Abstract
It is hard to produce dense refractory Ti5Si3 films at low temperature because of the extremely high melting point (2130 °C) of Ti5Si3. Herein, we report the facile electrodeposition of Ti5Si3 films from TiO2 and SiO2 in molten CaCl2-CaO at 850 °C. Crystalline Ti5Si3 films with tunable film thicknesses and morphologies can be obtained in a controlled manner. The dissolution-electrodeposition processes were systematically investigated by cyclic voltammetry (CV), in-situ X-ray diffraction (XRD), in-situ Raman analysis, etc., and the synthesized Ti5Si3 products were characterized by electron backscattered diffraction (EBSD), transmission electron microscopy (TEM), three-dimensional atomic probe (3DAP), etc. The results show that the morphology of the products can be significantly influenced by current density. By controlling current density at the range from 10 to 25 mA cm−2, Ti5Si3 products with different morphologies, i.e., dendritic particles, dense films, and porous powders, can be obtained in a controlled manner. Besides, Ti, Si atoms uniformly distributed in the films. The reaction mechanism of the formation of Ti5Si3 film was also proposed, which can be summarized as three periods: CaO-assisted dissolution of SiO2 and TiO2, the electrodeposition of Si and Ti, and the formation of stable Ti5Si3 phase. The CaO-assisted dissolution-electrodeposition process may provide a promising strategy for the production of Ti5Si3 alloy films and powders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of modern science and technology, silicides with a high melting point have become a hotspot in the field of high-temperature structural materials, which are widely used in the national defense and industrial fields. Among these silicides (such as TiSi2, NiSi, Ti5Si3, and CoSi), Ti5Si3 acts as a typical silicon intermetallic compound in the Ti-Si binary system,[1] as shown in Figure 1, and has received a tremendous amount of attention due to its distinct properties such as low density (4.32 g cm−3), low electrical resistivity (50 to 120 μΩ cm), high melting point (2130 °C), excellent oxidation resistance, and high specific strength.[2,3,4,5,6,7,8,9,10] In addition to the preceding ideal performances, Ti5Si3 also has high hardness, unique chemical composition, and strong covalent bond, which causes it to potentially be regarded as a wear-resistant and corrosion-resistant coating material.[11]
Phase diagram of Ti-Si system, adapted from Ref. 1
Based on these excellent properties and bright application prospects, many researchers have tried to prepare Ti5Si3 intermetallic compounds by various methods. However, previous studies about the synthesis of Ti5Si3 mainly focused on conventional methods, including self-propagating high-temperature synthesis, mechanical alloying, and spark plasma sintering.[5,6,7,12,13,14,15,16] Most of these methods involve a process of arc melting of the ultrapure elements Ti and Si, which are produced by energy intensive and tedious processes, respectively. To alleviate this problem, many recent works focused on the production of Ti5Si3 have been reported. Estruga et al.[17] reported a novel synthesis of Ti5Si3 nanoparticles (NPs) via the magnesio-reduction of TiO2 and SiO2 NPs in eutectic LiCl-KCl molten salts at 700 °C. Devyatkin et al.[18] successfully obtained Ti5Si3 powders on nickel cathodes through electrodeposition from the chloro-fluoride melts (NaCl-KCl-K2SiF6-K2TiF6). Peng et al.[19] also reported a novel method for preparing Ti5Si3 powders from Ti-bearing blast furnace slag, which provided a feasible route for the treatment of titanium-bearing blast furnace slag and the recovery of titanium resources. Jiao et al.[20] and our previous work[21,22,23,24] also reported the preparation of Ti5Si3 powders from their Ti/Si-containing metal oxide compounds using the Fray–Farthing–Chen Cambridge process and the solid oxide oxygen ion-conducting membrane-assisted electro-deoxidation process.
However, the methods mentioned previously can only produce Ti5Si3 powders other than dense films. It is still difficult to facilely produce dense crystalline Ti5Si3 films because of the high melting point of Ti5Si3. At present, Ti5Si3 films are mostly prepared by chemical vapor deposition and laser cladding.[2,25,26,27] These technologies have strict requirements on raw materials and the quality of the deposited products is hard to control. Therefore, searching for a cost-effective method is imminent at present.
In the present study, we report the successful demonstration of Ti5Si3 film electrodeposition in molten CaCl2-CaO containing titanium/silicon dioxides at a relatively low temperature (850 °C). The method described here is similar to the pulse electrodeposition of TiB2 thin films from fluoride melts.[28,29] The electrodeposition process was systematically investigated and the obtained Ti5Si3 products were characterized by electron backscattered diffraction (EBSD), transmission electron microscopy (TEM), three-dimensional atomic probe (3DAP), etc. Besides, the reaction mechanism for the electrodeposition of Ti5Si3 films was also proposed and discussed.
