Abstract
The CaO·3(Al,Fe)2O3, (C(A,F)3), phase forms a solid solution in the FeO-Fe2O3-CaO-Al2O3 system and is an end member of the SFCA (Silico-Ferrite of Calcium and Aluminum) suite of solid solutions observed in industrial iron ore sinters. SFCA acts as a bonding phase in these sinter materials so an accurate description of the phase equilibria and thermodynamic properties of this phase can be used to assist in the design and operation of Fe-sintering and Fe-making processes. New experimental data are reported on the liquidus and sub-liquidus phase equilibria in the iron-rich region of the FeO-Fe2O3-CaO-Al2O3 system in air. The study was undertaken using equilibration/quenching and microanalysis techniques enabling the compositions of the liquid and solid phases in equilibrium at high temperature to be directly and accurately measured. The compositional limits of the C(A,F)3 phase and associations with the primary phase fields of CA [CaO·(Al,Fe)2O3], CA2 [CaO·2(Al,Fe)2O3], CA6 [CaO·6(Al,Fe)2O3], Hematite [(Fe,Al)2O3], Spinel [(Fe,Ca)O·(Fe,Al)2O3)], C2F [2CaO·(Fe,Al)2O3], C2F3A (2CaO·3Fe2O3·Al2O3), CF [CaO·(Fe,Al)2O3], and CF2 [CaO·2(Fe,Al)2O3] have been determined in the high-iron, low-melting temperature region of the system. The C(A,F)3 is found to be stable over a wide range of temperatures (1213 °C to 1400 °C) and alumina compositions (3.3 to 33.6 mol pct AlO1.5). The solid-phase C2F3A (2CaO·3Fe2O3·Al2O3), which is isostructural to SFCA-I indicated by previous researchers, has been found to be stable up to 1225 °C in the FeO-Fe2O3-CaO-Al2O3 system in air.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Silico-Ferrite of Calcium and Aluminum (SFCA, where S, F, C, and A in this paper indicate SiO2, “Fe2O3,” CaO, and Al2O3, respectively) is a key phase in the iron ore-sintering process as it constitutes a large proportion of materials present in the industrial sinter products (see Figure 1) and is believed to enhance the reducibility and mechanical strength of the sinters.[1] This phase extends towards the FeO-Fe2O3-CaO-SiO2 sub-system as Silico-Ferrite of Calcium (SFC) and towards the FeO-Fe2O3-CaO-Al2O3 sub-system as the C(A,F)3 [CaO·3(Al,Fe)2O3] solid solution.
Previous experimental studies of phase equilibria relating to the SFCA phase have been principally focused on (i) the FeO-Fe2O3-CaO-SiO2 system in air;[2,3,4] (ii) the FeO-Fe2O3-CaO-SiO2 system at selected oxygen partial pressures[5,6]; and (iii) liquidus and sub-solidus regions in the FeO-Fe2O3-CaO-Al2O3 system[7,8,9,10,11,12,13]; and (iv) SFCA as well as the hematite liquidus in the FeO-Fe2O3-CaO-SiO2-Al2O3 system in air.[8,14] The aim of the present study is to determine the phase equilibria in the FeO-Fe2O3-CaO-Al2O3 system in air, with a focus on phase relations and thermodynamic limits of the C(A,F)3 [CaO·3(Al,Fe)2O3] solid solution.
Literature Review
The currently accepted version of the “Fe2O3”-CaO-Al2O3 liquidus surface in air in Slag Atlas,[15] was constructed based principally on the experimental studies by Dayal and Glasser,[16] and Lister and Glasser.[17] The system was investigated for temperatures from 1178 °C to 1527 °C using an equilibration and quenching method followed by optical microscopy, X-ray powder diffraction, and wet chemical analysis. The C(A,F)3 solid solution was reported to be stable on the liquidus surface up to 1435 °C, with its composition lying on the join between “CA3” (CaO·3Al2O3) and “CF3” (CaO·3Fe2O3) between 44.5 and 81.5 mol pct CF3. Malysheva et al.[18] studied this system using a combination of microprobe and wet chemical analyses. They reported variable CaO concentrations in the C(A,F)3 solid solution, although departing from the “CA3” – “CF3” join, within the following limits: 12-17 wt pct CaO, 2-30 wt pct Al2O3, and 58-51 wt pct Fe2O3 from 1130 °C to 1260 °C. Arakcheeva et al.[19,20] reported a new aluminum-calcium ferrite phase (CaFe3AlO7, expressed as “C2F3A (2CaO·3Fe2O3·Al2O3)” in the present study) at 1170 °C in the “Fe2O3”-CaO-Al2O3 system in air having a composition of \( {\text{Ca}}_{ 7. 1 2} {\text{Fe}}^{ 2+ }_{0. 8 8} {\text{Fe}}^{ 3+ }_{ 2 3. 8 2} {\text{Al}}_{ 8. 1 8} {\text{O}}_{ 5 6} \), this phase is not included in the phase diagram proposed by Dayal and Glasser.[16] This new compound, having an overall stoichiometry of M20O28, was later found to be isostructural to “SFCA-I.”[21,22] Mumme et al.[22] also reported a Si-free SFCA-I in the “Fe2O3”-CaO-Al2O3 system to be Ca3.18Fe 2+0.82 Fe 3+14.66 Al1.34O28, having a lower Al2O3 concentration than the SFCA-I reported by Arakcheeva et al.[19,20] In a later investigation,[21] Mumme reported another Si-free SFCA type (Ca2Fe7Al5O20, or CF1.25A1.75) having an overall stoichiometry of M14O20[23,24] formed in the “Fe2O3”-CaO-Al2O3 system. In the same study,[21] SFCA-II with a stoichiometry of M34O48 (Ca5.1Al9.3Fe 3+18.7 Fe 2+0.9 O48) was also reported, although it has not been found in industrial sinters.[25]
To date, different crystal structures of SFCA (SFCA, SFCA-I, and SFCA-II) have been reported,[19,20,21,22,23,24] although the phase relations between them in the FeO-Fe2O3-CaO-SiO2-Al2O3 system in air are yet to be fully characterized. The compositions of the different types of SFCA in the “Fe2O3”-CaO-SiO2-Al2O3 system are summarized and schematically shown in Figure 2.
