Abstract
Bloating of metal droplets in the BOF is important in developing fundamental models of the process. Bloating is controlled by the decarburization behavior of individual metal droplets in oxidizing slag. In this paper, a detailed investigation has been performed to elucidate the mechanism and rate controlling steps for decarburization of droplets over a range of droplet carbon concentration, droplet mass, slag basicity and slag FeO concentration. Four different carbon levels and three droplet masses were tested. Droplets typically exhibited three stages of decarburization: incubation period, where decarburization was mostly on the outer surface of the droplet, followed by a steady state reaction period where most decarburization was internal, and finally the end decarburization period. The rate of decarburization was found to be a mixed controlled process; the contributions of resistance from slag transport, interfacial chemical reaction, internal nucleation and growth of bubbels varied based on slag and metal chemistry. A mixed controlled kinetic model has been developed for steady state decarburization period and a partial validation of the mixed controlled kinetic model has been presented here. By varying slag FeO concentration and basicity for droplets containing 2.5 wt pct C, mixed controlled kinetics of decarburization reaction were explored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Decarburization kinetics play an important role in oxygen steelmaking. Decades of experimental research and phenomenological modeling have been conducted to understand the mechanism behind each of the stages of decarburization.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] Mainly four types of experimental investigation have been performed in this field—gas-metal bath, slag over metal bath, metal droplet reacting in slag and levitated metal droplet reacting in oxidizing gas atmosphere.
Philbrook and Kirkbride[1] conducted studies with a carbon saturated metal bath and a layer of slag. In their work the rate of reduction of FeO was found to be second order with respect to FeO concentration whereas Dancy[2] found it to be first order with respect to FeO under similar circumstances. Sarma[13] also found the rate of reduction of FeO by carbonaceous material to be limited by mass transport of FeO in the slag phase where rate of CO generation was \(\propto {\left(\text{wt pct} {\text{FeO}}\right)}^{1.67}.\) This was justified by the combined effect of FeO concentration on driving force and on the mass transfer coefficient; mass transfer coefficient, km \({\propto \left(\text{wt pct} {\text{FeO}}\right)}^{0.67}\) due to stirring from CO gas bubbles. Ito and Sano performed an experimental study on the rate of decarburization of a liquid metal bath reacting with oxidizing gas such as H2O-Ar[24,25] and CO2-Ar.[26,27] These authors proposed that initially oxygen transfer through a gaseous boundary layer controlled the rate and when the oxygen content in a layer near the top of metal bath increased to the saturation value, an oxide layer formed, transport of oxygen through which controlled the rate of decarburization. In another study by the same group of researchers, oxide forming elements such as Si, Cr and Mn were found to influence the end stage of decarburization, where the rate of decarburization decayed due to oxide layer formation. There have been several studies[4,5,28,30,31,32,33,34,35] performed using levitated droplets reacting with oxidizing gas and most of the workers found gas phase mass transport controlled decarburization kinetics above some critical concentration and followed by metal phase mass transfer control. It was observed in most of these cases that the droplet exploded after reacting for certain time, and some workers[28,31,34] observed that an outer oxide layer formed on the metal droplet and this layer preceded the explosion. In metal droplet/slag type decarburization studies,[7,8,9,10,11,19,36] most workers found carbon containing metal droplets to swell due to internal decarburization. Mulholland et al.[19] first visualized the metal droplet decarburization in slag by X-ray Fluoroscopy and confirmed the formation of CO bubbles within the droplet at the later stages of decarburization. Later Molloseau and Fruehan[10] also observed the swelling (termed “emulsification”) of droplet in oxidizing slag. The term ‘bloating’ was first introduced by Brooks et al.[14,37] when these workers recognized it’s importance in predicting the residence time of a droplet while decarburizing in BOF. Additionally the rate of decarburization was reported to be, under mixed control by Min and Fruehan,[8] slag mass transport control by Molloseau and Fruehan,[10] gas film diffusion and slag transport control above 2 to 3 wt pct C and electrochemical transport controlled below that level by Murthy et al.[9]
Gaye and Riboud[7] proposed decarburization to be interfacial reaction controlled initially and afterwards transport controlled whereas based on the linear relationship between rate and metal volume Chen and Coley[11,38] proposed it to be controlled by internal nucleation of CO. There have been several studies conducted by the group of Pal[9,36,39,40,41] regarding the end stage of decarburization. They have suggested that the reaction between FeO in slag and solute C in a metal bath is electrochemical in nature and proposed a rate enhancement mechanism on applying a DC potential across the slag layer. Apart from lab scale research, there have been reports from pilot scale converter trials from IMPHOS[42] project. Metal droplets, which were collected from emulsion with diameter of the order ~ 100 µm, dropped to very low concentrations for Si, Mn, P, V whereas C concentration remained above 1 wt pct due to kinetic limitation. Analysis of the slag metal samples collected by Holappa[43] and Cicutti[44] from an industrial scale converter shows that the droplets collected from the emulsion always have a carbon concentration higher than that predicted by thermodynamic equilibrium, although significantly less than that in the bath. Cicutti proposed partial decarburization to be due to short residence time of droplets, however Cicutti’s work predates the proposal of the bloated droplet concept which would lead to longer residence times for as long as internal decarburization was supported. This shows that although decarburization in the emulsion of a BOF is much higher compared to metal bath, the droplets do not reach thermodynamic equilibrium. Apart from liquid droplet decarburization studies, a recent study[45] to understand the reaction kinetics of direct reduced iron(DRI) pellets in oxidizing slag shows it to be a two stage process: initial stage controlled by heat transfer from slag to pellet and later stage controlled by FeO transport in the slag. There have been several attempts[15,18,20,21,22] to model the decarburization kinetics to predict the contribution of refining from emulsion in BOF and these are showing a much higher contribution (as high as above 70 pct) whereas Cicutti predicted the contribution to be 20-50 pct.
Despite the extensive research performed to-date, there is lack of clarity about the rate controlling mechanism at different stages of decarburization reaction. A sudden stop in decarburization has been observed by many researchers studying decarburization of droplets reacting in slag, but limited work has been done to understand the end stage of the decarburization process in details. In the present work, an attempt has been made to develop a mixed control kinetic model including slag transport, slag/metal interfacial reaction kinetics and internal nucleation and growth of bubbles for the steady state decarburization period when the decarburization is mostly internal. Additionally, an analysis have been performed on the end point carbon concentration on varying initial slag and metal chemistry to explore the effect of these parameters on shutting down the decarburization process.