Experimental
Solubility Test of TiO2/SiO2 in CaCl2-CaO Melts at 850 °C
The solubility test for the SiO2 pellet was reported in the previous work and in our recent work.[30,31,32,33] In this work, the solubility test for TiO2 is the same as for SiO2. 2 g TiO2 (99.5 pct, Sigma-Aldrich) powders were mixed with ~10 pct polyvinyl butyral by mechanical grinding, and then the milled mixture was pressed under 10 MPa to form a cylindrical pellet (15 mm in diameter and ~2 mm in thickness). The pellet was sandwiched by porous nickel foil and then immersed in 100 g molten CaCl2 (99 pct, Sigma-Aldrich) with the addition of 2 g CaO (99.9 pct, Sigma-Aldrich) at 850 °C for 24 hours. High-purity argon gas was continuously purged into the furnace during the entire process. Then the TiO2 pellet was taken out from the molten salts and washed by distilled water. The immersed pellet was characterized by X-ray diffraction (XRD) spectroscopy. Besides, a small amount of molten salt was taken out from the crucible and diluted with deionized water. Then the diluted solution was detected by an inductively coupled plasma atomic emission spectrometer (ICP-AES, 7300DV) to determine the solubility of TiO2 in molten CaCl2-CaO.
In-Situ XRD and In-Situ Raman Experiments
In-situ XRD and in-situ Raman experiments were performed to analyze the dissolution processes for SiO2 and TiO2 in molten CaCl2-CaO electrolyte. 0.1 g SiO2 (99.5 pct, Sigma-Aldrich), 0.1 g TiO2, 0.1 g CaO, and 0.3 g CaCl2 were weighed and mixed. Then the mixed powders were placed upon a ceramic sieve (pore size of 0.2 mm) so that the high-purity argon gas could penetrate the sample and sample holder. This in-situ XRD experiment was performed using an X-ray powder diffractometer (Bruker D8 Advance, with Cu Kα1 radiation). XRD patterns were collected at different temperatures (every 50 °C from 50 °C to 850 °C).
Before the in-situ Raman experiment, the sample (CaCl2:CaO:SiO2:TiO2 = 30:2:1:1, mol pct) was mixed and then predissolved in a crucible at 850 °C. When cooled to 100 °C, the electrolyte sample was taken out and ground to a fine powder. The electrolyte sample was loaded into a platinum crucible for the in-situ Raman experiment and then heated from room temperature to 850 °C with a heating rate of 10 °C /min. It was recorded using a Horiba Jobin Y’von LabRAM HR Evolution laser confocal Raman spectrometer, which was equipped with an intensified charge coupled device detector and a hot stage (High-Temperature Observational System IRF-DP). A visible pulsed laser beam of 532 nm was used as an excitation source.
Cyclic Voltammetry Measurements
Cyclic voltammetry (CV) analysis was conducted to investigate the electrochemical behaviors of the electrolyte containing 100 g CaCl2 with the addition of 2 g SiO2, 2 g TiO2, and 2 g CaO by using a three-electrode setup at 850 °C. Before the CV tests, the molten electrolyte was kept at 850 °C for 24 to 48 hours. The working electrode was a dense graphite plate (75-mm length × 2-mm width × 1-mm thickness). Graphite rods were employed as a counter electrode (6 mm in diameter) and a pseudo-reference electrode (3 mm in diameter), respectively. All electrodes were leaded by tungsten wires. More details about the CV experiment can be found in our previous work.[32,33]
Molten Salt Electrodeposition Experiments
A schematic diagram of the electrolytic cell for the electrodeposition of Ti5Si3 film is shown in Figure 2. In a typical experiment, 2 g SiO2, 2 g TiO2, and 2 g CaO were weighed and filled into a quartz crucible, and then 100 g CaCl2 was placed into the crucible. CaO would act as a kind of cosolvent to accelerate the dissolution processes of TiO2 and SiO2. Subsequently, the crucible filled with these materials was put into a one-end close fused quartz tube in a furnace. The furnace was heated to 400 °C for 24 hours to make sure the possible water residual can be removed completely. And then the furnace was heated to 850 °C and kept at the temperature for 24 to 48 hours. During this period, pre-electrolysis was first carried out at 2.5 V for 12 to 24 hours between two graphite electrodes (graphite rod: 6-mm diameter × 50-mm length) to remove the possible impurities contained in the molten salts. After that, Ti5Si3 films were electrodeposited on a graphite substrate (graphite plate: 10-mm width × 50-mm length × 1-mm thickness) by applying pulse current density from 10 to 25 mA cm−2, with a graphite rod as the anode. It should be noted that during the entire process, ultra-high-purity argon gas (99.999 pct) was continuously purged into the furnace to keep an inert atmosphere. After the electrolysis experiment, the deposited products were removed from the furnace and washed with tap water/distilled water as well as dried under vacuum.
