci. The greater Ci is, the smaller the influence of Ci on Cm and the more superior is the contact. For a perfect contact, Ci is infinite. A pitfall is the unsubstantiated assumption that Ci is infinite, so that 1/Ci equals 0 in Eq. 6. When Ci is not infinite, Cm is less than C, as indicated by Eq. 6. The assumption that Ci is infinite causes C to be under-estimated. Even if the contact material is highly conductive, the interface between the contact and specimen surface may not be perfect and, as a result, may be associated with a finite value of Ci.
The imperfect interface has been referred to as a “dead layer”. The interface is a region with a non-zero thickness. For example, microscopic pores can exist in this region, due to the roughness of the proximate surfaces and the inadequate conformability of the electrode material with the surface topography of the specimen.
Another issue is the possible accumulation of charge at the interface between the contact (electrode) and specimen surface. Charge accumulation may be due to space charge polarization, which is particularly common if the material has mobile ions (e.g., a cement-based material, which has mobile ions in the pore solution). It may also be due to the difference in the charge carrier relaxation time between the materials on the two sides of the interface (i.e., the Maxwell–Wagner effect, which commonly occurs when one material is more conductive than the other). In the presence of charge accumulation at the interface, the interfacial polarization can be significant, even possibly overshadowing the volumetric polarization in the material under investigation. The charge accumulation at the interface may increase or decrease the measured capacitance, depending on the effect of the charge accumulation on Ci. In other words, the charge accumulation may tighten or loosen the interface. Under this situation, the abovementioned decoupling of the interfacial and volumetric contributions to the capacitance is critically important.
Although the abovementioned decoupling method is simple scientifically, it is commonly not used, due to the inconvenience or impossibility of having specimens with various dimensions in the direction of the capacitance measurement. Without using the abovementioned experimental method of decoupling, one needs to resort to calculation (e.g., first-principles calculation) performed for a single value of the material dimension, with consideration of the voltage drop within the specimen and that at the interface. Such calculation is not simple and its accuracy is not certain.
Capacitance Response to a Stimulus
In case that the capacitance is studied during the application of a stimulus (e.g., voltage/current, stress/strain, temperature and damage), both C (which relates to κ) and Ci can change when the degree of the stimulus changes. Moreover, the changes in C and Ci can be in the same direction or in different directions under the same stimulus. For example, upon compression, C tends to increase due to the decrease in the specimen dimension in the direction of the capacitance measurement, but Ci may increase (in case that the electrode-specimen interface is improved by the compression) or decrease (in case that the electrode-specimen interface is degraded by the compression). Whether the electrode-specimen interface is improved or degraded by the compression depends on the properties of the materials involved, such as the brittleness of the electrode material and the quality of the interface prior to the compression. If the quality of the interface prior to compression is low, compression tends to improve the interface. If the material at the interface between the electrode and specimen surface is brittle, as in the case of silver paint after drying, the compression may degrade the interface. Therefore, the assumption that Ci is infinite throughout the variation in voltage/current, stress/strain, temperature or damage degree can cause unreliable determination of the effect of the stimulus on C.
Pitfall due to the Assumption that an LCR Meter Works Similarly for Both Conductors and Nonconductors
LCR meters are commonly used to measure the capacitance. However, LCR meters are not designed to measure the capacitance of a conductor. One can find the limitations of a meter in terms of the combination of capacitance and resistance by viewing the detailed specifications of the meter. In the case where the material being studied is a conductor, a thin dielectric film (i.e., a thin high-resistance film such as double-sided adhesive tape) must be positioned between the electrical contact and specimen surface, so as to increase the resistance of the material system to a level that is high enough for the LCR meter to function properly. The thickness of the dielectric film is ideally just enough to provide adequate resistance to the material system. This is because the larger the film thickness, the lower Ciis, and, based on Eq. 6, the lower Cm is (i.e., the less sensitive is the capacitance measurement). The presence of the dielectric film affects the capacitance, as it affects Ci Eq. 6. However, without the dielectric film, the measured capacitance may be very unreliable, due to the conductive nature of the specimen and the limitation of the meter. By testing the material at three or more different values of l and using Eq. 3, Ci can be decoupled from C (“ Pitfall Due to the Assumption that the Electrical Contacts are Perfect” Section) Such decoupling is needed to remove the effect of the electrode-specimen interface, which includes the dielectric film.