Experimental Methodology
The high-temperature equilibration/quenching/EPMA measurement approach used in the present study, developed by the authors,[26,27] has been described in detail in previous publications.[4,28,29,30] A 4-point test approach[27] was applied to confirm the achievement of equilibrium in each case, which includes (i) test of the effect of equilibration time; (ii) analysis of the homogeneity of the phases; (iii) evaluation of the effect of starting composition and phase assemblage on the approach toward the equilibrium point; and (iv) systematic analysis of reactions specific to the system.
The initial bulk mixtures used in the present study were prepared by mixing, i) high-purity powders of Fe2O3 (99.9 wt pct), Al2O3 (99.9 wt pct) supplied by Sigma-Aldrich Co, NSW, Australia, and ii) a pre-melted CaO-Fe2O3 master slag at 48 mol pct CaO. Each of the samples for the equilibration runs (< 0.3 g) was taken from the bulk mixture and pressed into a pellet using a tool steel die. The initial compositions of the mixtures were selected so that, at the equilibration temperature, one or more crystalline phases would be present in equilibrium with liquid slag. The sample bulk composition was selected targeting the volume fraction of solids to be below 50 pct, and preferably approximately 10 pct at the equilibration temperature. This enables rapid equilibration to be achieved and the liquid to be retained as an amorphous or glass phase on quenching by (i) reducing the availability of nuclei and surfaces for crystallization and (ii) reducing the “enthalpy” introduced by solids needed to be conducted into the quenching media.
The platinum substrates used for supporting the samples during equilibration were prepared by winding pure platinum wire (99.99 wt pct Pt, 0.5 mm diameter, supplied by Johnson Matthey Ltd, Tullamarine VIC, Australia) into a spiral shape. The design of this substrate maximizes the area of direct contact between the molten sample and the quenching medium, which enables rapid quenching of the sample.[3,4]
All equilibration experiments were carried out in a vertical reaction tube (impervious recrystallized alumina, 30-mm inner diameter) within an electrical resistance lanthanum chromite-heated furnace. The samples were placed in the uniform hot zone of the furnace immediately adjacent to a working thermocouple that was inserted into a recrystallized alumina sheath to monitor the actual sample temperature. The working thermocouple (Pt-Pt-13 pct Rh) was calibrated against a standard thermocouple (supplied by the National Measurement Institute of Australia, NSW, Australia). The overall absolute temperature accuracy of each experiment was estimated to be ±3 K. The bottom of the reaction tube remained open throughout the experiments to ensure ambient air atmosphere inside, p(O2) = 0.21 atm.
The samples were suspended in the hot zone of the furnace by Kanthal support wire (Fe-Cr-Al alloy, 0.7 mm diameter). For temperatures higher than 1450 °C, a 0.5-mm-diameter pure Pt wire was used to extend the support so that the Kanthal wire is not in the hot zone. The samples were pre-melted for 15 to 30 minutes at temperatures 5 °C to 100 °C above the equilibration temperature, to facilitate the formation of a homogeneous liquid slag in each case. Some samples were then pre-crystallized for another 15 to 30 minutes at temperatures 5 °C to 20 °C below the equilibration temperature, followed by equilibration at the final target temperature for the required time. The pre-melt and pre-crystallization procedures are designed to confirm the achievement of equilibrium from different thermal trajectories. At the end of the equilibration process, the samples were released and rapidly quenched into CaCl2-containing brine (<253 K (− 20 °C)). The samples were then washed thoroughly in water and ethanol before being dried on a hot plate and mounted in epoxy resin. Polished cross sections were prepared using conventional metallographic techniques.
