Abstract
High-chromium vanadium–titanium magnetite (HVTM) is an exceptional iron source that has not been mined on a large scale. Since using flux pellets facilitates the reduction in emissions and consumption, a reasonable way to utilize HVTM is by smelting it into flux pellets in a blast furnace. This study elucidates the reducibility and reaction mechanism of HVTM flux pellets. The reducibility, phase composition, and morphology were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy spectroscopy analysis (EDS). The influence of basicity on the reduction reaction mechanism was also analyzed, and 30 pct of conventional iron concentrate was added to optimize the reduction performance of pure HVTM flux pellets. The pellets were treated at 900 °C for 180 minutes in a reducing atmosphere of 30 pct CO+70 pct N2. When the basicity (CaO/SiO2) increased from 0.2 to 1.8, the reduction degree of pure HVTM pellets (100 pct HVTM pellets) increased from 60.3 to 85.3 pct. Meanwhile, the reduction degree of HVTM pellets comprising 30 pct conventional iron concentrate (70 pct HVTM pellets) increased from 63.1 to 89.5 pct. The post-reduction compressive strength of 100 pct HVTM pellets decreased from 965.8 to 223.8 N; the post-reduction compressive strength of 70 pct HVTM pellets first decreased from 1454.6 to 223.0 N and then increased to 630.9 N. Moreover, 70 pct HVTM pellets exhibited better reducibility and higher post-reduction compressive strength and could meet the blast furnace standards when the basicity exceeded 1.6 owing to the low TiO2 content and an appropriate amount of liquid phase. The reaction mechanism of 100 pct HVTM pellets was first controlled by the random nucleation and subsequent growth model as well as the two-dimensional diffusion model. However, after increasing the basicity, the reaction mechanism was only characterized by the random nucleation and subsequent growth model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although China's iron ore reserves rank fourth in the world, most iron concentrates exhibit an average grade of only approximately 35 pct. Owing to the development of grinding technology, certain lean minerals can now be used after multistage grinding and separation. However, ultra-fine ores are not suitable for sintering and are more useful for preparing pellets. In addition, the prominent pollutants and carbon dioxide emissions generated from the sintering process are not conducive to environmental protection. Therefore, it is an effective technique to improve the utilization of iron concentrate resources and, thus, increase the pelletizing capacity of blast furnaces under high pellet operation.
In general, slag-iron separation during blast furnace operation is observed when the basicity of blast furnace slag is 1.0 to 1.2.[1] Owing to the constant basicity of blast furnace slag, improving the basicity of pellets is bound to increase the proportion of pellets in the blast furnace.[2] Several studies on flux pellets[3,4,5,6,7,8,9,10,11] have concluded that a rise in basicity first increases the compressive strength of flux pellets and then decreases it; the degree of reduction gradually increases, and the reduction swelling rate increases first and then decreases.
Vanadium–titanium magnetite is widely distributed in Russia, Indonesia, China, Norway, and other countries.[12] The research on vanadium–titanium magnetite has mainly concentrated on high basicity sinters and acid pellets.[12,13,14,15,16] Nevertheless, the research on flux pellets of vanadium–titanium magnetite is still in its infancy. High-chromium vanadium–titanium magnetite (HVTM), a type of vanadium–titanium magnetite, is more precious because it comprises a higher chromium content. The Panzhihua area houses approximately 3.6 billion tons of HVTM,[17,18] which has not yet been mined on a large scale; thus, it is of great significance to explore the metallurgical properties associated with the flux pellets of HVTM.
Although numerous studies have been conducted on flux pellets, vanadium–titanium magnetite, especially HVTM, has rarely been researched. The presence of titanium in vanadium–titanium magnetite flux pellets renders them more complex, with their reduction mechanism and phase transition rule being different from those of ordinary flux pellets. In our previous studies, we investigated the effect of basicity on the compressive strength, phase evolution behavior, and oxidation induration mechanism of pellets and analyzed the oxidation induration mechanism of HVTM flux pellets.[19]
The lump zone plays a crucial role in the indirect reduction of iron-bearing burden. A more complete indirect reduction reaction reduces the extent of the subsequent direct reduction reaction, which in turn helps in reducing fuel consumption and improving gas utilization efficiency. Therefore, improving the reduction performance at medium temperatures facilitates energy saving, emission reduction, quality improvement, and consumption reduction.[20,21,22,23,24]
In this study, reduction experiments were performed on HVTM pellets with different basicities, and the reducibility, phase transition behavior, reaction mechanism, and model function of HVTM pellets were explored. Based on the conventional blast furnace smelting of vanadium–titanium magnetite, a conventional hematite concentrate was added in HVTM pellets to optimize reduction performances. Overall, this study aims to explore the reduction properties of HVTM pellets and achieve a better basicity scheme, while improving the theoretical basis of blast furnace smelting of HVTM and developing more reasonable burden structures.