Experimental Procedure
Experimental Setup
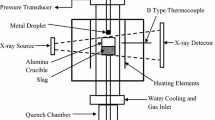
A resistance heated vertical tube furnace with 80 mm inner diameter alumina tube was used for all experiments. An X-ray imaging system was installed with the furnace to observe the bloating and other changes of droplets throughout the progress of the reaction. The X-ray video was recorded in the computer via One Touch Grabber Software. A differential pressure transducer with a maximum pressure limit of 13.8 kPa (FLW Southeast, Inc, 157C-W050NR, Very Low Pressure Transducer) with sensitivity of \(3\times {10}^{-5}\) atm was connected with the sealed reaction chamber to measure the change in pressure due to gas evolution from the decarburization reaction. The data was recorded by a computer software (RS232 Interface) at a selected frequency i.e. 10 Hz in this study. The pressure transducer data was converted to the number of moles of CO produced assuming all the pressure change is due to CO gas. The pressure change was calibrated after each experiment by injecting a fixed volume of air into the sealed furnace.
Sample Preparation
Metal droplets were prepared by mixing electrolytic iron of 99.99 pct purity, graphite and Fe-S alloy and heating to 1550 °C in a vertical tube furnace under an inert Argon atmosphere and kept for 1 hour for homogenizing and then sampling was done by pipetting through quartz tube and quenching in water. The resulting cylindrical metal rods were then polished and cut into sections of nominal mass 1.0,1.5 and 2.0 g sections. For use in calculations, the actual mass of each section was measured to within ± 0.0005 g. The carbon and sulfur concentration of these sections from each batch was measured with LECO carbon sulfur analyzer to make sure the desired composition was achieved. These individual cylindrical sections from each batch were remelted in an Electric Arc Melter to further homogenize and produce droplets. The composition of the droplets were further confirmed by testing samples from each batch with LECO analysers. The oxygen concentration of the samples was measured to be around 50 ± 30ppm. To prepare the slag, a proportionate mixture of calcium oxide, silica and alumina powders were premelted in a platinum crucible at 1550 °C and homogenized for 1 hour and then quenched on top of a steel slab in air. The premelted slag was then crushed and mixed with FeO powder to prepare a batch of slag weighing 25.0 ± 0.5 g for each experiment. The composition of post reacted slag was analyzed using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). The metal and slag chemistry employed in this study are listed in Tables I and II. The first set of experiments were carried out with four different carbon levels and three different sizes of droplets. Then other set of experiments were performed by varying the slag FeO concentration, slag basicity.
Experimental Procedure
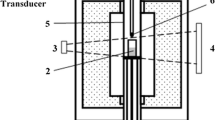
A schematic of the experimental set up is shown in Figure 1. A metal droplet (A) of specific size was held at the end of an alumina tube using a magnet (H). A 99.99 pct pure alumina crucible (C) with outer diameter of 40mm, containing 25 g of slag (B) was inserted from the bottom of the furnace and placed in the hot zone of the furnace using a support rod (E). The furnace was sealed and evacuated to 700 millitorr (i.e. 93.33 Pa). The furnace was then backfilled with high purity argon. After backfilling the outlet valve on the furnace was opened to allow argon to flow continuously. The argon was passed through a gas purifying system to absorb moisture and oxygen. The argon flow continued till the target temperature was attained. At the target temperature, the flow was interrupted, and the valves connected to furnace were closed to make a sealed chamber. The droplet was then allowed to fall by removing the magnet. The droplet could not pass through the hole at the bottom of the delivery tube until it melted, at which point it dropped into the slag and the progress of the decarburization reaction was recorded by capturing the pressure change from a pressure transducer(M). X-ray video was recorded of the droplet from the time where the magnet was removed until the end of the experiment. The melting time of the droplet before releasing the magnet was determined from X-ray Video and is presented in Table III for the cases of varying carbon cases and for varying droplet mass.
The melting time varied with the size of the droplet as well as with droplet carbon concentration. For droplets with the same carbon concentration smaller droplets needed less time to melt, whilst lower carbon concentration droplets needed a longer melting time due to their higher melting point. The time at which the melted droplet fell into the slag was defined as time zero for kinetic analysis. For initial experiments two calibration methods were used to determine the volume of gas generated during the experiment. In one method the pressure data was calibrated by comparing the difference between the final and initial carbon content of the droplet with the constant volume pressure increase. In another technique, 10ml, 20ml and 30ml of air were injected into the sealed furnace and the pressure change recorded. This data was used to calibrate the pressure change against the known gas addition. The calibration was then used to calculate the volume of CO generated during the decarburization reaction. There was no significant (±10 pct) difference between these two methods in calculating the number of moles of CO generated during the reaction. Therefore, the method of calibration using the carbon concentration measurement, before and after was employed for all datasets. The total CO generation data which was calculated from the pressure data was then filtered in MATLAB to reduce the noise introduced during data collection and presented in the following sections.
Experimental Results
The Effect of Cabon Concentration and Mass on Decarburization of Droplets
The kinetics of the decarburization reaction was investigated by varying the droplet initial carbon concentration and the droplet mass. Droplets containing 0.01 wt pct S and carbon concentrations between 0.5 and 4.4 wt pct were reacted with slag having 16 wt pct FeO and basicity (\({V}_{\text{ratio}} =\frac{CaO}{Si{O}_{2}}\)) of 0.91 at a temperature of 1580 °C.
