Abstract
Electrochemical behavior of Nd was studied in NaCl-KCl melt on W and Cu electrodes via cyclic voltammetry and chronopotentiometry. Generally, the reduction of Nd3+ takes place in two consecutive steps in molten chlorides, such as LiCl-CaCl2, LiCl-BaCl2, CaCl2-NaCl, LiCl-KCl melts. However, the reduction of Nd3+ ions was found to be through a one-step process: Nd3+ + 3e− → Nd. The co-reduction behavior of Nd3+ and Cu2+ ions and the mechanisms of alloy formation were investigated in NaCl-KCl melt on W electrodes at 988 K (715 °C). Four potential plateaus corresponding to four different kinds of Cu-Nd intermetallic compounds were detected. Cu-Nd alloys were prepared on Cu electrodes at 988 K (715 °C) and 1143 K (870 °C). At 988 K (715 °C), Cu5Nd phase was identified by X-ray diffraction. The morphology and micro-zone chemical analysis of the alloys were characterized by scanning electron microscopy equipped with energy-dispersive spectrometry. The alloy film was observed on the Cu electrodes. Moreover, at 1143 K (870 °C), a globate bulk Cu-Nd alloy with Cu5Nd, Cu4Nd, Cu2Nd, CuNd, and Cu phases, as liquid in the melt, was obtained at the bottom of the crucible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Full recovery and reasonable treatment of spent nuclear fuel are key concerns for future nuclear power stations. To reduce the output and long-lived radiotoxicity of spent fuel and high-level waste, partitioning and transmutation (P&T) strategies have become a potential option for the advanced fuel cycle and an alternative for complementary waste management.[1] The first requirement of P&T is to extend the performance of Pu separation to 99.9 pct, separating Np, Am, and Cm, either as a group or individually, and in any case to remove lanthanide (Lns) contaminates as much as possible. The separated transuranium elements (TRUs) should then be “transmuted” (or “burned”) in a neutron field.[2] However, Lns can effectively absorb neutrons and prevent neutron capture by TRUs. Therefore, TRUs have to be separated from Lns to avoid affecting their transmutation efficiency; molten salts employed as solvent media have been proposed as a promising option to achieve this.[3] On the other hand, the accumulation of Lns in the molten salts will modify their physical and chemical properties and contaminate the final cathodic product. When Lns concentration exceeds about 10 wt pct in the melt it must generally be regenerated to avoid lowering the TRUs/Lns separation efficiency[4] and to recycle the molten salts, because the reprocessing agent should be stable and immediately available for reuse, without further treatment.[5]
Molten chlorides are reported to be convenient solvents for obtaining the detailed chemistry of fission products in molten salts due to their high radiation and thermal resistance, low neutron cross section, and high solubility of fuel components.[5] Nd is abundant in the fission products with a large transverse section of neutron capture and is one of the surrogate elements of Am3+ in molten salts.[6,7,8] The electrochemical behavior of Nd3+ has been widely studied in molten chloride. The reduction of Nd3+ takes place in two consecutive steps in LiCl-CaCl2, LiCl-BaCl2, CaCl2-NaCl, LiCl-KCl melts.[6,9,10,11,12] In molten chlorides, like other valence Lns (Sm, Eu, Tm, and Yb), Nd has two stable electro-active forms, Nd2+ and Nd3+. However, for Nd and Tm, they are a little different from Sm, Eu, and Yb. This is because the deposition potentials of Nd2+ and Tm2+ are more positive than those of common solvents (Li+, Na+, and K+) while those of Sm2+, Eu2+, and Yb2+ are closer to or more negative than common solvents. Therefore, it is possible to deposit Nd and Tm on an inert electrode in common solvents. However, corrosion reactions occur between Nd(Tm) metal and Nd3+(Tm) in the molten chloride media, which are expressed as follows: 2Nd3+ + Nd ↔ 3Nd2+. Taking this into account, if the preparation of metal Tm(Nd) is carried out on inert electrodes, a low current yield in the electrolysis and a low stability of the deposits are expected.[13] Therefore, employing electro-reduction of Nd3+ on a reactive electrode to extract Nd seems to be a sensible choice because alloy formation can prevent the corrosion reaction.
Interestingly, Nohira and colleagues[14] have reported that Nd3+ reduction was by a one-step process with a three-electron exchange in NaCl-KCl melt. They prepared different phases of Ni-Nd alloys (NdNi2, NdNi3, Nd2Ni7, and NdNi5) on Ni electrodes and determined the corresponding equilibrium potentials. However, so far, only a one-step Nd3+ reduction has been reported in molten fluoride, such as LiF-CaF2, LiF-NaF, LiF-CaCl2, and LiF melts.[3,15,16,17] Massot and colleagues[18] have investigated the co-reduction process of Al-Nd alloys in LiF-CaF2 melt (79 to 21 mol. pct) on an inert W electrode. Extraction of Nd was performed via potentiostatic electrolysis with an efficiency of about 95 pct. In addition, they[3,19] have investigated Nd3+ reduction on Cu and Ni electrodes in LiF-CaF2 melt. Cu is considered to be a better cathode material than Ni, for their experimental temperature 988 K (715 °C), Cu and Nd form liquid intermetallic compounds. As the kinetics of the alloy layer growth is controlled by the intermetallic diffusion within the surface alloy layer,[3] liquid alloy formation can keep the surface of the cathode free of diffusion layer.