Experimental
Materials
The HVTM concentrate used in this study was sourced from Panzhihua, Sichuan Province, China. Meanwhile, a conventional hematite concentrate was obtained from the Panzhihua Steel Company. The main components of these concentrates are shown in Table I. The Cr2O3 content in HVTM exceeded 1 mass pct, and the total iron content in the conventional iron concentrate reached 57.94 pct.
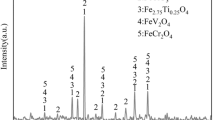
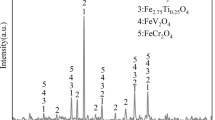
The micro-morphology and energy spectrum analysis of HVTM concentrates are shown in Figure 1. Magnetite and titanomagnetite were disproportionately distributed, while gangue mainly existed in the form of silicate and magnesia-alumina spinel. Figure 2 shows the X-ray diffraction pattern of the HVTM concentrate. The HVTM concentrate comprised magnetite (Fe3O4), ilmenite (FeTiO3), titanomagnetite (Fe2.75Ti0.25O4), chromohercynite (FeCr2O4), and ferrovanadium spinel (FeV2O4). The calcium-containing additive (CaCO3) used to prepare flux pellets was pure (Sinopharms Chemical Reagent Co.). Bentonite was used as the binder for pelletizing, and its main physical properties are shown in Table II. The HVTM and conventional iron concentrates were ground, after which 80 pct of the pellets exhibited a particle diameter of < 74 μm.
Procedures
The experimental process included mixing, pelletizing, drying, oxidation roasting, compressive strength testing, and reducibility testing. The HVTM concentrate, conventional concentrate, bentonite, and CaCO3 were mixed and pelletized according to the batching scheme. The batching plant of HVTM pellets with different basicity (R) and conventional iron concentrate ratios is shown in Table III. The diameter of the disk pelletizer was 2 m, and the rotating speed was 20 r/min. Green pellets with a diameter of 12.5 mm were obtained by pelletizing for 40 minutes with 8.5 mass pct of water. After the green balls were dried at 105 °C for 300 minutes to remove moisture, the roasting experiment was performed in air in a muffle furnace. After preheating at 900°C for 10 minutes and roasting at 1250 °C for 20 minutes, roasted pellets were obtained.
The reducibility test of pellets was performed according to the GB/T 34211-2017 standard: iron concentrate determination of reducibility. A schematic of the reduction device is displayed in Figure 3. The device comprises a heating system, gas supply system, cooling system, and data recording system. The inner diameter of the sealed reactor is 75 mm, and the middle section has a sieve plate to hold the pellet; the crucible bottom has a hole that is 2.5 mm in diameter to let in air. Subsequently, 500 g pellets were placed in the reactor and transferred to the reduction furnace. Then, the thermocouple and electronic balance were loaded and the air tightness of the device was checked. Data were collected using an automatic recording software. After increasing the temperature to 900 °C at a rate of 10 °C/min and maintaining this temperature for 30 minutes, reduction gases (70 pct N2+30 pct CO gas mixtures) were introduced into the furnace at a total gas flow rate of 15 L/min to initiate reduction. After 180 minutes of reduction, the pellets were cooled down in the furnace using a 5 L/min gas flow of N2. The heating profile and atmosphere are shown in Figure 4.
The reaction mechanism provides an understanding of the reduction mechanism and reaction process of HVTM pellets. Moreover, the effect of basicity on the reaction mechanism of HVTM pellets was studied using classical approaches, including the model-fitting method and ln-ln method. The advantage of the model-fitting method lies in the simplicity of the transformation mechanism during the reaction. The ln-ln method is based on the Avrami-Erofeev equation, and the reaction series is determined using linear fitting. Subsequently, the reaction mechanism is ascertained. In this study, both these methods were used to determine the reaction mechanism.