The cumulative CO generation profiles for 1, 1.5 and 2 g droplets with 4.4, 2.5, 1.5 and 0.5 wt pct C are presented respectively in Figures 2(a) through (d). The bloating behavior of droplets having different masses is also reported here in Figures 2(e) through (h) which show as a function of time, the volume of CO retained in the droplets normalized with respect to original droplet volume. These data have been extracted at one second intervals from the recorded X-ray videos. The measure droplet area from the 2-D image was converted to volume by calibrating the measured area at reaction time zero with the initial liquid droplet volume.[46] Finally, the normalized retained CO gas volume was calculated according to Eq. [1].
where V(t) is the volume of the bloated droplet at time t, and V(0) is the original volume of the droplet. All curves of CO gas generation exhibit a similar decarburization behavior. Some droplets showed an initial slow decarburization rate which increased to a relatively constant rate, referred to as peak decarburization rate, which eventually decayed and finally stopped altogether. In most of the cases, the initial slow period, termed the incubation period, was absent but the data showed an approximately steady-state rate followed by a characteristic slowing and stopping. It can be observed from the CO generation profile at 4.4 wt pct C that the peak rate of decarburization increased with increasing droplet size from 1 g to 2 g. Here, the peak decarburization rate was determined from the slope of each curve in the period of faster, relatively constant rate. Based on the curves drawn in Figure 2 the 1.5 g droplet showed a higher peak rate compared to 1 and 2 g droplets, however this depends very much on exactly where the incubation time ends. Given the uncertainty over this choice, and the observation of other workers that CO generation rate scales with droplet mass, it is probably fair to say thast the current results are not sufficiently precise to dispute that finding. The normalized retained volume also demonstrated that with increasing droplet size the maximum volume of gas retained within the droplet increased from 2, to 3.5 to 4 times of the original volume respectively for 1, 1.5 and 2 g droplet. It is to be noted that 1 and 2 g droplets showed ~ 6 seconds of incubation period whereas the incubation period was shorter, ~2 seconds, for 1.5 g droplet. As found in previous research in the authors’ laboratory, during the reaction the slag separated into two layers; a foamy layer lying over a dense layer. The droplets were observed in the X-ray image to bloat and rise up into the foamy slag, shrink then sink back into the dense slag. The entire sequence of behavior is indicated by individual peaks in the normalized retained volume plot and was found to occur 7 times in the case of 1g droplet and 2 to 3 times for 1.5 and 2 g droplets respectively. The droplets in some cases were observed to remain bloated and sit between the dense and foamy slag.
Figure 2(b) presents the decarburization behavior of droplets containing 2.5 wt pct C. It shows that the peak rate of decarburization increased significantly with increase in droplet size from 1.5 to 2 g but the increase in peak rate was not significant with an increase in droplet size from 1 to 1.5 g. The extent and rate of bloating was found to be similar for droplets of different sizes based on Figure 2(f). The rebloating phenomena usually did not occur in the case of droplets with 2.5 wt pct carbon, for all three droplet sizes presented here. Furthermore, the decarburization behavior did not show an incubation period according to Figures 2(b) and (f).
For the case of droplets with 1.5 wt pct C of 1.5 and 1 g, Figures 2(c) and (g) show that the peak decarburization rate increased with increasing droplet size and the maximum amount of retained gas within droplet increased from 1.5 to 2 times the original volume as the droplet mass increased from 1 g to 1.5 g. Compared to the case of the 1.0 g droplet, the 1.5 g droplet remained bloated for a longer time period as shown in Figure 2(g). No rebloating was observed for droplets containing 1.5 wt pct C.
The decarburization behavior for droplets with 0.5 wt pct C at three different masses is presented in Figures 2(d) and (h). The peak decarburization rate was found to increase with increasing droplet size and the droplets also showed bloating at this low level of carbon concentration. The extent of bloating was found to be smallest in the case of the 1g droplet and increased with increasing droplet mass, which is consistent with all other droplets studied here regardless of carbon concentration.
The Effect of Slag FeO Concentration on Decarburization of Droplets
Another set of experiments were performed at 1580 °C, to observe the decarburization behavior of 2 g droplets with 2.5 wt pct C and 0.01 wt pct S over a range of oxygen potential of the slag as defined by the wt pct FeO in the slag. Experiments were conducted with 2.5, 5, 10 and 16 wt pct FeO in the slag, while keeping the V-ratio constant at 0.91. The CO generation and the bloating behavior under these conditions are presented in Figures 3(a) and (b). These figures show that the peak rate of decarburization increased with increasing FeO concentration in the slag which is consistent with findings in the literature.[10] The droplets were found to bloat in all cases except that with 2.5 wt pct FeO. The maximum gas volume within bloated droplets increased from 5 wt pct FeO (3 times) to 10 wt pct FeO (4.8 times) followed by a decrease to 3.5 times at 16 wt pct FeO. The CO generation rate for droplets reacting with slag containing 2.5 wt pct FeO was found to be linear with respect to time, which along with the observation that those droplets did not bloat indicates that they are most likely experiencing external decarburization and that the rate is most likely controlled by mass transport of oxygen in the slag or by chemical reaction at the slag metal interface. The transport of carbon towards slag/metal interface seems to be fast enough compared to the transport of oxygen towards the slag/metal interface from bulk slag in 2.5 wt pct FeO slag, otherwise there would have been oxygen transport into the bulk metal leading to bloating. Decarburization for droplets ceased, in order of increasing FeO concentration, at 1.79, 1.67, 1.43 and 1.1 wt pct C, whereas the thermodynamic estimation from FactSage suggests that carbon concentration can be reduced to 0.08, 0.03, 0.01 and 0.005 wt pct with increasing order of FeO concentration. Calculation of the lower limit of carbon concentration from the supersaturation limit suggests that the end point carbon should have been 1.25, 0.67, 0.4 and 0.3 wt pct with increasing order of FeO concentration. In calculating the limiting supersaturation pressure, the authors considered the barrier to nucleation rather than thermodynamic equilibrium. Many practical studies indicate that this barrier for CO nucleation will be overestimated if one employs classical nucleation theory. For this reason, the authors employed the methodology used by Chen and Coley[11] to address this issue. In any case, this offers a conservative calculation of the endpoint carbon and still shows that the decarburization terminated prior to reaching the limiting carbon concentration (thermodynamic equilibrium limit as well as nucleation barrier limit) for range of slag FeO concentrations.