Materials Characterization
The deposited products were characterized using XRD (Bruker-AXS D8 Advance), and the morphology of the synthesized films was examined by a scanning electron microscope (SEM, Quanta 650 FEG). The elemental composition was analyzed by an energy-dispersive X-ray spectroscope (EDS, XFlash Detector 5010) attached to the SEM. Before being analyzed by TEM (JEOLFootnote 1 2100F) and 3DAP (LEAP 4000X HR), a dual beam focused ion beam (FIB, JEOL JIB-4500) was used to prepare the thin section out of Ti5Si3 film grown on the graphite substrate using the lift-out method. EBSD (Oxford Nordly max3) was also used to analyze the crystallographic orientation of deposited crystalline Ti5Si3.
Results and Discussion
Dissolution Behaviors of TiO2/SiO2 in Molten CaCl2-CaO at 850 °C
For the TiO2 dissolution process, the solubility of TiO2 in molten calcium chloride is generally low; however, the addition of CaO in molten CaCl2 would also facilitate the dissolution process.[34,35] The use of CaO to enhance the solubility of TiO2 in molten calcium chloride is similar to the previous work in fluorides.[36,37] In this work, the dissolution of TiO2 in CaCl2-CaO melts was determined by measuring the concentration of the titanium element in the melts. After the calculation, the solubility of TiO2 in molten CaCl2-2 wt pct CaO at 850 °C was determined to be approximately 0.43 mol pct by ICP analysis. The ICP results suggest that titanium oxides can dissolve in molten CaCl2 with the addition of CaO, and the solubility of TiO2 generally depends on the content of CaO dissolved in the molten CaCl2.[34,35]In our previous work,[31,33] it was confirmed that SiO2 would be converted into silicates ions (such as SiO44−) in molten CaCl2-CaO; then silicate ions could be reduced to form Si during electrodeposition. The solubility of SiO2 in molten CaCl2-CaO also generally depends on the content of CaO.[30] Thanks to the high solubility of CaO (~ 20 mol pct) in molten CaCl2,[38] the solubility of SiO2 in CaCl2-2 wt pct CaO at 850 °C can be controlled at about 1.5 wt pct. It has been proved that the solubilities of TiO2 and SiO2 both increase with increasing temperature,[30,34,35] which implies that the solubilities of TiO2 and SiO2 can be controlled by varying the temperature and the CaO concentration in the molten salts. Accordingly, the tunable solubilities of SiO2 and TiO2 offer access to the direct electrodeposition of titanium silicides in molten CaCl2-CaO.
In order to explore the dissolution behaviors of TiO2/SiO2 in molten CaCl2 with the addition of CaO, the intermediate products of the TiO2 and SiO2 pellets after being immersed in molten CaCl2-CaO at 850 °C for 24 hours were characterized by XRD, as shown in Figure 3. According to the results shown in Figure 3(a), only one calcium silicate (Ca2SiO4) appears on the SiO2 pellet’s surface after immersion, which is different from our assumption that various calcium silicates (such as CaSiO3, Ca2SiO4, and Ca3Si2O7) may be formed during the dissolution process. This finding indicates that Ca2+ and O2– dissolved in molten CaCl2 electrolyte would react with SiO2 to form Ca2SiO4,[39] as expressed by Reaction [1]; then, Ca2SiO4 would dissolve into molten CaCl2 to form SiO44– (Reaction [2]).
Based on Figure 3(b), only one calcium titanate (CaTiO3) is observed as the intermediate product on the surface of the TiO2 pellet, which confirms that TiO2 can react with Ca2+ and O2– to form CaTiO3,[40,41,42] as described by Reaction [3]. Then, CaTiO3 would dissolve into the molten salts through Reaction [4]. It should be noted that Reactions [1] through [4] occur simultaneously during the dissolution process.