The samples were first examined by optical microscopy and then carbon coated, and the phase compositions were measured using an electron probe X-ray microanalysis technique with wavelength dispersive detectors (JEOL 8200L EPMA, Japan Electron Optics Ltd., Tokyo, Japan). In the present study, the EPMA is operated at 15 kV accelerating voltage and 20 nA probe current using the standard Duncumb–Philibert atomic number, absorption, and fluorescence (ZAF) correction supplied by JEOL.[31,32,33] The standards used (supplied by Charles M. Taylor Co., Stanford, CA) include wollastonite (CaSiO3) for calcium (Kα) calibration, hematite (Fe2O3) for iron (Kα) calibration, corundum (Al2O3) for alumina (Kα) calibration, and in some cases, platinum (Mα) to test for possible contamination. Fe is always present in the slag in two oxidation states: Fe2+ and Fe3+. Only the concentrations of the metal cations were measured by EPMA in the present study. All cation concentrations measured with EPMA were then recalculated to selected oxidation states (i.e., FeO1.5, CaO, and AlO1.5) and normalized to 100 pct for presentation purposes only. The normalized phase compositions and original sums of elements or oxides from EPMA analyses are provided in tabular form. The total compositions before normalization to 100 pct are also reported in Table I, indicated as the “original total.”
The ability to quench the liquid slag phase to ambient temperature without the onset of crystallization was found to depend on the composition of the slag and the equilibration temperature. The most significant problems were observed with low-Al2O3 slags at high temperatures, at or above 1300 °C, where the glassy homogeneous areas, believed to be formed at the surfaces directly contacting the quenching medium, were only a small proportion of the quenched melt. For these samples, the approach taken to obtain accurate, repeatable, and objective measurements of the average compositions of the liquid slag phase by the use of EPMA was similar to that described by Nikolic et al.[34]: an average of at least 20 points in the best quenched area (usually, near the surface) was used, with a standard deviation of composition not exceeding 1 mol pct. In cases where melt crystallization was rapid, the experiments were repeated until an area of sufficiently well-quenched microstructure was found. Increasing the probe diameter to 5 to 50 μm resulted in reduced variability of the measured liquid slag compositions.[35]
The standard ZAF correction supplied by JEOL was further improved following the same approach as described in detail in previous publications.[4,28] Using stoichiometric Ca2Fe2O5, CaFe2O4 and CaFe4O7 as secondary standards, an analytical expression is developed to correct all cation concentrations in the “FeO1.5”-CaO-Al2O3 system:
where x(CaO, FeO1.5, AlO1.5) are the cation molar fractions following JEOL ZAF correction, and x(CaO, FeO1.5, AlO1.5)corr are the final corrected cation molar fractions. This approach is similar to that employed in the previous studies by the authors.[29,36] The accuracy of the compositions measured was estimated to be within 1 wt pct.
Results
Phase Equilibria and Microstructures
Backscattered electron micrographs of typical microstructures obtained on equilibration in the iron-rich region in the FeO-FeO1.5-CaO-AlO1.5 system in air, are shown in Figures 3(a) through (f). From the results of electron probe microanalysis, the following phases have been identified to be present in the range of compositions and temperatures investigated: liquid (Liq), hematite [(Fe,Al)2O3], spinel [(Fe,Ca)O·(Fe,Al)O2O3)], CA [CaO·(Al,Fe)2O3], CA2 [CaO·2(Al,Fe)2O3], CA6 [CaO·6(Al,Fe)2O3], C2F [2CaO·(Fe,Al)2O3], CF [CaO·(Fe,Al)2O3] and CF2 [CaO·2(Fe,Al)2O3], C(A,F)3 [CaO·3(Al,Fe)2O3], and C2F3A (2CaO·3Fe2O3·Al2O3).
Backscattered electron images showing typical microstructures of samples in the pseudo-ternary “FeO1.5”-CaO-AlO1.5 in air and the corresponding experiment numbers. (a) C2F3A + CA + C2F, exp. 44; (b) liquid + C(A,F)3 + C2F3A, exp 38; (c) liquid + C(A,F)3 + CA, exp 36; (d) liquid + C(A,F)3 + CA2, exp. 34; (e) liquid + spinel + C(A,F)3, exp 27; and (f) liquid + CA6 + CA2, exp. 41
Figure 3(a) shows the microstructure of a sub-solidus sample containing three solids: C2F3A, C2F, and CA. Figures 3(b) through (e) show microstructures containing of the C(A,F)3 phase in equilibrium with a liquid phase, and the C2F3A, CA, CA2, and spinel phases, respectively. Figure 3(f) shows the liquid in equilibrium with CA6 and CA2.
The liquid phase is retained as a glass on rapid quenching of the samples from the equilibration temperature. The presence of fine microcrystalline in, or instead of, the glassy liquid phase is the result of crystallization that has taken place in the high fluidity slag during the cooling of the samples; these phases and associated structures are artifacts and are not included in the description of the equilibria.