The Effect of Slag Basicity on Decarburization
The decarburization behavior for 2.5 wt pct C to 0.01 wt pct S 2g droplets reacting with different basicity slags, V-ratio varied from 0.9 to 2, was investigated at 1580 °C and the results are presented in Figure 4. The gas volume within the droplet increased up to 7 times its original volume for reaction with slag V = 2.0, compared to 6 times in the case of V = 1.5 and 3 times in the case of V = 0.9. The CO generation for V = 2.0 has highest peak rate of decarburization compared to the cases of V = 1.5 and V = 0.9. The extent of decarburization is much greater, and the end point carbon goes down to 0.33 wt pct for V = 2.0 compared to 0.96 wt pct for the case of V = 1.5 and 1.1 wt pct for V = 0.9. In the case of the higher basicity slag, the decarburization proceeded to a carbon concentration close to that predicted by considering the barrier to nucleation in the supersaturation limit(~ 0.3 wt pct). This value is higher than that predicted for thermodynamic equilibrium but is consistent with the idea that the nucleation barrier would set the supersaturation limit beyond which carbon could not decrease. At that point, the droplet sank back into the dense slag as observed by X-ray fluoroscopy. Gaye and Riboud[7] studied the decarburization and dephosphorization with much higher basicity slag(V-ratio of 9.6) than used in the current work at 1550 °C. The end point carbon concentration was reported to be between 0.3 to 0.5 wt pct over a range of oxidizing slag (NO/NFe from 1.43 to 1.17) whereas Min and Fruehan[8] and Molloseau and Fruehan[10] reported a much higher end point carbon concentration. In Min and Fruehan’s studies, the end point carbon concentration varied between 3.5 to 2 wt pct as the slag FeO concentration was changed from from 3.2 to 15.2 wt pct with V-ratio of 1 at 1400°C. Molloseau and Fruehan conducted studies with slag of V-ratio 1.2, and reported end point carbon concentration was 2.65 to 1.65 wt pct for slag FeO concentration from 3 to 30 wt pct at 1440°C. It appears that for higher basicity slags in the current work and in the published literature decarburization stops at a point consistent with the calculated supersaturation limit for nucleation of CO bubbles. Whereas for lower basicity slags the reaction stops at much higher carbon concentrations.
Discussion
Decarburization of Droplets; Effect of Carbon Concentrations and Droplet Mass
Decarburization data for droplets with different carbon concentrations, presented in Figure 2, show that the rate of decarburization goes through a short incubation period followed by a peak, steady-state decarburization period and a subsequent slow decarburization period i.e the end stage of decarburization. In this section a qualitative discussion is conducted of the underlying mechanism during the incubation period along with a quantitative discussion of the peak decarburization period. Additionally, few interesting observations will be discussed for the final stage. To assist in discussion of the mechanism a theoretical framework is proposed in the following section.
Theoretical Framework
A mixed controlled kinetic model is developed in this section to describe the steady state period during which the rate is fastest. At steady state, the rate of generation of CO would likely be controlled by oxygen transport in the slag, interfacial chemical reaction, internal nucleation of CO, internal growth of CO bubbles or a combination of the four. If the rate of CO generation is controlled by oxygen transport in the slag, then the rate of CO generation(mol/s) is represented by Eq. [2] where oxygen transport is considered phenomenologically as the transport of FeO.
\({k}_{\text{s}}\) is the mass transfer coefficient, \({A}_{\text{s}-\text{m}}(t)\) instantaneous slag-metal surface area, and \((\Delta {C}_{{\text{FeO}}})\) is the difference between interface and bulk concentration of FeO in the slag. In this work, the maximum change in FeO concentration in the slag was ~ 2 pct, so the changes in slag mass transfer coefficient due to variation in composition was ignored. If the rate of CO generation(mol/s) at steady state is controlled by transfer of oxygen by interfacial chemical reaction, then
where \(\overrightarrow{k}\) is the forward reaction rate constant, \({\theta}_{\text{S}}\) is the fraction of site blocked by surface active species sulfur, \({a}_{{\text{FeO}}}^{i}\) and \({a}_{\text{O}}^{i}\) are the activities of reactant, FeO, and product, dissolved oxygen, respectively.
When the rate of CO generation(mol/s) is controlled by internal nucleation[11,47] of CO bubbles within droplet, then
where \(J\) is the rate of nucleation per unit volume, \({n}_{\text{e}}\) is the no of molecules in an embryo, \({N}_{\text{A}}\) is the Avogadro’s number and \({V}_{\text{m}}\) is the volume of the metal droplet. \({N}_{\text{O}}\) is the number concentration of CO embryos in the liquid, \(\Delta {G}_{\text{crit}}\) critical free energy barrier for CO nucleation, \(\Delta H\) is the heat of formation of CO molecule, \(m\) is the mass of one molecule of CO,\(\sigma \) is the surface tension of liquid metal, \({W}_{\text{m}}\) is the mass of the metal droplet, \({V}_{\text{m}}\) is the volume of metal, \( {\text{[\% O]}} \) is the bulk oxygen concentration in metal, \({M}_{\text{O}}\) is the molar mass of [O], \({k}_{\text{B}}\) is the Boltzman constant and T is the temperature of the system. The CO bubbles are observed to form inside the metal droplet, this introduces intensive stirring, hence, carbon and oxygen transport within the liquid metal droplet are not likely rate determining steps during bloating. Growth of bubbles may also contribute to CO generation in which case Eq. [5] for chemical reaction at the gas/metal interface may describe the CO generation rate(mol/s) or more likely a combination of Eqs. [4] and [5] will describe the rate.
where \({k}_{{\text{gr}}}\) is the growth rate constant, \({A}_{\text{g-m}}\left(t\right)\) is the total gas/metal surface area within the metal droplet at time instant t, \({f}_{\text{C}}\) and \({f}_{\text{O}}\) are the Henrian activity coefficient of carbon and oxygen with respect to 1 wt pct Henrian standard state, \(\left[{\text{pct C}}\right]\) is the wt pct of bulk carbon concentration. The total gas/metal surface area (\({A}_{\text{g-m}}\left(t\right)\)) at any time instant within the metal droplet is a function of rate of nucleation and rate of escape of gas bubbles.
The rate of change of carbon concentration in metal droplets can be calculated from the rate of CO generation as
The carbon concentration at any time can be evaluated as
where \( \left[ {{\text{pct}}\;{\text{C}}} \right]^{0}\) is the starting carbon concentration, \({n}_{\text{CO}}\left(t\right)\) is the total of CO generated in moles after t sec. In this analysis, the total CO generation profiles will be investigated with respect to \(A\left(t\right)\) and \({V}_{\text{m}}\) to qualitatively understand the combination of reaction steps responsible for rate control in the decarburization process. To represent the overall system, a circuit analogy is presented in Figure 5 in terms of several resistances.