Electrochemical Characteristics of the Process
The CV curve of the electrodeposition process in molten CaCl2-CaO containing SiO2 and TiO2 is shown in Figure 4(a). It can be seen that three reduction peaks and two oxidation peaks appeared. Peaks A1 and C1 at around 0/–0.6 V are mainly attributed to the reduction of Ca2+ and the dissolution of Ca, respectively. Peak C3 at approximately 0.75 V is attributed to the reduction of silicate ions.[31] During the dissolution process, CaO would dissolve into molten CaCl2 to form Ca2+ and O2– and then O2– would react with SiO2 to form SiO44–. SiO44– was reduced to silicon at a potential of approximately 0.75 V, as confirmed in our previous work.[31,33] Compared to the CV curve obtained in molten CaCl2-CaO dissolved only SiO2,[31] it is obvious that the peak C2 can be ascribed to the reduction of titanate ions. Accordingly, peak A2 starting at 0.5 V is the oxidation peak of the formed silicide TiSix. Based on the CV analysis, it is believed that TiO2 particles would also react with CaO (Ca2+, O2–) to form titanate ions, i.e., TiO32–; then, the titanate ions can be reduced to Ti through electrodeposition.[34,35] The detailed reaction mechanisms, including the dissolution and the electrodeposition processes, will be discussed and summarized later. In this work, the pulse electrodeposition process (15 seconds at a current density of 10 to 25 mA cm−2 for electrodeposition and then 5 seconds at a current density of 0 mA cm−2 for the depletion layer to recover and bring ions to the electrode surface) was used to deposit TiSix films. Figure 4(b) presents the typical potential-time curves of the pulse electrodeposition process, which shows a relatively stable electrodeposition process. Commonly, excessive TiO2 and SiO2 can be added into the molten CaCl2-CaO, and these excessive TiO2 and SiO2 would remain at the bottom of the electrolytic cell. When the TiO2/SiO2 dissolved in CaCl2 is consumed, the excess TiO2/SiO2 remaining at the bottom could be continuously dissolved. Therefore, the dissolution and electrodeposition processes happen simultaneously, which means that the electrodeposition process is a continuous production process. Actually, continuous production of silicon through the dissolution-electrodeposition processes was demonstrated in our recent work.[43]
Characterization of the Ti-Si Products
The deposited Ti-Si films were analyzed using XRD, as shown in Figure 5. The result shows that the Ti-Si film is Ti5Si3 (JCPDS: 08-0041), and the lattice constants of Ti5Si3 can also be determined (a = 7.429 Å, c = 5.139 Å). Besides, the appearance of carbon peak is due to the graphite substrate. For comparison, the typical XRD patterns of the starting raw materials (TiO2 and SiO2) are also presented in Figure 5. It is evident that crystalline Ti5Si3 has been successfully produced from molten CaCl2-CaO dissolved with TiO2 and SiO2 by using pulse electrodeposition. The Ti5Si3 deposited at the current density of 15 mA cm−2 was also characterized by SEM and EDS analyses, as shown in Figure 6. It is evident that Ti5Si3 films with different thicknesses were deposited on the graphite substrate (Figures 6(a) through (c)) by changing the electrodeposition time, and the thickness of Ti5Si3 films can reach about 20 μm within 7 hours, as shown in Figure 6(c). It is suggested that the thickness and micromorphology of the film can be controlled by changing the current density, electrodeposition time, etc. Figures 6(d) through (f) are the elemental maps of the SEM image shown in Figure 6(c). The EDS mappings show that Ti, Si elements are uniformly distributed in the films, which further confirms the formation of Ti5Si3 film on the graphite substrate. Besides, the surface morphology observations (Figures 6(g) through (i)) further demonstrate that the crystal size of the deposited films can be controlled by changing the deposition time. At the beginning, the size of grains is not uniform, as shown in Figure 6(g). When the electrodeposition time reaches 3 hours, dense film gradually forms and the grains become more homogeneous.
SEM images of the cross sections of the Ti5Si3 films obtained at 850 °C for (a) 1 h, (b) 3 h, and (c) 7 h with a current density of 15 mA cm−2; (d) through (f) corresponding EDS elemental mappings over the image (c); (g) through (i) SEM images of the surfaces of the Ti5Si3 films obtained at 850 °C for 1, 3, and 7 with a current density of 15 mA cm−2, respectively
In order to further characterize the deposited Ti5Si3 film, 3DAP analysis was performed. Figure 7 presents the elemental distribution of Ti and Si atoms in the Ti5Si3 film deposited at 15 mA cm−2 for 3 hours. It can be seen that Ti and Si elements are uniformly distributed in the Ti5Si3 film. To understand the growth process and crystal orientation of the deposited Ti5Si3 film, EBSD analysis was also performed. Figure 8(a) shows the SEM image of the scanning area. Based on the EBSD analysis result, it can be seen that equiaxed grains with a random orientation start to deposit on the graphite substrate during the initial period (Figures 8(b) and (c)). Gradually, the grains show a preferred orientation with a fiber texture of (0001) along the normal direction and present a long column (Figures 8(b) through (d)), which is similar to the solidification behavior of columnar crystals. The misorientation angle distribution in Figure 8(e) also reveals that there are many low-angle grain boundaries with grain misorientations less than 5 deg, and high-angle grain boundaries are mainly distributed under 45 deg, which results from the fiber texture.