Characterization
The phase compositions of HVTM pellets were identified by X-ray diffraction (XRD). The XRD analysis was carried out by an X’PERT PRO MPD/PW3040 (PANalytical B. V. Corp., Netherlands) using Cu Ka radiation. The detector type was PIXcel 1Dsuper energy detector. All HVTM pellets were ground, after which 100 pct of the pellets exhibited a particle diameter of >74 μm. Meanwhile, the scanning range, scanning time, and scanning speed were 2θ =10 to 90 deg, 10 minutes, and 8 deg/min, respectively. The microscopic observation and analysis of the element distribution in the samples were conducted by scanning electron microscopy (SEM, TESCAN VEGA III) equipped with energy-dispersive spectrometry (EDS) (INCA Energy350). An electronic voltage of 20 kV and a sample current of 20 μA were used for analysis.
The porosity was measured using image processing. By utilizing the Image Pro Plus 6.0 software, pellet porosity was determined by coloring the different gray-level images comprising the phase and pores and calculating the area of different colored regions.[13,25] The microscopic images used to calculate the phase composition were collected from five points in the radial uniform distribution of pellets to eliminate the differences caused by the internal and external pores as well as the pellet compositions. Accordingly, the phase composition was determined by averaging the collected data. It is worth noting that this method leads to 10–15 pct error due to the randomness of the field size and data collection points.
Results and Discussion
Effect of Basicity on Reducibility
The reduction degree (α) was calculated according to Eq. [1]:
where m0 is the weight of pellets before reduction (500 g), mt is the weight at a certain time during reduction (t in minutes), m1 is the initial mass of the pellets, W1 is the mass pct of FeO before reduction, and W2 is the mass pct of TFe before reduction.
The curve of reduction degree with basicity under different schemes is illustrated in Figure 5. Figure 5a shows that after implementing reduction for 180 minutes at 900 °C, the reduction degree of acidic 100 pct HVTM pellets was 60.3 pct. When the basicity reached 1.8, their reduction degree increased to 85.2 pct. Meanwhile, the reduction degree presented a parabolic shape over time; that is, the reaction rate increased first and then decreased. The reduction degree of HVTM pellet with 30 pct conventional iron concentrate (70 pct HVTM pellet) was increased from 63.1 pct to 89.5 pct. It is worth noting that the reducibility of HVTM pellets under the II-0.8–II-1.4 schemes fluctuated. To further clarify the reduction degree of HVTM pellets, the oxidized pellets and post-reduction pellets were ground, after which 100 pct of the pellets exhibited a particle diameter of < 74 μm. The chemical composition of the pellets was also analyzed, and the results are shown in Table IV.
The reducibility of both 100 and 70 pct HVTM pellets rose with increasing basicity. This increase in basicity resulted in the formation of calcium ferrite[19] which directly improved the reducibility of flux pellets.
Table IV shows that there was more TFe in 70 pct HVTM pellets. The reactions of titanium-iron oxide and iron oxide with CO are shown in Eqs. ([2] through [7]). Titanium-iron oxide shows worse reducibility and can only be reduced when it is included in flux pellets with higher basicity, which is consistent with the known thermodynamic principles.
Table IV brings up the critical fact that with increasing basicity, the FeO content of 100 pct HVTM pellets decreased. Meanwhile, the MFe content increased after reduction, indicating that a rise in basicity increased the pellet reduction; consequently, the step-by-step reduction of iron oxides occurred more extensively. The changing trends of FeO and MFe after the reduction of 70 pct HVTM pellets were the same as those of 100 pct HVTM pellets. However, an anomaly was observed with the II-0.8–II-1.4 schemes; that is, there was more FeO content and less MFe content in the post-reduction pellets. This anomaly is generally associated with pellet reduction swelling. Figure 6 shows the macroscopic morphology of 100 and 70 pct HVTM pellets after reduction. There was no noticeable change on the surface of the 100 pct HVTM pellets after reduction; however, cracks were evident under the II-0.8–II-1.4 schemes. Figure 7 shows the change in the porosity of pellets with basicity after reduction. The porosity under the II-0.8~II-1.4 scheme increased abnormally. The presence of 30 pct conventional iron concentrate led to more hematite in 70 pct HVTM pellets. The volume change during hematite reduction has been one of the reasons behind crack formation.[26,27] The reduction swelling index of vanadium–titanium magnetite pellets is generally low,[28,29] while the reduction swelling index of hematite pellets was larger, while that of hematite pellets is higher. The reduction swelling index of hematite pellets tends to increase first and then decrease with a rise in basicity,[8,30,31] which is consistent with the results presented in this paper.
It is advantageous to improve the reduction degree of the iron-bearing burden in the lump zone of the blast furnace since it allows the lower FeO content to be reduced by the burden. Furthermore, the higher the melting point of the initial slag, the higher the softening temperature and the smaller the melting area of the lump zone. An increase in the metallization rate of the concentrate decreased the differential pressure of the soft melt belt, which in turn is conducive to improving breathability.[32] These properties are beneficial to the blast furnace operation.