Phase Compositions
The initial bulk mixture compositions, and the measured average molar compositions and standard deviations of the measurements of each phase, are given in Table I. It should be noted that the Fe species in the slag are present in two oxidation states: Fe2+ and Fe3+. The proportions of Fe3+ in the slags increase with increasing oxygen partial pressures and decreasing temperatures.[37] Previous measurements by Dayal and Glasser[16] have shown that the concentration of ferrous ions in the liquid slags in air are low, typically less than 2 wt pct FeO even for temperatures up to 1450 °C. Thermodynamic predictions using the FactSage public database[38,39,40] also indicate that the Fe2+ concentrations in the slags are low in this system within the range of conditions investigated in the present study. Therefore, all Fe concentrations are recalculated to Fe3+ for presentation purposes to unambiguously report the compositions of the phases.
The estimated liquid compositions and temperatures of the invariant points in the pseudo-ternary “FeO1.5”-CaO-AlO1.5 system in air are summarized in Table II. The invariant points in the regions where no experimental data have been obtained in the present study are predicted using the FactSage package[40] with a separate internal database. The binary invariant temperatures and compositions are taken from Eriksson and Pelton (CaO-AlO1.5),[41] Prostakova et al. (“FeO1.5”-AlO1.5),[42] and Hidayat et al (“FeO1.5”-CaO)[43] with minor modifications from recent experimental studies by the authors.[44] The use of the database, which incorporates all the thermodynamic and phase equilibria data available on the system, ensures that any extrapolations or interpolations undertaken to predict the positions of the phase boundaries obey thermodynamic principles.
Liquidus Surface
Based on the experimental data points obtained in the present study given in Table I, the invariant points from Table II, and the thermodynamic database, the liquidus surface of the “FeO1.5”-CaO-AlO1.5 pseudo-ternary system in air has been constructed. Figure 4 shows the liquidus across the whole of the pseudo-ternary. The system contains the following primary phase fields: lime, corundum, hematite, spinel, CA6, C(A,F)3, CA2, CA, C2F3A, C3A, CF2, CF, and C2F. The presence of the primary phase field for the ternary compound 2CaO·3Fe2O3·Al2O3 (C2F3A) has not previously been reported in the literature.
Proposed liquidus surface of the “FeO1.5”-CaO-AlO1.5 system in air. Temperatures in °C. Phase boundaries and isotherms greater than 1450 °C are estimated and indicated by dash lines. Details of invariant points (a-z) are listed in Table II
The experimental liquidus data obtained in the present study have been obtained primarily at liquidus temperatures between 1200 °C and 1450 °C, in the compositional region of the C(A,F)3 primary phase field, and the boundaries and isotherms in these cases are marked in solid lines. The phase boundaries and the liquidus isotherms on the diagram at temperatures above 1450 °C are estimated using the thermodynamic database and are marked in both cases with dashed lines. The estimated temperatures (°C) of the invariant and saddle points (a-z) identified in Table II are also marked on the diagram.
Figure 4 shows that the liquidus of the system is dominated by the presence of the primary phase fields of compounds having fixed Ca/(A+F) ratios, these features reflect the extensive solid solutions present in these compounds that have been verified in previous[7,9,10,11,12,18] and the present experimental studies.
The diagram differs from that previously presented in Slag Atlas[15] in two principal respects:
-
(a)
the C12A7 phase has been previously shown to be a hydrate Ca12Al14O32(OH)2 and not present in the binary CaO-AlO1.5 system.[45,46] Based on that consideration, the CaO-AlO1.5 binary[41] and the phase boundaries in this region in the “ternary” system in the present study have been amended to reflect the presence of the eutectic between CA-C2F-C3A at 43.3 mol pct FeO1.5, 8.0 mol pct FeO1.5, 48.8 mol pct CaO, and 1324 °C (point l on the diagram in Figure 4.
-
(b)
the primary phase field for C2F3A has been included, and the phase relations in the low-temperature region centered around the C(A,F)3 primary phase field has been amended based on findings in the present study.
Figure 5 shows a detail of the liquidus of the “FeO1.5”-CaO-AlO1.5 system in the compositional region of the C(A,F)3 primary phase field. The liquidus temperature in the C(A,F)3 primary phase field (marked T) increases with decreasing CaO concentration, from a minimum value of 1189 °C at the point v, corresponding to the C(A,F)3-CF-C2F eutectic, to a maximum of 1413 °C, at point q, which indicates the equilibrium between the C(A,F)3-C(A,F)6-spinel phases. The range of temperatures over which the C(A,F)3 primary phase field is present is in close agreement with Dayal and Glasser[16] (1175 °C ~ 1435 °C).