(a) SEM image of the cross section for the Ti5Si3 film deposited at 15 mA cm−2 for 3 h; (b) corresponding inverse pole figure (IPF)-Y (normal direction) and (c) IPF-Z; (d) distribution of orientation along the normal direction; and (e) fraction of misorientation angles for the microstructure of Ti5Si3 in (a)
Except Ti5Si3 film, Ti5Si3 powders can also be produced by electrodeposition. Figure 9 displays the SEM images of products obtained at different current densities. Figure 9(a) shows the SEM image of the surface of Ti5Si3 film deposited at 15 mA cm−2 for 3 hours and its corresponding elemental mappings. Apparently, Ti and Si elements are distributed uniformly on the surface. When the current density increases to 25 mA cm−2, the nucleation rate increases; thus, porous Ti5Si3 powders are formed. On the contrary, when the current density decreases to 10 mA cm−2, the nucleation rate decreases and the formed nuclei have sufficient time to grow to form dendritic particles, as shown in Figure 9(c). In this work, the current efficiency for the electrodeposition of Ti5Si3 film is hard to accurately calculate because a small amount of powders would be generated on the surface of Ti5Si3 films. However, according to our previous work[31,32,33] and based on the similar experimental observations, the current efficiencies for the electrodeposition of Ti5Si3 products at 10, 15, and 25 mA cm−2 are believed to be approximately 50 to 60 pct.
The typical Ti-Si products deposited on graphite substrate were then analyzed by TEM, and the TEM images are shown in Figures 10(a) through (d). Figure 10(a) and (c) are the high-resolution TEM (HRTEM) images, while Figures 10(b) and (d) are their corresponding FFT diffraction patterns. The FFT image in Figure 10(b) displays typical features of an equilateral triangle, which matches a [001] crystal belt axis diffraction pattern. According to the crystal interplanar spacing marked in Figure 10(a), the lattice constant can be estimated to be 7.44 Å, which is consistent with the XRD results. Figure 10(c) shows the characteristic spacings of 0.627, 0.402, and 0.399 nm for the (0\( \bar{1} \)0), (101), and (1\( \bar{1} \)1) lattice planes (Figure 10(d)). It can be inferred from Figure 10(c) that the lattice constants are 7.44 Å and 5.114 Å, which also agree with the XRD results. Thus, a hexagonal Ti5Si3 compound (P63/mcm) was confirmed and its crystal structure is inserted in Figure 10(d).
Reaction Mechanisms
As expected, Ti5Si3 films have been directly electrodeposited from molten CaCl2-CaO dissolved with TiO2 and SiO2. Based on the experimental results and the previous studies,[30,33,34] the general reaction mechanism of the electrodeposition of Ti5Si3 can be summarized as three periods: (1) CaO-assisted dissolution of SiO2 and TiO2 in molten CaCl2 to form silicate ions (SiO44–) and titanate ions (TiO32–), respectively; (2) electrodeposition of Si and Ti from silicate ions and titanate ions, respectively; and (3) formation of stable Ti5Si3 through Si, Ti → TiSi2 → Ti5Si3.
-
(1)
It has been proved that the addition of CaO can promote the dissolution of SiO2 and TiO2 in molten CaCl2.[30,35] To reveal the CaO-assisted dissolution process more intuitively, in-situ XRD analysis and an in-situ Raman experiment were performed to explore the phase transformation during the dissolution process. Figure 11(a) plots the accumulated in-situ XRD data collected during the process. When the temperature is below 400 °C, the phase composition does not change. With the increase of temperature, Ca4OCl6 begins to appear. When the temperature reaches about 750 °C or higher, the peaks of SiO2, TiO2, and CaO disappear and Ca2SiO4, Ca3SiO4Cl2, and CaTiO3 appear, which is consistent with the preceding results. Based on these results, the main chemical reactions that occurred during the entire heating process can be seen in Figure 11(b).
Fig. 11 Figure 12 shows the in-situ high-temperature Raman spectra and their corresponding fitting results. Based on previous studies,[44,45,46] the Raman peaks in Figures 12(a) and (b) correspond to [TiO3]2– and [SiO4]4–, respectively, which is consistent with the in-situ XRD results (Figure 11). When adding SiO2 and TiO2 into the molten CaCl2-CaO simultaneously, the silicate ions and titanate ions coexist in the molten salts, as shown in Figure 12(c). The Raman peaks at 160, 223, 280, 337, 673, and 797 cm−1 in Figure 12(c) are assigned to [TiO3]2–. The vibrational modes of 223, 280, and 337 cm−1 are associated with the O-Ti-O bending vibrations.[47] The Raman peak 673 cm−1 originates from the Ti-O symmetrical vibrations.[44] Besides, the remaining peaks at 443 and 979 cm−1 are related to [SiO4]4–.[48] These results indicate that CaO can accelerate the dissolution processes for SiO2 and TiO2 in molten CaCl2.