Effect of Basicity on Post-reduction Compressive Strength
The relationship between basicity and post-reduction compressive strength of 100 pct HVTM pellets is shown in Figure 8. When the basicity increased from 0.2 to 1.8, the post-reduction compressive strength of 100 pct HVTM pellets decreased from 965.8 to 223.8 N after reduction, respectively. The macroscopic cross section of the reduced 100 pct HVTM pellet is shown in Figure 9. The reduction reaction occurred from the outside to the inside with obvious stratification, and the stratification disappeared until the basicity reached 0.8. When the basicity exceeded 1.2, the reduced pellets showed a uniform phase distribution, no prominent bright or dark areas were observed, and the internal structure became looser. The cross-sectional morphology reflected the post-reduction compressive strength of pellets to some extent.
The porosity of 100 pct HVTM pellet increased with basicity.[19] Meanwhile, the reducing gas entered the pellet more effectively, thereby improving the kinetic conditions. Although an increase in porosity alleviated the reduction swelling of HVTM pellets to some extent, the pellet strength decreased after reduction due to a high perovskite content. In general, the amount of perovskite produced gradually increases with basicity.[33,34] The presence of 10 to 11 pct TiO2 in 100 pct HVTM pellets provides favorable conditions for the generation of perovskites in flux pellets. Based on the thermodynamic scale, perovskite is bound to generate more easily than calcium ferrate.[33,35,36] The ΔGf value of CaO·TiO2 and CaO·Fe2O3 was -21.617 kJ/mol and -9.431 kJ/mol at 1250 °C, respectively. Furthermore, perovskite exhibits poor bonding strength[37] and reducibility,[38] which adversely affected the post-reduction compressive strength of flux pellets. An excessively low post-reduction compressive strength leads to the crushing of pellets in the lump zone, which reduces the permeability of the lump zone, reduces the softening temperature of the charge, and worsens the melting property of the charge. In general, the post-reduction compressive strength of a pellet does not exceed 300 N, which is insufficient to meet the blast furnace requirements.[39] Therefore, the basicity of 100 pct HVTM pellet should be at most 1.0.
The post-reduction compressive strength of 70 pct HVTM pellets first decreased from 1454.6 to 223.0 N and then increased to 630.9 N with basicity. The post-reduction compressive strength of 70 pct HVTM pellets was greater than that of 100 pct HVTM pellets under each basicity scheme. The main reasons behind this result were the low perovskite amount and the excessive CaO content in 70 pct HVTM pellets. Table V lists the theoretical TiO2 and CaO contents of 100 pct HVTM pellets and 70 pct HVTM pellets after oxidation induration. Owing to the presence of 30 pct conventional iron concentrate, the total TiO2content in 70 pct HVTM pellet was lower. This directly reduced the amount of perovskite produced, which in turn increased the post-reduction compressive strength of 70 pct HVTM pellets.
When the basicity exceeded 1.4, the compressive strength increased gradually, and the compressive strength of 70 pct HVTM flux pellets at a basicity of 1.6 and 1.8 met the blast furnace standard. TiO2 reacted completely with CaO; that is, when the ratio of n(TiO2)/n(CaO) exceeded 1, TiO2 was consumed under the ideal circumstances. Thus, the CaO content became excessive at a basicity of 1.0 and 1.4 for 70 pct HVTM pellets and 100 pct HVTM pellets, respectively. Excess CaO reacted with Fe2O3 to produce calcium ferrate, which increased the liquid phase content and reducibility of the pellets. Based on the phase diagram of CaO-Fe2O3-TiO2 in air (Figure 10), the liquid phase of 70 pct HVTM pellets appeared earlier and the melting point of this system decreased with increasing basicity.[40] An increase in the liquid phase facilitated its bonding with other solid phases in the pellet. Consequently, the reducibility improved, and the generated iron content enabled the pellet to resist deformation.
Owing to the formation of excessive amount of liquid phase,[1,5,41] the compressive strength of the pellets decreased significantly, thus, not allowing the basicity of conventional hematite concentrate pellets to exceed 1.4.[42] The combination of HVTM and conventional hematite concentrate facilitates the liquid phase development,[13] while meeting the blast furnace standards at a basicity exceeding 1.6. Currently, the basicity of the basicity sinter is 1.8–1.9; thus, 70 pct HVTM flux pellets meet the blast furnace standards for reduction and increase the proportion of flux pellets. Performance optimization was achieved in 70 pct HVTM flux pellets under this scheme at a basicity exceeding 1.6.