Detail of liquidus in the “FeO1.5”-CaO-AlO1.5 system in air showing the phase relations of the compositional region of C(A,F)3 primary phase field. Temperatures in °C. Hatched area is the range of solid solution of T. Details of invariant points (a-z) are listed in Table II. Univariant lines are labeled with numbers and the phases in equilibrium are indicated by the capital letters given in the legend for the ease of presentation
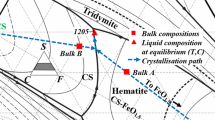
The liquid compositions measured in the present study in the compositional region of the C(A,F)3 primary phase field are shown in Figure 6. The phase boundaries between C(A,F)3 and all surrounding phases, hematite, spinel, CA6, CA2, CA, C2F3A, CF, and CF2 have been determined. It can be seen that the low-temperature region extends from point j on the “FeO1.5” – CaO pseudo-binary through points x-v-u-y-s until approximately 20mol pct AlO1.5. There are ternary eutectics at point s (liquid-C(A,F)-C2(F,A)-C2F3A) at 1205 °C and point v (liquid-C(F,A)-C(A,F)3-C2(F,A)) at 1189 °C.
Detail of the liquidus of the C(A,F)3, primary phase field in the “FeO1.5”-CaO-AlO1.5 system in air. Temperatures in °C. The measured liquid compositions and compositions of the corresponding C(A,F)3 (T) solid solution are also included. Experimental points on the univariant lines are underlined, details of the invariant points (a-z) are listed in Table II
The C2F3A phase is shown to be an incongruently melting compound with primary phase field bounded by points r-s-y-u-z-r, including the following features:
-
(i)
the r-z-u boundary between liquid-C(A,F)3-C2F3A;
-
(ii)
the maximum temperature to which C2F3A is stable is estimated to be 1230 °C (point z), above which it incongruently melts into liquid and C(A,F)3;
-
(iii)
the s-y-u boundary between liquid-C2(F,A) and C2F3A, with the local maximum at the saddle point y.
The invariant points: the peritectic reactions [liquid-C(A,F)-C2(F,A)-C(A,F)3)] at 1210 °C point r and [liquid-C(A,F)-C2(F,A)-C(A,F)3] at 1194 °C point u, and the ternary eutectics [liquid-C(A,F)-C2(F,A)-C2F3A] 1205 °C point s in Figure 5 and in Table II, and [liquid-C(F,A)-C (A,F)3-C2(F,A)] 1189 °C (point v) proposed in the present study differ from the ternary eutectic points [liquid-CA-CF-C2F] E3 at 1190 °C, [liquid-CA-CF-C(A,F)3] E4 at 1180 °C, and [liquid- C(A,F)3-CF-CF2] E5 at 1175 °C reported by Dayal and Glasser.[16] These differences in findings between the studies are principally due to the presence of the C2F3A phase. The reason why the absence of C2F3A in Dayal and Glasser’s study[16] may have been be due to the difference in experimental techniques. Dayal and Glasser relied principally on the use of optical microscopy and limited X-ray diffraction analysis for phase identification. In contrast, in the present study, significantly more weight has been given to the use of the direct and accurate measurement of phase compositions with EPMA. The C2F3A is more easily identified under backscattered electron mode rather than under reflected light. The narrow range over which the C2F3A phase can be formed also helps explain why the presence of this C2F3A primary phase field was not recognized in earlier investigations.
Isothermal Sections at Selected Temperatures
The estimated equilibria for the “FeO1.5”-CaO-AlO1.5 system in air, in the form of isothermal sections at 1200 °C, 1250 °C, 1300 °C, and 1400 °C, are shown in Figures 7(a) through (d). They are estimated with an internal database using FactSage,[40] in which the experimental data for the conjugate lines in binary and ternary phase fields obtained in the present study have been incorporated. The extent of solid solutions of the phases at these particular temperatures are taken from the ternary data obtained in the present study (Table I) for the hematite, spinel, C(A,F), C(A,F)2, C(A,F)3, C(A,F)6, and C2(F,A), C(F,A), C(F,A)2, and C2F3A phases (plotted on Figures 7(a) through (d)) and the following that were not measured in the current study (corundum, C2(F,A) >1300 °C or with high Al2O3, C3(A,F) and CaO), were predicted from the internal database in which previously available experimental data were assessed.[7,9,10,11,12,13,43,47] To assist in the readability of the diagram and understanding of the phase relations this complex system, each phase has been designated by capital letters A – V; the legend is provided in Figure 7(a). It can be seen that C(A,F)3 (designated T) is stable over a wide range of compositions at a given temperature with its relative stability decreasing with increasing temperature. Phase relations between the solid-phase C2F3A and liquid, CA, C2F, C(A,F)3 phases at 1200 °C are presented in Figure 7a.