\({R}_{\text{I}}\) is the resistance to transport of oxygen in the slag from the bulk to slag/metal interface, \({R}_{\text{II}}\) is the resistance to dissociation at the slag/metal interface, \({R}_{\text{III}}\) and \({R}_{\text{IV}}\) combine to give the resistance to CO generation at the slag metal interface i.e. external decarburization. \({R}_{\text{III}}\) represents the resistance to CO formation and \({R}_{\text{IV}}\) is the resistance to transport of carbon in the metal phase. \({R}_{\text{V}}\) is resistance to transport oxygen in metal phase, \({R}_{\text{VI}}\) is the resistance to nucleation of CO bubbles within the metal droplet and \({R}_{\text{VII}}\) is the resistance to growth of these bubbles. The overall resistance can be expressed as
The decarburization results presented earlier show that the decarburization is mostly internal during the peak decarburization period. The resistance from external decarburization can be ignored at this period of decarburization. It is also to be noted that due to stirring introduced by CO bubbles within droplet, there is negligible resistance of transport in the metal during this period. With an assumption that at steady state, decarburization will only be by internal decarburization, the analgous circuit simplifies to that presented in (Figure 6) and the overall resistance can be expressed as in Eq. [9]
These resistances are \({R}_{\text{I}}\propto \frac{1}{{k}_{\text{s}}A\left(t\right)}\), \({R}_{\text{II}}\propto \frac{1}{\overrightarrow{k}(1-{\theta }_{\text{S}})A\left(t\right)}\), \({R}_{\text{VI}}\propto \frac{1}{{V}_{\text{m}}}\) and \({R}_{\text{VII}}\propto \frac{1}{{A}_{\text{g-m}}\left(t\right)}\). After few rearrangements, combining the Eqs. [2] through [5], the overall rate constant(mol/s) for liquid metal droplet reacting in oxidizing slag can be expressed as
The rate constants \({K}_{\text{a}}\), \({K}_{\text{b}}^{\text{nuc}}\) and \({K}_{\text{b}}^{\text{gr}}\), for a specific slag and metal chemistry are
Here the activity coefficient of FeO (\({\gamma }_{\text{FeO}}\)) in the slag was calculated for this specific slag composition using the correlation proposed by Basu et al.[48], the density of metal (\({\rho }_{\text{m}})\) was calculated from Jimbo and Cramb’s correlation and the surface tension (\(\sigma )\) was evaluated using Chung and Cramb [49] correlation.
At steady state under mixed controlled kinetic conditions, the overall rate equation can be stated as
If one assumes that there is no contribution from growth (growth rate constant,\({K}_{\text{b}}^{\text{gr}}\) 0) to simplify the analysis further, the overall rate constant and the rate equation simplifies as
It can be observed from this correlation, that under mixed controlled kinetic conditions, the rate would show a linear behavior with \({A}_{s-m}\left(t\right)\) and nearly linear with \({V}_{\text{m}}\) except at very small droplet sizes region and non-linear variation with area/volume ratio if the droplet mass is varied linearly. If the droplet size is varied in very small droplet size region, the variation of volume of metal would show a parabolic increase of FeO flux. The overall rate constant is analyzed qualitatively to understand the rate controlling steps in decarburization kinetics at different level of carbon concentration reacting in oxidizing slag.
The decarburization reaction kinetics of the three different stages are discussed in detail in the following section.
Incubation period
The bloating profile along with the CO generation profile shows an elongated incubation period for droplets with 4.4 wt pct C concentration whereas the incubation period is very short for droplets with lower carbon concentrations (2.5,1.5 and 0.5 wt pct). During this period, mostly external decarburization occurs with very limited amount of internal decarburization where droplets retain their original size and shape. In a decarburizing droplet, there is a competition between two processes:1/transport of oxygen from the bulk slag to the slag/metal interface crossing that interface and transporting into the bulk metal, and 2/ the transport of carbon from bulk metal to the slag-metal interface. If the former is faster the oxygen will build up in the metal until the supersaturation pressure for CO nucleation is reached at which point carbon and oxygen will react to form CO bubbles. If the latter process is faster CO will nucleate at the slag/metal interface. It is also possible under many conditions for both processes to proceed simultaneously. In that case where transport of oxygen to the slag/metal interface is slightly faster than transport of carbon in the metal, CO may form on the surface and excess oxygen may diffuse into the melt and eventually exceed the supersaturation pressure for internal CO nucleation. As the carbon at the surface becomes depleted CO nucleation at the slag metal interface may stop further increasing oxygen supply into the melt. The interplay between these effects has a strong influence on the incubation time for bloating. With increase in droplet initial carbon concentration, the transport of carbon towards slag metal interface becomes faster, whereas the oxygen transport rate in the slag remains the same. For high carbon droplets, there would be sufficient carbon supply to the slag-metal interface to support external decarburization for a longer period, thereby delaying the onset of bloating, by consuming oxygen which would have otherwise been transported into the bulk droplet. For lower carbon droplets, lower oxygen consumption at the slag-metal interface would enable higher oxygen flux into the droplet and thus a very short incubation period for bloating is observed (Figure 7).
Steady state decarburization period
To investigate the rate controlling step/s for decarburization of droplets with different carbon concentrations at steady state, the total CO generation profiles were divided by the time averaged slag/metal area (\({A}_{avg}\left(t\right)\)) and the volume of the metal (\({V}_{metal}\)) and presented in Figures 8 through 11 in order to understand the underlying mechanism for different droplet sizes. The importance of using time averaged area with this type of data was demonstrated by Rhamdhani et al.[50] The time averaged area is calculated as
Based on Eqs. [2] and [3] we can see that if the steady state rate of decarburization changes linearly with \({A}_{s-m}\left(t\right)\), then the reaction rate is likely to be controlled by either slag-metal interfacial reaction or transport in the slag whereas, if the rate is proportional to \({V}_{metal}\) then internal nucleation of bubbles within the metal droplet is the most likely rate determining step as illustrated by Eq. [4]. As shown in Figures 7 through 10 the data does not offer a perfect fit when normalized with respect to either area or volume. This is consistent with the reaction being under mixed controlled kinetics.
It is not within the scope of the current work to develop and validate the type of numerical model required to describe the full complexity of the reaction mechanism. However, by developing a conceptual model capturing the essential details of the system, the following section will estimate the contribution of the rate control of each reaction step involved. This will be done by employing parameters from the literature obtained under reasonably comparable conditions to those used in the present work.