-
(2)
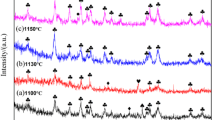
After 24 to 48 hours of the dissolution process at 850 °C, sufficient silicate ions and titanate ions are formed and dissolved in the CaCl2 melt. When a pulse current is applied, these silicate ions and titanate ions around the cathode are electroreduced to Si and Ti metals, respectively, and the related electrochemical reactions are listed as Reactions [5] and [6]. Figure 13 shows the XRD result of the products collected from the graphite cathode when applying a pulse current of 25 mA cm−2 for 0.5 hours. It can be seen that silicon is formed during this process, which suggests that silicate ions would be reduced to form silicon prior to the electroreduction of titanate ions. Although no titanium is observed, the lower oxide Ti5O9 appears; this observation is similar to the report of Suzuki.[49] It is suggested that the reduction of titanate ions involves a multistep reaction process in the CaCl2 melt (Reactions [7] and [8]), which is consistent with the previous work.[22,50]
$$ {\text{SiO}}_{4}^{4 - } + 4e^{-} \to {\text{Si}} + 4{\text{O}}^{2 - } , $$(5)$$ {\text{TiO}}_{3}^{2 - } + 4{\text{e}}^{-} \to {\text{Ti}} + 3{\text{O}}^{2 - } , $$(6)$$ 5{\text{TiO}}_{3}^{2 - } + 2{\text{e}}^{-} \to {\text{Ti}}_{5} {\text{O}}_{9} + 6{\text{O}}^{2 - } , $$(7)$$ {\text{Ti}}_{x} {\text{O}}_{(2x + y)}^{2y - } + 4x{\text{e}}^{-} \to x{\text{Ti}} + \left( {2x + y} \right){\text{O}}^{2 - } . $$(8) -
(3)
The electrodeposited titanium and silicon would react with each other to form various kinds of titanium-silicon silicides (Reactions [9] through [12]), such as TiSi, TiSi2, Ti5Si3, and Ti5Si4.[18,51] With prolonged time, Ti5Si3 finally would be obtained (Si, Ti → TiSi2 → Ti5Si3) because of its excellent stability among the titanium silicides,[52] which was also discussed in our previous studies.[22]
$$ {\text{Ti}} + 2{\text{Si}} \to {\text{TiSi}}_{2} , $$(9)$$ {\text{TiSi}}_{2} + {\text{Ti}} \to 2{\text{TiSi,}} $$(10)$$ 4{\text{TiSi}} + {\text{Ti}} \to {\text{Ti}}_{5} {\text{Si}}_{4} , $$(11)$$ 3{\text{Ti}}_{5} {\text{Si}}_{4} + 5{\text{Ti}} \to 4{\text{Ti}}_{5} {\text{Si}}_{3} . $$(12)
It is noteworthy that during the entire process, the formation steps mentioned previously are not necessarily in a chronological order; they can occur simultaneously during the electrodeposition process. When silicate ions and titanate ions in the molten salts are reduced to Si and Ti, the continuous dissolution process takes place accordingly and the dissolution-electrodeposition processes proceed simultaneously. We note that the reaction mechanism can only be considered as a general guideline to understand the electrodeposition process; further investigation is currently being undertaken.
Conclusions
We have demonstrated that Ti5Si3 films can be successfully electrodeposited in molten CaCl2-CaO dissolved with TiO2 and SiO2 at a relatively low temperature (850 °C) by applying pulse current. The morphology of the products can be significantly influenced by current density. By controlling current density at the range from 10 to 25 mA cm−2, Ti5Si3 products with different morphologies, i.e., dendritic particles, dense films, and porous powders, can be obtained in a controlled manner. Besides, Ti, Si atoms uniformly distributed in the films. The mechanism of the formation of Ti5Si3 film can be divided into three periods: (1) CaO-assisted dissolution of SiO2 and TiO2 in molten CaCl2 to form silicate ions and titanate ions, respectively; (2) electrodeposition of Si and Ti from silicate ions and titanate ions, respectively; (3) formation of stable Ti5Si3 through Si, Ti → TiSi2 → Ti5Si3. The CaO-assisted dissolution-electrodeposition strategy may have great potential to be used for the production of refractory Ti5Si3 alloy films or powders. This strategy may also have implications for the production of other refractory alloys.
Notes
*JEOL is a trademark of JEOL, Tokyo.