Effect of Basicity on Phase and Morphology of Post-reduction Pellets
XRD patterns and phase changes of 100 pct HVTM pellets and 70 pct HVTM pellets at different basicities after reduction are displayed in Figure 11. Figure 11(a) shows that the main phases in the reduced 100 pct HVTM pellets are Fe, FeO, Fe2.5Ti0.5O4,and FeTiO3 at natural basicity. When the basicity was increased to 0.4, FeTiO3 disappeared; when it increased to 0.8, perovskite appeared. The peak of Fe2.5Ti0.5O4 disappeared when the basicity increased to 1.6. With increasing basicity, the characteristic peaks of metallic iron increased gradually.
Figure 11(b) shows the main phases of 70 pct HVTM pellets, which are similar to those of 100 pct HVTM pellets. However, there were subtle differences under the II-0.8–II-1.4 schemes. A diffraction peak of the new phase (Fe0.925O) appeared in the area represented by a red rectangle in Figure 11(b). Fe0.925O is an iron oxide, which generally appears as a reduction transition product. The new physical phase that appeared under the II-0.8–II-1.4 schemes verified the presence of a high FeO content. Meanwhile, the cracks caused due to undesirable reduction swelling helped the reducing gas enter the pellet interior, which improved the indirect reduction degree of the pellet.
The micro-morphologies of the HVTM pellets after reduction at different basicities are shown in Figure 12. Figure 11(a) shows that the main phases in the acid pellet after reduction were silicate, iron-titanium oxide (FexTi(3−x)O5), and iron/iron oxide. When the basicity was 0.8 [Figure 12(b)], iron phase coexisted with slag and iron-titanium oxide disappeared gradually. The composition and morphology of the slag changed, showing two kinds of slag phases with high and low calcium contents. According to atomic ratio analysis, perovskite and ferrous oxide were the main components in the high calcium slag phase. Magnesium aluminum silicate was the main component of the low calcium slag phase. When the basicity was 1.8 [Figure 12(c)], the calcium content in the slag had converged, and perovskite dispersed in the slag phase as a separate phase. Meanwhile, the iron-titanium oxide ratio further reduced, with a small amount of Ti entering the reduced iron. The morphology and EDS analysis of 70 pct HVTM pellets [Figures 12(d) through (f)] were similar to those of 100 pct HVTM pellets. The Ti content derived during the EDS analysis was low due to the presence of 30 pct conventional iron concentrate. Moreover, the presence of cracks proved the occurrence of undesirable reduction swelling [Figure 11(e)]. The map-scanning images of 100 pct HVTM pellets after reduction under the I-1.8 scheme are shown in Figure 13.
Effect of Basicity on Reduction Mechanism
Owing to reduced swelling, cracks appeared in the 70 pct HVTM pellets under certain basicity schemes (II-0.8–II-1.4). These cracks altered the original reducing mechanism. Therefore, the discussion regarding the reaction mechanism in this section is only applicable to 100 pct HVTM pellets.
Model-fitting method
The model-fitting method represents a dynamic mechanism analysis, which involves drawing a curve between t/t0.5 and the chemical kinetics mechanism function g (α), thereby comparing the analytical curve with the theoretical curve of the known mechanism function. If the analytical curve coincides well with the theoretical curve, the chemical kinetics mechanism is considered to be the same as the theoretical one.
According to Sharp,[43]
where α is reduction degree, t is time, t0.5 is time when α is 0.5, G(α)α=0.5 is the α=0.5 model function, and G(α)α=1.0 is the α=1.0 model function.
The reduced chemical kinetics model can be divided into unreacted nucleus model, diffusion model, and reaction series model. More than 40 kinetic model functions have been found thus far, and nine common ones are listed in Table VI.[44,45,46,47]
The fitted and theoretical curves are plotted in Figure 14. When the basicity of pellets is 0.2 to 1.2 and the reduction degree is less than 0.5, the analysis curve fits well with the F1 model. When the reduction degree is greater than 0.5, the analysis curve is between F1 and D2 models, which shows that the first half of the reduction reaction is a random nucleation and subsequent growth model (F1); the latter half is controlled by a random nucleation and subsequent growth model (F1) as well as a two-dimensional diffusion model (D2). Figure 13c shows that with increasing basicity, the analysis curve of the reduction reaction approached the model of random nucleation and subsequent growth until the basicity reached 1.6, and the analysis curve fitted the F1 curve; that is, the entire reaction was controlled by the random nucleation and subsequent growth model.