Considering mixed controlled kinetics, in the overall rate constant expression, the first two terms in Eq. [10] mainly determine oxygen transport from bulk slag into the metal droplet whereas the third term corresponds to the rate of internal nucleation and growth of bubbles. The overall rate constant (simplified in Eq. [16]) increases with increase in slag-metal interfacial area (\({A}_{s-m}(t)\)) and metal droplet volume (\({V}_{\text{m}}\)). Due to bloating at steady state, there is a rise in instantaneous slag/metal area by between 50 pct and 200 pct in the range of carbon concentration 0.5 to 4.4 wt pct. The volume of the metal is fixed for a given droplet.
An attempt has been made to validate the mechanism of decarburization during the steady state stage, based on the mixed controlled model described in Eq. [16]. The parameter \({K}_{\text{a}}\) (mol -m-2 -s-1) is constant for a fixed slag mass transfer coefficient and interfacial chemical reaction condition and should vary with temperature, slag composition and stirring conditions in the slag. It has been approximated as 0.02 mol -m-2 -s-1 using values for mass transfer coefficient in the slag(\({k}_{\text{s}}\)), the chemical reaction rate constant of dissociation of FeO at the s/m interface(\(\overrightarrow{k}\)) and the fraction of the surface poisoned by sulfur (\(\theta \)). A literature value obtained under similar conditions to the present work, was used for mass transfer coefficient.[8] It is worth noting that previous work in authors’ laboratory shows that there is a significant effect of void fraction on the slag mass transfer coefficient in foamy slag. However, the mass transfer coefficient determined experimentally under similar conditions already includes this effect. In selecting this value, the authors compared values from several workers studying decarburization of droplets in FeO based slag. The range of value was 10-5 to 10-4 m/s and the average value 5 × 10-5 was selected for this calculation. \(\theta \) was calculated using the Langmuir isotherm based on a well established value for the adsorption coefficient for sulfur[49] and the chemical reaction rate constant (\(\overrightarrow{k}\)) was taken from parallel work by the authors[51].
\({K}_{\text{b}}^{\text{nuc}}\)(mol -m-3 -s-1 ) may be calculated using Eq. [13] and requires knowledge of the parameters \(\Delta {G}^{crit}\) and \({n}_{\text{e}}\). The variation of \(\Delta {G}^{\text{crit}}\) and \({n}_{\text{e}}\) with the variation of carbon concentration has been estimated based on the approach suggested by Chen and Coley[11] employing a surface tension modifying parameter (ψ) determined from the authors parallel work[51]. The calculated values of \({K}_{\text{b}}^{\text{nuc}}\) are given in column 2 of Table IV.
The parameter \({K}_{\text{b}}^{\text{nuc}}\) can also be determined from experimental data using Eq. [16]. The volume(\({V}_{\text{m}})\) is known from droplet mass and density.[46] The surface area \({A}_{\text{s}-\text{m}}(t)\) of the droplet was determined using X-ray video images. \({k}_{\text{o}}\) was determined from the peak reaction rate and the other parameters(\({K}_{\text{a}}\)) for Eq. [16] were determined as described above. The values for \({K}_{\text{b}}^{\text{nuc}}\) determined by this method are presented in column 3 of Table IV.
The values presented in Table IV, for the two different calculation methods follow a similar trend with the exception of the value for 4.4 pct C. The rate constant, \({K}_{\text{b}}^{\text{nuc}}\) decreases with decraesing carbon concentration in the metal droplet for the range of 2.5 to 0.5 wt pct C and this is due to lowering of supersaturation pressure with decreasing carbon concentration, whereas for the case of 4.4 wt pct, there may be significant contribution from external decarburization which wouldn’t validate this calculation method. It should also be noted that the values calculated using the current experimental data are quite a bit higher than the predicted values. It seems likely that both deicrepancies arise from the same source that the prediction is theoretically based whereas the calculation based on experimental data assumes that all CO production comes from nucleation. The latter assumption is clearly untrue but the authors were not able to distinguish between nucleation and growth.
There are complex interrealtions among the parameters and to fully explore the complex interacting effects in the mixed controlled decarburization of droplets, a detailed numerical modeling is required. The authors are currently working to develop this type of model.
At steady state, the metal droplet bloats due to a difference between gas generation and gas escape rate. The gas escape behavior depends on the interfacial tension, viscosity, average size of escaping bubbles, pressure within the bubbles [52,53] and the available surface area through which it escapes. The rate of bloating is observed to increase with increase in droplet initial carbon concentration due to increase in rate of generation of CO gas whereas no specific trend is observed with the variation of droplet mass.
End decarburization period
With the progress of reaction, decarburization slows down irrespective of end point carbon concentration eventually leading the droplet to sink into the dense slag due to lack of buoyancy. A comparison of the carbon concentration, before and after reaction, is presented in Table V. It can be observed that decarburization for the case of 2 g droplet containing 4.4 wt pct C ended when the remaining carbon concentration was 3.04 wt pct. When experiments were conducted with 2.5 wt pct C 2 g droplets, i.e. lower than 3.04 wt pct, bloating occurred due to decarburization and suddenly shut down the reaction when the carbon reached 1.1 wt pct. When this ‘decarburized’ droplet with 1.1 wt pct C were reacted again in fresh slag of same composition as earlier (16 wt pct FeO and V-ratio 0.9), the decarburization started again and went through a peak decarburization period with bloating followed by cessation of the reaction at ~ 0.5 wt pct carbon. Another experiment with a ‘fresh’ 2 g droplet with 0.5 wt pct C also showed decarbuisation with bloating and finally the reaction stopped at 0.11 wt pct carbon. However, for decarburizing droplets with fixed slag oxygen potential, the end point carbon concentration should be a fixed value (i.e. 0.3 wt pct C from supersaturation limit and 0.05 wt pct C from thermodynamic limit) which should not vary with droplet mass or droplet initial carbon concentration. It has been mentioned earlier that for low basicity slag, the decarburization stopped at much higher carbon concentration than that predicted from supersaturation limit or thermodynamic limit and this was consistently observed for all initial carbon concentrations. One can conclude from this observation that the shutdown of decarburization is not due to lack of carbon or to insufficient FeO remaining in the slag. This leaves the authors to conclude that some mechanism is in play which prevents sufficient oxygen from coming into contact with sufficient carbon. The authors are currently not able to offer an explanation for this observation.