References
1. T.B. Massalski, P.R. Subramanian, H. Okamoto, and L. Kacprzak: Binary Alloy Phase Diagrams, 2nd ed., ASM International, Materials Park, OH, 1990.
2. Z. Ren, Y. Huang, M. Shen, C. Song, W. Weng, G. Han, N. Ma, and P. Du: J. Non-Cryst. Solids, 2011, vol. 357, pp. 2802–09.
3. K. Ito, T. Hayashi, and H. Nakamura: Intermetallics, 2004, vol. 12, pp. 443–50.
4. L. Zhang and J. Wu: Acta Mater., 1998, vol. 46, pp. 3535–46.
5. K. Kasraee, M. Yousefpour, and S.A. Tayebifard: J. Alloys Compd., 2019, vol. 779, pp. 942–49.
6. S. Li, X. Zou, K. Zheng, X. Lu, C. Chen, X. Li, Q. Xu, and Z. Zhou: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 790–802.
7. W.H. Lee, Y.W. Cheon, Y.H. Yoon, C.H. Jeong, C.J. Van Tyne, and H.G. Lee: Int. J. Refract. Met. Hard Mater., 2019, vol. 80, pp. 174–80.
8. J.H. Schneibel and C.J. Rawn: Acta Mater., 2004, vol. 52, pp. 3843–48.
9. S. Agarwal, E.J. Cotts, S. Zarembo, R. Kematick, and C. Myers: J. Alloys Compd., 2001, vol. 314, pp. 99–102.
10. D. Szwagierczak: Ceram. Int., 2004, vol. 30, pp. 757–64.
11. L. Liu, J. Xu, P. Munroe, and Z.-H. Xie: Electrochim. Acta, 2014, vol. 115, pp. 86–95.
12. C.L. Yeh, W.H. Chen, and C.C. Hsu: J. Alloys Compd., 2007, vol. 432, pp. 90–95.
13. J. Liu, Y. Bai, P. Chen, N. Cui, and H. Yin: J. Alloys Compd., 2013, vol. 555, pp. 375–80.
14. S.G. Vadchenko, A.E. Sytschev, D.Y. Kovalev, A.S. Shchukin, and A.F. Belikova: Int. J. Self-Propag. High-Temp. Synth., 2014, vol. 23, pp. 141–44.
15. K. Kasraee, A. Tayebifard, and E. Salahi: J. Mater. Eng. Perform., 2013, vol. 22, pp. 3742–48.
16. A. Calka, A.P. Radlinski, R.A. Shanks, and A.P. Pogany: J. Mater. Sci. Lett., 1991, vol. 10, pp. 734–37.
17. M. Estruga, S.N. Girard, Q. Ding, L. Chen, X. Li, and S. Jin: Chem. Commun., 2014, vol. 50, pp. 1454–57.
S.V. Devyatkin, O.I. Boiko, N.N. Uskova, and G. Kaptay: Z. Naturforsch. A: Phys. Sci., 2001, vol. 56, pp. 739–40.