ln–ln method
Ln-ln method is based on the Avrami-Erofeev equation:
where α is the reduction degree, K is the rate constant, n is the Avrami index, and t is time. By taking logarithms on both sides of Eq. [3], the following expression is obtained:E
The slope n can be obtained by linear fitting ln[− ln(1 − α)] and lnt. Since the reduction degree associated with each basicity scheme increased first and then decreased, the derivative of reduction degree curve can be obtained [Figure 15(a)]. The reduction degree increased gradually with increasing basicity. The reduction degree increased rapidly at the beginning of the reaction, and slowly decreased to approximately 0 after reaching its peak value. Therefore, the reduction reaction exhibits two stages due to a change in the reduction degree. The reduction degree of the first stage, which is shown in Figure 15(b), does not exceed 20 pct under each basicity scheme; it is considered that the reduction degree of each basicity scheme under 900°C is controlled by the second stage.
The linear fitting between ln[− ln(1 − α)] and lnt is shown in Figure 16, and the fitting parameters of the fitting line are shown in Table VI. The second stage (n) of each basicity scheme was close to 1, the N values were between the F1 model (n = 1) and the D2 model (n = 0.57), and slowly decreased to approximately 0 after reaching its peak value. Therefore, the reduction reaction exhibits two stages due to a change in the reduction degree. The reduction degree of the first stage, which is shown in Figure 15(b), does not exceed 20 pct under each basicity scheme; it is considered that the reduction degree of each basicity scheme under 900°C is controlled by the second stage and slowly decreased to approximately 0 after reaching its peak value. Therefore, the reduction reaction exhibits two stages due to a change in the reduction degree. The reduction degree of the first stage, which is shown in Figure 15(b), does not exceed 20 pct under each basicity scheme; it is considered that the reduction degree of each basicity scheme under 900°C is controlled by the second stage.
These results show that the two reaction mechanism analyses obtained similar conclusions. That is, the reaction mechanism of each basicity scheme was mainly controlled by the random nucleation and subsequent growth model, and the two-dimensional diffusion model and the random nucleation and subsequent growth model coexist at a basicity of less than 1.2 (Table VII).
The reaction mechanism transformation of 100 pct HVTM pellets can be described as follows:
The reducing gas first contacted the outer layer of the pellet, and the reducing reaction occurred from outside to inside. After the initial reaction of random nucleation and subsequent growth, the product layer was coated on the outer pellet layer, and the inner layer reaction was not as complete as the outer layer reaction; the reduction reaction was affected by diffusion resistance after random nucleation. With an increase in basicity, the porosity increased gradually, the diffusion of reducing gas occurred easily, and calcium ferrite-based materials began to appear at high basicities. The chemical kinetics conditions associated with reactivity and reduction in the pellet gradually improved, the thickness of the outer product layer increased until no obvious outer product layer appeared, and the reduction reaction occurred more uniformly in the radial direction of the pellet. Overall, the chemical kinetics mechanism of the entire pellet was random nucleation and subsequent growth, and the reduction performance gradually improved.
Conclusion
-
1.
When the basicity was increased from 0.2 to 1.8 by heating at 900 °C for 180 minutes, the reduction degree of 100 pct HVTM pellets increased from 60.3 to 85.3 pct, respectively; meanwhile, the reduction degree of 70 pct HVTM pellets increased from 63.1 to 89.5 pct, respectively. The reduction degree of 70 pct HVTM pellets, which fluctuated due to undesirable swelling and increased with basicity, was higher than that of 100 pct HVTM pellets.
-
2.
When the basicity was increased from 0.2 to 1.8, the post-reduction compressive strength of 100 pct HVTM pellets decreased from 965.8 N to 223.8 N, respectively; meanwhile, the post-reduction compressive strength of 70 pct HVTM pellets first decreased from 1454.6 N to 223.0 N and then increased to 630.9 N. Thus, the 70 pct HVTM flux pellet had a better post-reduction strength.
-
3.
The basicity of 100 pct HVTM pellets should be at most 1.0. The basicity of 70 pct HVTM flux pellets met the blast furnace standards when it was higher than 1.6. The reducibility of 70 pct HVTM pellets was optimized because of the low TiO2 content and an appropriate amount of liquid phase.
-
4.