Decarburization of Droplets Reacting with Slag at Different FeO Concentrations
The decarburization along with the normalized volume profile for droplets with 2.5 wt pct C of 2 g with a range of FeO (5 to 16 wt pct) concentration does not show any incubation period before bloating whereas for 2.5 wt pct FeO case, the droplet did not bloat at all. The transport of oxygen from bulk slag to slag/metal interface and then into the bulk metal droplet is fast enough compared to the transport of carbon from the bulk metal to the slag/metal interface, resulting early bloating for cases with 5, 10 and 16 wt pct FeO concentration in the slag and for 2.5 wt pct FeO case, the carbon transport towards slag/metal interface being faster consumes all the oxygen at the slag/metal interface.
The rate at the peak decarburization period is observed to increase due to increase in bulk slag oxygen potential with increase in slag FeO concentration. The bulk slag oxygen potential determines both the rate of transport of oxygen from bulk slag to slag/metal interface and the rate of oxygen dissolution at the slag/metal interface as illustrated in Eqs. [2] and [3]. Under mixed controlled kinetics the rate of decarburization by internal or external nucleation or by both are in balance with the rate of transport of oxygen in slag and the rate of the interfacial FeO dissociation reaction. In all of the cases, rate of decarburization is influenced by bulk slag oxygen potential. With decreasing slag oxygen potential, a threshold point is reached where all the oxygen is consumed at the slag/metal interface. In the current work the threshold is in the range 2.5 to 5 wt pct FeO. This threshold will vary based on the temperature, slag basicity and conductivity as well as carbon concentration.
The decarburization has been observed to shut down at a much higher carbon concentration than that predicted by supersaturation limit or by thermodynamic limit presented in Figure 11(a), for over a range of slag FeO concentrations. The path of reaction either via internal decarburization or external decarburization or both doesn’t improve the shutting down condition.
With increasing slag FeO concentration, the rate of bloating is found to increase, and this may be attributed to the higher rate of CO generation with higher oxygen potential slag. Between 10-16 wt pct FeO in the slag, the rate of bloating does not increase appreciably.
Decarburization of Droplets Reacting with Slags of Different Basicity
The absence of an incubation period in the decarburization profile for 2 g droplets with 2.5 wt pct C, over a range of slag V-ratio, can be attributed to faster transport of oxygen from the bulk slag into bulk the metal compared to carbon transport within the metal towards slag/metal interface.
The peak rate of decarburization has been observed to increase with increasing slag basicity (Figure 4). The increase in slag V-ratio lowers the slag viscosity leading to higher rates of transport of oxygen in the slag for the same total Fe. The overall rate constant in Eq. [10] increases with increase in slag mass transfer coefficient (\({k}_{\text{s}})\) with slag V-ratio, resulting in higher rate of CO generation.
The comparison of end-point carbon concentration in Figure 11(b) shows that with increasing slag basicity, the decarburization reaches close to the limiting carbon concentration as predicted from supersaturation limit, although this is far beyond the thermodynamic limit. The authors do not have a definitive explanation for this observation but it appears that increasing the slag basicity, prevents the decarburization from shutting prematurely by facilitating sustained the oxygen transport. Ongoing work in the authors’ laboratory seeks to provide a detailed explanation.
The rate of bloating was found to increase with increasing slag basicity which can be attriburted to the higher rate of gas generation along with negligible variation in gas escape.
This analysis suggests that there are multiple factors working simultaneously to control the decarburization kinetics at different stages. Qualitative agreement with a mixed control kinetic model, which includes slag mass transport, interfacial chemical reaction and internal nucleation, is found at the steady state period.
Another important finding of this analysis is that the cessation of decarburization does not occur due to lack of carbon in metal or lack of FeO in the slag, but seems to be most likely caused by an interruption of oxygen supply to the metal droplet. In all the studied cases, decarburization stops earlier than can possibly be justified by either the thermodynamic equilibrium or by the nucleation barrier despite having sufficient reactants. In case of higher basicity, the decarburization gets close to the supersaturation limit suggesting that the cessation of decarburization cannot be related to conventional transport breaking down, because transport circumstances do not change significantly. It may be that the oxygen in the slag is facing a barrier to transport at the slag/metal interface due to charge accumulation[9,36]. In this work, the authors are not able to present a definitive mechanism due to insufficient evidence. Currently, the authors are pursuing an experimental program to elucidate the mechanism by which decarburization of droplets is inhibited.
Conclusions
-
1.
The overall decarburization kinetics are under mixed control at steady state: including mass transport in slag, interfacial chemical reaction and either internal nucleation and growth of bubbles or external decarburization or both internal and external decarburization. The dominant rate determining step changes with slag and metal chemistry and reaction progress.
-
2.
The transition from non-bloating to bloating is driven by increasing driving force for oxygen transfer into the bulk of the droplet. FeO concentration in the slag and at 10 wt pct FeO concentration, highest rate of bloating has been observed.
-
3.
The end point carbon concentration of decarburizing droplets occurs long befor reaching equilibrium or the supersaturtion limit of CO. This fact and the observed dependency on the slag FeO concentration and basicity suggests that the mechanism is related to oxygen transport. The authors have suggested that decarburization is inhibited by the build up of charge at the droplet/slag interface, blocking oxygen transfer to the drolet. Although there is no definitive proof for this mechanism it is supported by the fact that factors which mitigate this effect generally increase electrical conductivity of the slag.
References
W.O. Philbrook and L.D. Kirkbride: J. Met., 1956, vol. 8, pp. 351–6.
T.E. Dancy: J. Iron Steel Inst., 1951, vol. 169, pp. 17–24.
R.S. Kaplan and W.O. Philbrook: Metall. Trans., 1972, vol. 3, pp. 487–91.
P.G. Roddis: J. Iron Steel Inst., 1973, vol. 211, pp. 53–8.
N.H. El Kaddah and D.G.C. Robertson: J. Colloid Interface Sci., 1977, vol. 60, pp. 349–60.
N.H. El Kaddah and D.G.C. Robertson: Metall. Mater. Trans. B., 1988, vol. 19B, pp. 831–7.
H. Gaye and Riboud.P.V: Metall. Trans. B, 1977, vol. 8, pp. 409–15.
D.J. Min and R.J. Fruehan: Metall. Trans. B., 1992, vol. 23B, pp. 29–37.