19. B. Peng, L. Wu, J.-H. Zhu, Y. Hou, and G.-H. Zhang: Metall. Res. Technol., 2020, vol. 117, p. 614.
20. H. Jiao, Q. Wang, J. Ge, H. Sun, and S. Jiao: J. Alloys Compd., 2014, vol. 582, pp. 146–50.
21. X. Zou, X. Lu, Z. Zhou, W. Xiao, Q. Zhong, C. Li, and W. Ding: J. Mater. Chem. A, 2014, vol. 2, pp. 7421–30.
22. X. Zou, X. Lu, Z. Zhou, C. Li, and W. Ding: Electrochim. Acta, 2011, vol. 56, pp. 8430–37.
23. X. Zou, X. Lu, Z. Zhou, and C. Li: Electrochem. Commun., 2012, vol. 21, pp. 9–13.
24. X. Zou, X. Lu, C. Li, and Z. Zhou: Electrochim. Acta, 2010, vol. 55, pp. 5173–79.
25. D. Gu, J. Liu, G. Meng, C. Li, Y. Li, and Y. Shen: Mater. Sci. Technol., 2011, vol. 27, pp. 1736–40.
26. D. Gu, Y. Shen, and Z.J.M.L. Lu: Mater. Lett., 2009, vol. 63, pp. 1577–79.
27. D. Vojtech, P. Novak, P. Machac, A. Mort’anikova, and K. Jurek: J. Alloys Compd., 2008, vol. 464, pp. 179–84.
28. L. Jun and L. Bing: Mater. Lett., 2007, vol. 61, pp. 1274–78.
29. G. Ett and E.J. Pessine: Electrochim. Acta, 1999, vol. 44, pp. 2859–70.
30. W. Xiao, X. Wang, H. Yin, H. Zhu, X. Mao, and D. Wang: RSC Adv., 2012, vol. 2, pp. 7588–93.
31. X. Zou, L. Ji, J. Ge, DR. Sadoway, E.T. Yu, and A.J. Bard: Nat. Commun., 2019, vol. 10, pp. 5772–79.
32. X. Zou, L. Ji, X. Yang, T. Lim, E.T. Yu and A.J. Bard: J. Am. Chem. Soc., 2017, vol. 139, pp. 16060–16063.
X. Yang, L. Ji, X. Zou, T. Lim, J. Zhao, E.T. Yu, and A.J. Bard: Angew. Chem., Int. Ed., 2017, vol. 56, pp. 15078–82.
34. T. Takenaka, K. Shimokawa, K. Nishikawa, H. Okada, and T. Morishige: Mater. Trans., 2017, vol. 58, pp. 350–54.
35. H. Okada, K. Shimokawa, T. Morishige, and T. Takenaka: Mater. Trans., 2018, vol. 59, pp. 690–93.
S. Su, T. Villalon, Jr., U. Pal, and A. Powell: Proc. 10th Int. Conf. on Molten Slags, Fluxes and Salts (MOLTEN16), 2016, pp. 465–75.
37. J. Guo, T. Villalon, U. Pal, and S. Basu: J. Am. Ceram. Soc., 2018, vol. 101, pp. 3605–16.
38. D.A. Wenz, I. Johnson, and R.D. Wolson: J. Chem. Eng. Data, 1969, vol. 14, pp. 250–52.
39. Y. Katasho, Y. Norikawa, T. Yamamoto, K. Yasuda, and T. Nohira: Electrochem. Commun., 2020, vol. 115, p. 106740.
40. R. Bhagat, D. Dye, S.L. Raghunathan, R.J. Talling, D. Inman, B.K. Jackson, K.K. Rao, and R.J. Dashwood: Acta Mater., 2010, vol. 58, pp. 5057–62.
41. M. Hu, T. Ma, L. Gao, P. Lai, Z. Qu, L. Wen, D. Li, S. Zhang, and M. Hu: Mater. Trans., 2019, vol. 60, pp. 416–21.
42. G.Z. Chen and D.J. Fray: J. Electrochem. Soc., 2002, vol. 149, p. 455.
43. X. Zou, L. Ji, Z. Pang, Q. Xu, and X. Lu: J. Energy Chem., 2020, vol. 44, pp. 147–53.
44. F. Shi, G.E. Fu, E.C. Xiao, and J. Li: J. Mater. Sci.: Mater. Electron., 2020, vol. 31, pp. 18070–18076.
45. H. Zheng, I.M. Reaney, G.D.C. Csete de Györgyfalva, R. Ubic, J. Yarwood, M.P. Seabra, and V.M. Ferreira: J. Mater. Res., 2004, vol. 19, pp. 488–95.
M.L. Moreira, E.C. Paris, G.S. do Nascimento, V.M. Longo, J.R. Sambrano, V.R. Mastelaro, M.I.B. Bernardi, J. Andrés, J.A. Varela, and E. Longo: Acta Mater., 2009, vol. 57, pp. 5174–85.
47. U. Balachandran and N.G. Eror: Solid State Commun., 1982, vol. 44, pp. 815–18.
48. C. Remy, B. Reynard, and M. Madon: J. Am. Ceram. Soc., 1997, vol. 80, pp. 413–23.
49. R.O. Suzuki, K. Ono, and K. Teranuma: Metall. Mater. Trans. B, 2003, vol. 34B, pp. 287–95.
50. D.T.L. Alexander, C. Schwandt, and D.J. Fray: Electrochim. Acta, 2011, vol. 56, pp. 3286–95.
51. J. Trambukis and Z.A. Munir: J. Am. Ceram. Soc., 1990, vol. 73, pp. 1240–45.
52. Y.J. Du, K.P. Rao, J.C.Y. Chung, and X.D. Han: Metall. Mater. Trans. A, 2000, vol. 31A, pp. 763–71.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 52022054, 51974181, and 52004157), the Shanghai Rising-Star Program (Grant No. 19QA1403600), the Iron and Steel Joint Research Fund of National Natural Science Foundation and China Baowu Steel Group Corporation Limited (Grant No. U1860203), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (Grant No. TP2019041).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted January 21, 2021; accepted March 23, 2021.
Rights and permissions
About this article
Cite this article
Tang, W., Li, G., Pang, Z. et al. Facile Electrodeposition of Ti5Si3 Films from Oxide Precursors in Molten CaCl2. Metall Mater Trans B 52, 1985–1996 (2021). https://doi.org/10.1007/s11663-021-02158-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02158-x