Discussion
C(A,F)3 and C2F3A Solid Compositions
The measured compositions of C(A,F)3 and C2F3A solid solutions in equilibrium with the liquid phase at selected temperatures (see Table I) are plotted in Figure 8. The C(A,F)3 phase identified in the present study is found to have variable C/(A+F) stoichiometry that decreases with increasing temperature rather than lying on the “CA3”–“CF3” join (constant 14.3 mol pct CaO), as assumed by Dayal and Glasser.[8] This observation is in agreement with the findings of Malysheva et al.[18] who also reported variable CaO concentrations (12-17 wt pct CaO) in the C(A,F)3 solid solution from 1130 °C to 1260 °C.
Isotherms of the C(A,F)3 solid solution in equilibrium with liquid and compositions of the C2F3A solid obtained in the present study (compositions of different types of SFCA in this system previously reported by the authors[19,20,21,22] with their respective preparation temperatures are also plotted as points 1, 2, 3, 4, indicated in Fig. 2)
The apparent movement of the towards lower CaO concentration with increasing temperature could be explained by the change in the Fe2+/ Fe3+ concentration ratio in the slag with increasing temperature.[16] This can result in the partial replacement of Ca2+ ions in the solid by Fe2+ ions. It is also possible that some of the iron ions in the lattice sites occupied by Al3+ and Fe3+ ions are also replaced by Fe2+ ions, thus, reducing the Ca2+ requirement to obtain charge balance.
The AlO1.5 concentration in the C(A,F)3 solid solution in equilibrium with liquid is found to be in the range from 3.3 to 33.6 mol pct AlO1.5 from 1213 °C to 1400 °C, which extends to a lower alumina concentration compared to Dayal and Glasser’s study[16] (18.5 ~ 55.5 mol pct CA3 on the “CA3”-“CF3” scale, which corresponds to 15.8 ~ 47.6 mol pct AlO1.5) on liquidus temperatures at 1140 °C to 1330°C.
The C2F3A phase found in the present study is observed to have consistently lower CaO concentration (~18.6 mol pct) than its stoichiometric composition (20.0 mol pct). This is close to the composition \( {\text{Ca}}_{ 7. 1 2} {\text{Fe}}^{ 2+ }_{0. 8 8} {\text{Fe}}^{ 3+ }_{ 2 3. 8 2} {\text{Al}}_{ 8. 1 8} {\text{O}}_{ 5 6} \) (which corresponds to approximately 17.8 mol pct of CaO) reported by Arakcheeva et al.[19,20] In addition, the C2F3A phase obtained in the present study appears to be a solid solution with the Fe3+ ↔ Al3+ substitution taking place from 18.6 to 21.7 mol pct AlO1.5 from 1150 °C to 1225 °C.
Extent of AlO1.5 in C(A,F)3 Solid Solutions
The C(A,F)3 compositions listed in Table I are also projected as their AlO1.5 concentrations against equilibrium temperatures (Figure 9). The invariant points, univariant lines, and the solid phases in equilibrium are also identified by joining these solid compositions as plotted in Figure 9. The C(A,F)3 solid solution in equilibrium with liquid is found to be stable over the range 3.3~33.6 mol pct AlO1.5 from 1189 °C to 1413 °C; this is greater than previously reported by Dayal and Glasser[8] but close to that of Malysheva et al.
Equilibria Between Liquid and Spinel and Hematite Phases
Details of the equilibria between “FeO1.5”-CaO-AlO1.5 liquids in air and the hematite (H) and spinel (M) phases obtained in the present study are presented in Figure 10 also included on this diagram are the invariant points associated with the equilibria with the hematite and spinel phases. It is found that alumina is preferentially distributed into the liquid phase over both the hematite and spinel solid phases within the temperature and compositional ranges that have been studied.
Tie-lines joining solids (hematite and spinel) and liquids in equilibrium for the system “FeO1.5”-CaO-AlO1.5 in air, invariant points are listed in Table II, temperatures next to solid compositions in °C
In the case of the hematite, the mean distribution ratio of (mol pct Al in the liquid/mol pct Al in the solid) is found to be 1.73 between 1250 °C and 1350 °C; no significant solubility of CaO was found in hematite. For the spinel phase, the mean distribution ratio of (mol pct Al in the liquid/mol pct Al in the solid) is 1.24 between 1370 °C and 1450 °C, and the mol pct CaO in spinel decreases from 3.3 pct at 1370 °C to 1.4 pct at 1450 °C.
The experimental data obtained in the present study have helped to improve the understanding of phase formations during iron ore sintering and will be used for further revision and development of thermodynamic model of the FeO-Fe2O3-SiO2-CaO-Al2O3-MgO system for iron-making industries.