G.G.K. Murthy, A. Hasham, and U.B. Pal: Ironmak. Steelmak., 1993, vol. 20, pp. 191–200.
C.L. Molloseau and R.J. Fruehan: Metall. Mater. Trans. B, 2002, vol. 33B, pp. 335–44.
E. Chen and K.S. Coley: Ironmak. Steelmak., 2010, vol. 37, pp. 541–5.
K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B, 2017, vol. 48B, pp. 2984–3001.
B. Sarma, A.W. Cramb, and R.J. Fruehan: Metall. Mater. Trans. B., 1996, vol. 27B, pp. 717–30.
Subagyo, G.A. Brooks, and K.S. Coley: Can. Metall. Q., 2005, vol. 44, pp. 119–30.
N. Dogan, G.A. Brooks, and M.A. Rhamdhani: ISIJ Int., 2011, vol. 51, pp. 1093–101.
R. Sarkar, P. Gupta, S. Basu, and B.N. Ballal: Metall Mater. Trans. B., 2015, vol. 46B, pp. 961–76.
D. Dering, C. Swartz, and N. Dogan: Processes., 2020, vol. 8, pp. 1–23.
A. Kadrolkar and N. Dogan: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 2912–29.
E.W. Mulholland, G.S.F. Hazeldean, and M. Davies: J. Iron Steel Inst., 1973, vol. 211, pp. 632–9.
B.K. Rout, G. Brooks, M.A. Rhamdhani, Z. Li, F.N.H. Schrama, and A. Overbosch: Metall. Mater. Trans. B., 2018, vol. 49B, pp. 1022–33.
B. Deo, P. Ranjan, and A. Kumar: Steel Res., 1987, vol. 58, pp. 427–31.
H. Sun: ISIJ Int., 2006, vol. 46, pp. 1560–9.
H. Sun, K. Gao, V. Sahajwalla, K. Mori, and R.D. Pehlke: ISIJ Int., 1999, vol. 39, pp. 1125–33.
K. Ito and K. Sano: Tetsu-to-Hagane., 1964, vol. 50, pp. 873–7.
K. Ito and K. Sano: Tetsu-to-Hagane., 1965, vol. 51, pp. 1252–9.
Y. Niiri, K. Ito, and K. Sano: Tetsu-to-Hagane., 1969, vol. 55, pp. 437–45.
K. Ito, K. Sano, and S. Arino: Iron Steel Inst Jpn-Trans., 1969, vol. 9, pp. 465–71.
P.A.A. Distin, G.D.D. Hallett, and F.. D. Richardson: J. Iron Steel Inst., 1968, vol. August, pp. 821–33.
L.A. Baker and R.G. Ward: J. Iron Steel Inst., 1967, vol. 205, pp. 714–7.
L.A. Baker, N.A. Warner, and A.E. Jenkins: Trans. Metall. Soc., 1967, vol. 239, pp. 857–64.
M. Hayer and S.G. Whiteway: Can. Metall. Q., 1973, vol. 12, pp. 35–44.
M. Sano, H.-Y. Tao, and M. Katoh: Sixth Int. Iron Steel Congr., 1990, vol. 1, pp. 370–7.
D. Widlund, D.S. Sarma, and P.G. Jönsson: ISIJ Int., 2006, vol. 46, pp. 1149–57.
J.B. See and N.A. Warner: J. Iron Steel Inst., 1973, vol. 211, pp. 44–52.
S. Jahanshahi: Imperial College of Science and Technology, London, 1980.
G. Murthy, Krishna, G, Y. Sawada, and F. Elliot, J: Ironmak. Steelmak., 1993, vol. 20, pp. 179–200.
G. Brooks, Y. Pan, and K.S. Coley: Metall. Mater. Trans. B., 2005, vol. 36B, pp. 525–35.
E. Chen and K.S. Coley: McMaster University, 2010.
D.E. Woolley and U.B. Pal: Ironmak. Steelmak., 2002, vol. 29, pp. 125–32.
U.B. Pal, S.A. Macdonald, D.W. Woolley, and A.C. Powell: Metall. Mater. Trans. B., 2005, vol. 36B, pp. 209–18.
D.E. Woolley and U.B. Pal: ISIJ Int., 1999, vol. 39, pp. 103–12.
M. Millman, A. Kapilashrami, M. Bramming, and D. Malmberg: Imphos: Improving Phosphorus Refining, 2011.
L. Holappa: Scand. J. Metall., 2005, vol. 34, pp. 66–78.
C. Cicutti, M. Valdez, T. Perez, J. Petroni, A. Gomez, R. Donayo, and L. Ferro: in 6th International Conference on Molten Slags, Fluxes and Salts, vol. 367, 2000.
J. Li and M. Barati: Metall. Mater. Trans. B, 2009, vol. 40B, pp. 17–24.
I. Jimbo and A.W. Cramb: Metall. Trans. B., 1993, vol. 24B, pp. 5–10.
M. Blander and J.L. Katz: AIChE J., 1975, vol. 21, pp. 833–48.
S. Basu, A.K. Lahiri, and S. Seetharaman: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 447–56.
Y. Chung and A.W. Cramb: Metall. Mater. Trans. B., 2000, vol. 31B, pp. 957–71.
M.A. Rhamdhani, K.S. Coley, and G.A. Brooks: Metall. Mater. Trans. B., 2005, vol. 36B, pp. 219–27.
J. Biswas and K.S. Coley: A Decarburisation Model for a Fe-C Droplet Reacting in Oxidizing Slag, McMaster University, Unpublished Research, 2021.
A. Rozario, N.N. Viswanathan, and S. Basu: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 10–5.
T.X. Zhu: Bubble Escape Model, Private Communication, Hamilton, 2018.
Acknowledgments
The authors wish to thank McMaster Steel Research Center and Natural Science and Engineering Research Council of Canada for funding this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted 23 December 2020; accepted 2 October 2021.
Rights and permissions
About this article
Cite this article
Biswas, J., Gu, K. & Coley, K.S. Decarburization of Bloated Droplets: An Experimental Study to Understand the Kinetics of Decarburization of Metallic Iron Droplets in FeO Containing CaO-SiO2 Slags. Metall Mater Trans B 52, 4215–4229 (2021). https://doi.org/10.1007/s11663-021-02344-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02344-x