The reduction mechanism of 100 pct HVTM pellets at a basicity of 0.2 to 1.2 was controlled by both the random nucleation and subsequent growth model and the two-dimensional diffusion model. When basicity increased, the reduction mechanism shifted to only the random nucleation and subsequent growth model. At a basicity of 1.8, random nucleation and subsequent growth became the reduction mechanism. This result has been verified using two mechanism analyses, phase analysis, and morphology analysis.
-
5.
As a burden, HVTM flux pellets effectively enhance the blast furnace process and reduce CO2 emissions. The study on the reducibility and kinetics helps clarify the basicity range of HVTM flux pellets and to understand the reduction behavior of pellets in blast furnaces, which lays a theoretical foundation for the efficient utilization of HVTM.
References
X. Fan, M. Gan, T. Jiang, L. Yuan, and X. Chen: J. Cent. South Univ. Sci. Technol., 2010, vol. 17, pp. 732–37.
K. Bai, L. Liu, Y. Pan, H. Zuo, J. Wang and Q. Xue: Ironmak. Steelmak., 2021, vol. 48, pp. 1048–1063.
Q. Gao, X. Jiang, H. Zheng, and F. Shen: Minerals, 2018, vol. 8, pp. 389–407.
M.K. Mohanty, S. Mishra, B. Mishra, and S. Sarkar: Arab. J. Sci. Eng., 2018, vol. 43, pp. 5989–98.
R.K. Dishwar, A.K. Mandal, and O.P. Sinha: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 617–21.
K.-K. Bai, H.-B. Zuo, W.-G. Liu, J.-S. Wang, J.-S. Chen, and Q.-G. Xue: J. Iron Steel Res. Int., 2021, vol. 29, pp. 1185–93.
P. Prusti, K. Barik, N. Dash, S.K. Biswal, and B.C. Meikap: Powder Technol., 2021, vol. 379, pp. 154–64.
Y.-B. Zhang, X.-J. Chen, Z.-J. Su, S. Liu, F. Chen, N.-Y. Wu, and T. Jiang: J. Iron Steel Res. Int., 2021, vol. 29, pp. 1381–92.
A.R. Firth and J.F. Garden: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 524–33.
J. Li, H.-F. An, W.-X. Liu, A.-M. Yang, and M.-S. Chu: J. Iron Steel Res. Int., 2019, vol. 27, pp. 239–47.
F.R. Silva, L.R. Lemos, P. de Freitas Nogueira, and M. Bressan: Metall. Mater. Trans. B, 2020, vol. 52B, pp. 69–76.
L. Zhang, S. Yang, W. Tang, H. Yang and X. Xue: Ironmak. Steelmak., 2019, vol. 47, pp. 821–827.
Z. Gao, G. Cheng, X. Xue, H. Yang, and P. Duan: Steel Res. Int., 2018, vol. 89, p. 1700543.
Y.L. Sui, Y.F. Guo, T. Jiang, and G.Z. Qiu: Ironmak. Steelmak., 2016, vol. 44, pp. 185–92.
W. Zhao, M. Chu, Z. Liu, H. Wang, J. Tang, and Z. Ying: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 1878–95.
W. Tang, S. Yang, G. Cheng, Z. Gao, H. Yang, and X. Xue: Minerals, 2018, vol. 8, pp. 263–77.
W. Li, G. Fu, M. Chu, and M. Zhu: Powder Technol., 2020, vol. 360, pp. 555–61.
G. Cheng, X. Zhang, Z. Gao, H. Yang and X. Xue: Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2020, vol. pp. 1-18.
B. Chen, J. Wen, T. Jiang, L. Li, G. Yang, and T. Zhao: Metall. Mater. Trans. B, 2022, vol. 53B, pp. 178–89.
C.H. Borgert, L.R. Neto, F.F. Grillo, J.R. de Oliveira, J.L. Coleti, J.A.S. Tenório, D.C.R. Espinosa, T.E.A. Frizon, M.V.G. Zimmermann, and E. Junca: Jom, 2022, vol. 74, pp. 439–7.
S. Prakash, M.C. Goswami, A.K.S. Mahapatra, K.C. Ghosh, S.K. Das, A.N. Sinha, and K.K. Mishra: Ironmak. Steelmak., 2013, vol. 27, pp. 194–201.
R.K. Dishwar and O.P. Sinha: Fuel, 2021, vol. 296, p. 120640.
X. Gao, J. Wang, W. Lv, J. Xiang, and X. Lv: Can. Metall. Q., 2018, vol. 58, pp. 177–86.
A. Hammam, Y. Li, H. Nie, L. Zan, W. Ding, Y. Ge, M. Li, M. Omran, and Y. Yu: Min. Metall. Explor., 2020, vol. 38, pp. 81–93.