Summary
Experimental measurements have been undertaken to determine the liquidus surface of the pseudo-ternary system “Fe2O3”-CaO-Al2O3 in air with a particular focus on the stability field of C(A,F)3 (one of the end members of SFCA) in the system. The C(A,F)3 is an incongruently melting compound having a primary phase field extending from 1189 °C to 1413 °C. The extent of the alumina concentrations in C(A,F)3 varies with temperature, the minimum is 3.3 mol pct AlO1.5 at 1213 °C, and the maximum is 33.6 mol pct AlO1.5 at 1400 °C from the present investigations. The measured solid compositions also indicate a variable CaO concentration in C(A,F)3 phase extending from below to above stoichiometric CaO concentrations with increasing temperature from 1213 °C to 1400 °C. The extent of the C(A,F)3 primary phase field is limited by the formation of the following solid phases: CA [CaO·(Al,Fe)2O3], CA2 [CaO·2(Al,Fe)2O3], CA6 [CaO·6(Al,Fe)2O3], Hematite [(Fe,Al)2O3], Spinel [(Fe,Ca)O·(Fe,Al)2O3)], C2F [2CaO·(Fe,Al)2O3], C2F3A (2CaO·3Fe2O3·Al2O3), CF [CaO·(Fe,Al)2O3], and CF2 [CaO·2(Fe,Al)2O3].
The solid-phase C2F3A is also found to be an incongruently melting compound, which has been shown to be a solid solution containing between 18.6 and 21.7 mol pct of AlO1.5 and is stable up to 1225 °C. The C2F3A solid phase is indicated to be isostructural to SFCA-I according to previous researchers.
The additional phase equilibria measurements obtained in the present study provide a more complete and more accurate description of the Si-free end member of the SFCA solid solution, an important phase encountered in iron ore-sintering processes.
References
P. Dawson, Ironmak. Steelmak. 1993, vol. 20, pp. 137-143.
M.I. Pownceby, J.M.F. Clout and M.J. Fisher-White, Trans. Instn Min. Metall. Sect.C: Mineral Process Extr. Metall. 1998, vol. 107, pp. C1-C9.
J. Chen, M. Shevchenko, P. Hayes and E. Jak, ISIJ Int. 2018, vol. 59, pp. 795-804.
S. Cheng, M. Shevchenko, Peter C. Hayes and E. Jak, Metall. Mater. Trans. B 2019, vol. 51, pp. 1587–1602.
J. Chen, M. Shevchenko, P. Hayes and E. Jak, ISIJ Int. 2019, vol. 59, pp. 805-809.
M. I. Pownceby and J. M. F. Clout, Trans. Inst. Min. Metall., Sect. C 2000, vol. 109, pp. C36-C48.
A. Burdese and C. Brisi, Annali Chim. 1961, vol. 41, pp. 564-568.
J. Chen, S. Cheng, M. Shevchenko, P. C. Hayes and E. Jak, Metall. Mater. Trans. B 2021, vol. 52, pp. 517-527.
W. C. Hansen, L. T. Brownmiller and R. H. Bogue, J. Am. Chem. Soc. 1928, vol. 50, pp. 396-406.
H. F. McMurdie, J. Research NBS 1937, vol. 18, pp. 475-484.
C. Brisi, Annali Chim. 1954, vol. 44, p. 510.
T. F. Newkirk and R. D. Thwaite, J. Res. Nat. Bur. Stand. 1958, vol. 61, pp. 233-245.
C. M. Schlaudt and D. M. Roy, Nature (London) 1965, vol. 206, p. 819.
T. R. C. Patrick and M. I. Pownceby, Metall. Mater. Trans. B 2002, vol. 33B, pp. 79-89.
M. Allibert, H. Gaye, J. Geiseler, D. Janke, B. J. Keene, D. Kirner, M. Kowalski, J. Lehmann, K. C. Mills, D. Neuschutz, R. Parra, C. Saint-Jours, P. J. Spencer, M. Susa, M. Tmar and E. Woermann: Slag Atlas, 1995, p. 127.
R. R. Dayal and F. P. Glasser, Sci. Ceram. 1967, vol. 3, pp. 199, 203, 205-207.
D. H. Lister and F. P. Glasser, Trans. Brit. Ceram. Soc. 1967, vol. 66, pp. 293-305.
T. Y. Malysheva, I. V. Ostrovskaya, V. Y. Lyadova, M. S. Model and V. I. Ponomarev, Izv. Akad. Nauk SSSR, Met. 1976, vol. No 5, pp. 78-86.
A. V. Arakcheeva, O. G. Karpinskii and V. Ya. Lyadova, Kristallografiya 1991, vol. 36, pp. 603-611.
A. V. Arakcheeva, Zh. Strukt. Khim. (Zhurnal Strukturnoi Khimii) 1994, vol. 35, pp. 85-96.
W. G. Mumme, Neues Jahrb. Mineral., Abh. 2003, vol. 178, pp. 307-335.
W. G. Mumme, J. M. F. Clout and R. W. Gable, Neues Jahrb. Mineral., Abh. 1998, vol. 173, pp. 93-117.
W. G. Mumme, Neues Jahrb. Mineral., Monatsh. 1988, pp. 359-66.
J. D. G. Hamilton, B. F. Hoskins, W. G. Mumme, W. E. Borbidge and M. A. Montague, Neues Jahrb. Mineral., Abh. (Neues Jahrbuch fuer Mineralogie, Abhandlungen) 1989, vol. 161, pp. 1-26.