Numerous papers have been published in various journals, reporting incorrectly high values of the relative permittivity for conductive materials, due to the pitfall associated with not recognizing the limitation of the meter in capacitance measurement. Even values that are too high by orders of magnitude have been reported. For example, the permittivity is measured as a function of the conductive filler content in a composite with a nonconductive matrix. Below the percolation threshold, the conductivity of the composite is low; above the percolation threshold, the conductivity is much higher. The assumption that the permittivity can be measured in exactly the same way, regardless of the filler content, can cause significant error in the results on the effect of the filler content on the permittivity.
Pitfall due to the Fringing Electric Field
In case that the material is a poor conductor, fringing electric field may be significant at the exposed surface of the specimen, thus causing inaccuracy in the capacitance measurement. In case of a parallel-plate capacitor, the fringing field at the specimen edge causes the area of the capacitor to be effectively larger than the true area. As a consequence, the capacitance is higher than the value without the fringing field. In case of coplanar electrodes, the fringing field is even more significant, due to the relatively large amount of exposed area.
In case of the parallel-plate capacitor configuration, the use of a large-area electrode, a small specimen thickness and the use of a third electrode (called a guard electrode) to surround one of the two electrodes two-dimensionally would alleviate this problem. In the case of coplanar electrodes, the use of a short distance between the electrodes helps reduce the fringing field. On the other hand, for a material that is a good conductor, the fringing field is negligible, as the electric field is trapped in the material.
Impedance Measurement
Impedance measurement suffers from the same pitfalls as those mentioned in “Resistance Measurement” Section and “Capacitance Measurement” Section for resistance and capacitance measurements, respectively. The real part of the complex impedance is the resistance; the imaginary part relates to the capacitance and inductance. However, this commentary does not address the inductance. The fact that the impedance is for AC rather than DC does not alleviate the issues. The analysis of the frequency dependence of the real and imaginary parts of the impedance using plots such as the Nyquist plot also does not remove the issues.
Unfortunately, impedance measurements reported in the literature commonly involve the two-probe method, with the assumption that the two electrodes are perfect or nearly perfect at all frequencies, i.e., with zero (or negligible) resistance and infinite (or very high) capacitance at the electrode-specimen interface at all frequencies, and with the assumption that the same measurement method can be the used, regardless of the resistance of the material. The specifications of the impedance meter need to be consulted to obtain the range of the combination of resistance and capacitance that the meter can handle properly. This range varies with the frequency.
The frequency may affect both the electrode-specimen interface characteristics and specimen characteristics, and these effects may be qualitatively or quantitatively different. The excursion distance of the charged species decreases with increasing frequency. The smaller this distance, the less is the effect of the microstructure, such as the grain boundaries. The available distance is small at the interface compared to that in the volumetric bulk. Furthermore, the degree of the dipole friction may differ between the volumetric bulk and the interface.
To alleviate the problems mentioned above concerning impedance measurement, one should perform decoupling of the volumetric and interfacial contributions to the impedance, using the decoupling methods mentioned in “Four-Probe Method Versus Two-Probe Method” Section and “Decoupling the Volumetric Capacitance and the Electrode Interface Capacitance” Section. For the decoupling, the impedance should be measured for various specimen dimensions. In the case of the impedance measurement as a function of the frequency, the decoupling should be performed at each frequency, because the frequency may affect the volumetric impedance and interfacial impedance differently. A change in frequency is a type of stimulus, akin to a change in temperature being a stimulus. The pitfalls associated with measurement of the electrical response to a stimulus have been discussed in “Resistance Response to a Stimulus” Section and “Capacitance Response to a Stimulus” Section.
The equivalent circuit model commonly derived from analysis of the Nyquist plot by curve fitting assumes that the circuit elements in the model are independent of the frequency. In fact, both the resistance and capacitance can vary with the frequency, particularly when the frequency is varied over a wide range. A wide frequency range is required for the analysis based on the Nyquist plot. In addition, the circuit model is typically not unique (as the plot is typically not simple in shape), making the values of the circuit elements in the model not very meaningful from the viewpoint of the basic science (as opposed to the empirical science).
Conclusion
Resistance, capacitance and impedance measurements are basic in the study of the electrical behavior of materials of all types, regardless of the level of conductivity or the level of permittivity. They appear to be simple, as performed by using meters, but they are associated with common pitfalls. The main pitfalls and the measurement methodology are covered in this commentary.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chung, D.D.L. Pitfalls and Methods in the Measurement of the Electrical Resistance and Capacitance of Materials. J. Electron. Mater. 50, 6567–6574 (2021). https://doi.org/10.1007/s11664-021-09223-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-021-09223-w