B. Chen, J. Wen, T. Jiang, L. Li, G. Yang, and T. Zhao: Metall. Mater. Trans. B, 2021, vol. 53B, pp. 178–89.
K. Bai, H. Zuo, B. Lv, J. Wang, Y. Pan, and Q. Xue: Jom, 2022, vol. 74, pp. 2010–18.
Y. Fan, Y. Zhang, Z. Li, Y. Chai, Y. Wang, G. Luo and S. An: Ironmak. Steelmak., 2021, vol. 48, pp. 1158–1168.
W. Li, G. Fu, M. Chu, and M. Zhu: Powder Technol., 2019, vol. 343, pp. 194–203.
W. Li, G.-Q. Fu, M.-S. Chu, and M.-Y. Zhu: Int. J. Hydrogen Energy, 2017, vol. 42, pp. 24667–674.
G.-C. Zhang, G.-P. Luo, P.-F. Jia, Y.-C. Wang, and Y.-F. Chai: High Temp. Mater. Processes (Lond.), 2021, vol. 40, pp. 193–203.
Z. Yang, Z. Liu, M. Chu, L. Gao, C. Feng, and J. Tang: ISIJ Int., 2021, vol. 61, pp. 1431–38.
K. Higuchi, S. Matsuzaki, K. Saito, and S. Nomura: ISIJ Int., 2020, vol. 60, pp. 2218–7.
M. Yang, J. Xiang, C. Bai, X. Zhou, Z. Liu, and X. Lv: Metall. Mater. Trans. B, 2021, vol. 52B, pp. 1436–49.
W.-D. Tang, S.-T. Yang, L.-H. Zhang, Z. Huang, H. Yang, and X.-X. Xue: J. Cent. South Univ., 2019, vol. 26, pp. 132–45.
S. Xuan, X. Lv, C. Ding, K. Tang, G. Li, G. Pei, and S. Wu: Steel Res. Int., 2018, vol. 89. pp. 1700452–1700460.
C. Ding, X. Lv, Y. Chen, G. Li, W. He, and X. Lv: J. Alloy Compds., 2019, vol. 789, pp. 537–46.
W.-D. Tang, X.-X. Xue, S.-T. Yang, L.-H. Zhang, and Z. Huang: Int. J. Miner. Met. Mater., 2018, vol. 25, pp. 871–0.
M. Zhou, S. Yang, T. Jiang, and X. Xue: Jom, 2015, vol. 67, pp. 1203–3.
G. Qing, K. Wu, Y. Tian, G. An, X. Yuan, D. Xu, and W. Huang: Ironmak. Steelmak., 2016, vol. 45, pp. 83–9.
S. Kimura and A. Muan: Am. Mineral., 1971, vol. 56, pp. 1332–46.
S. Dwarapudi, T.K. Ghosh, A. Shankar, V. Tathavadkar, D. Bhattacharjee, and R. Venugopal: Int. J. Miner. Process., 2011, vol. 99, pp. 43–53.
T.Y. Malysheva, Y.S. Yusfin, and S.V. Plotnikov: Steel Transl., 2011, vol. 41, pp. 705–07.
J.H. Sharp, G.W. Brindley, and B.N.N. Achar: J. Am. Ceram. Soc., 1966, vol. 49, pp. 379–82.
W. Lv, X. Lv, Y. Zhang, S. Li, K. Tang, and B. Song: Powder Technol., 2017, vol. 320, pp. 239–48.
M.B. Gillot, E. Guendouzi, and M. Kharroubi: Mater. Chem. Phys., 1989, vol. 24, pp. 199–208.
M. Mozammel, S.K. Sadrnezhaad, A. Khoshnevisan, and H. Youzbashizadeh: J. Therm. Anal. Calorim., 2012, vol. 112, pp. 781–9.
A. Khawam and D.R. Flanagan: J. Phys. Chem. B, 2006, vol. 110, pp. 17315–328.
Acknowledgments
This research was financially supported by the Programs of the National Natural Science Foundation of China (Nos. 52174277 and 52204309).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, B., Jiang, T., Wen, J. et al. Reducibility Optimization and Reaction Mechanism of High-Chromium Vanadium–Titanium Magnetite Flux Pellets. Metall Mater Trans B 54, 2503–2518 (2023). https://doi.org/10.1007/s11663-023-02851-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-023-02851-z