S. Nicol, J. Chen, M. I. Pownceby and Nathan A. S. Webster, ISIJ int. 2018, vol. 58, pp. 2157-72.
E. Jak, P. C. Hayes and H. G. Lee, Met. Mater. (Seoul, Repub. Korea) 1995, vol. 1, pp. 1-8.
E. Jak, In 9th Intl. Conf. on Molten Slags, Fluxes and Salts (MOLTEN12), The Chinese Society for Metals: Beijing, China, 2012, p. W077.
J. Chen, M. Shevchenko, P.C. Hayes and E. Jak, ISIJ Int. 2019, vol. 59, pp. 795-804.
M. Shevchenko and E. Jak, Metall. Mater. Trans. B, 2019, vol. 50, pp. 2780-2793.
M. Shevchenko and E. Jak, Ceram. Int. 2019, vol. 45, pp. 6795-6803.
J. Philibert, X-Ray Opt. X-Ray Microanal., 3rd Intl. Symp., Stanford Univ. 1963, pp. 379-92.
P. Duncumb and S.J.B. Reed: Quantitative Electron Probe Microanalysis, 6th ed., K.F.J. Heinrich, ed., NBS Spec. Publ. 298, US Dept. Commerce, 1968, pp. 133–54.
P. Duncumb, Electron Microsc. Anal., Proc. Anniv. Meet., 25th 1971, pp. 132-37.
S. Nikolic, P. C. Hayes and E. Jak, Metall. Mater. Trans. B, 2009, vol. 40B, pp. 892-899.
A. Fallah-Mehrjardi, T. Hidayat, P. C. Hayes and E. Jak, Metall. Mater. Trans. B 2017, vol. 48, pp. 3002-3016.
M. Shevchenko and E. Jak, J. Phase Equilib. Diff. 2019, vol. 40, pp. 319-355.
M. Timucin and A. E. Morris, Metall. Trans. 1970, vol. 1, pp. 3193-3201.
E. Jak, P. Hayes, A. D. Pelton and S. A. Decterov, In Proc. VIII Int’l Conf. on Molten Slags, Fluxes and Salts, (Santiago, Chile, 2009), pp 473-90.
S. A. Decterov, I.-H. Jung, E. Jak, Y.-B. Kang, P. Hayes and A. D. Pelton, In SAIMM Symposium Series S36 (VII Int. Conf. on Molten Slags, Fluxes & Salts), ed. C. Pistorius (The South African Institute of Mining and Metallurgy, Johannesburg, Republic of South Africa: 2004), pp 839-50.
FactSage. (Ecole Polytechnique, Montréal, http://www.factsage.com/, 2008).
G. Eriksson and A. D. Pelton, Metall. Trans. 1993, vol. 24, pp. 807-816.
V. Prostakova, D. Shishin, M. Shevchenko and E. Jak, Calphad 2019, vol. 67, p. 101680.
T. Hidayat, D. Shishin, S.A. Decterov and E. Jak, Metall. Trans. B 2016, vol. 47, pp. 256-281.
S. Cheng, M. Shevchenko, P.C. Hayes and E. Jak, Experimental Phase Equilibria Studies in the FeO-Fe2O3-CaO system, private communication, PYROSEARCH, The University of Queensland, Brisbane, QLD, 2020.
R. W. Nurse, J. H. Welch and A. J. Majumdar, Trans. Brit. Ceram. Soc. 1965, vol. 64, pp. 409-418.
R. W. Nurse, J. H. Welch and A. J. Majumdar, Trans. Brit. Ceram. Soc. 1965, vol. 64, pp. 323-332.
D. Shishin, V. Prostakova, E. Jak and S. Decterov, Metall. Mater. Trans. B 2016, vol. 47, pp. 397-424.
Acknowledgments
The authors would like to acknowledge the Australian Research Council Linkage Program and Altonorte Glencore, Atlantic Copper, Aurubis, Olympic Dam Operation BHP Billiton, Kazzinc Glencore, PASAR Glencore, Outotec Oy (Espoo), Anglo American Platinum, Umicore, and Kennecott Rio Tinto for financial support to enable this research to be carried out, the Centre for Microscopy and Microanalysis, at the University of Queensland for providing electron microscope facilities and the scientific and technical assistance, and Ms. Suping Huang, Ms. Marina Chernishova, and Mr. Shuyi Lou for assistance in experimental work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted November 4, 2020; accepted April 18, 2021.
Rights and permissions
About this article
Cite this article
Cheng, S., Shevchenko, M., Hayes, P.C. et al. Experimental Phase Equilibria Studies in the FeO-Fe2O3-CaO-Al2O3 System in Air. Metall Mater Trans B 52, 2416–2429 (2021). https://doi.org/10.1007/s11663-021-02198-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02198-3