Therefore, this paper is concerned with the electrochemical behavior of Nd3+ in NaCl-KCl melt and its reduction mechanism on W and Cu electrodes, respectively.
Experimental
NaCl-KCl melt (99.5 pct NaCl, 99.5 pct KCl, and mole ratio of sodium to potassium = 50.6:49.4 pct) was dried under vacuum for more than 72 hours at 473 K (200 °C) to remove excess H2O before being used. Cu2+ and Nd3+ ions were introduced into NaCl-KCl melt in the form of anhydrous CuCl2 (99.5 pct) and NdCl3 (Aladdin Chemistry Co. Ltd. ≥99.95 pct) powder, respectively. All experiments were carried out in an inert atmosphere which was maintained by purging with purified Ar gas to avert exposure to O2 and H2O.
Cyclic voltammetry, chronopotentiometry, and galvanostatic electrolysis tests were performed using an Autolab PGSTAT302N potentiostat/galvanostat controlled with the Nova 1.8 software package. The transient electrochemical measurements were carried out in an electrochemical quartz cell with a three-electrode setup. The working electrodes were W wire (d = 1 mm), Cu wire (d = 2 mm), and Cu plate (10 mm × 10 mm × 0.2 mm). The lower ends of the working electrodes were polished thoroughly with SiC paper and then cleaned in ethanol using ultrasound to remove the oxide film and impurities on the electrode surface. The active surface area of working electrode was determined after each experiment by measuring the immersion depth of the electrode. The counter electrode was a spectral pure graphite rod (d = 6 mm). Ag+/Ag couple was used as reference electrode, which consisted of AgCl (1.0 wt pct) in NaCl-KCl molten mixture. The temperature of the melts was measured with a chromel–alumel thermocouple.
Cu-Nd alloys were prepared via galvanostatic electrolysis on Cu electrodes. After electrolysis, the samples were washed with ethylene glycol to remove solidified salts attached on the surface of the alloy samples. Then the deposits were successively polished with 360#, 600#, 1500#, and 2000# metallographic emery papers to make a cross section. The samples were analyzed by X-ray diffraction (XRD) (Rigaku D/max-TTR-III diffractometer) using Cu Kα radiation at 40 kV and 150 mA. Scanning electron microscopy (SEM) equipped with energy-dispersive spectrometry (EDS) (JSM-6480A; JEOL Co., Ltd.) was used to analyze the microstructure and micro-zone chemical composition of bulk Cu-Nd alloys.
Results and Discussion
Electrochemical Behavior of Nd3+ on a W Electrode
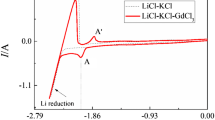
Figure 1 exhibits cyclic voltammograms obtained on a W electrode in NaCl-KCl melt before (dotted line) and after (solid line) the addition of 2 wt pct NdCl3 at 988 K (715 °C). In NaCl-KCl melt, the cathodic limit is related to the formation/dissolution of metal Na. It is noteworthy that the reduction current corresponding to Na deposition is much larger than the reoxidation current. The reason is that some deposited Na dissolved in the melt and Na fog is formed in the cathodic process. After the addition of Nd3+, compared to the dotted line, the solid line shows that the reduction of Nd3+ starts at around −1.70 V, which makes the larger reduction current at potentials more negative than −1.70 V. In the anodic direction, only signal B′, which peaks at about −1.82 V, is observed, and is thought to be related to the dissolution of Nd because the peak potential of signal B′ in the solid line is about 0.2 V more positive than that of signal C′ in the dotted line related to the oxidation of Na. The reason for the absence of Na dissolution signal is that the deposited Nd in the working electrode accelerates the dissolution of metal Na in the melt. Therefore, on the basis of the above discussion, the reduction of Nd3+ in NaCl-KCl melt was through a one-step process. The reaction is thought to be as follows: Nd3+ + 3e → Nd. In other molten chlorides, Nd3+ reduction was through two consecutive steps. The reason for one-step reduction of Nd3+ in NaCl-KCl melt is that Nd2+ is not stable in NaCl-KCl melt due to its disproportionate reaction in molten chlorides: 3Nd2+ → 2Nd3+ + Nd. Castrillejo et al.[20] have reported that the stability of Nd2+ is related to molten salt systems. Therefore, there may be almost no Nd2+ ions in NaCl-KCl melt, or the concentration probably is very low.
Open circuit chronopotentiometry was employed to further study the Nd3+ reduction. Figure 2 shows open circuit chronopotentiometry curves obtained on the W electrode in NaCl-KCl melt before (curve 1) and after (curves 2 and 3) the addition of NdCl3 at 988 K (715 °C). In NaCl-KCl melt (curve 1), only plateau C at about −1.97 V is detected corresponding to the reduction of Na+. After the addition of NdCl3, apart from the plateau C, a new plateau B is observed, which is attributed to the reduction of Nd3+ to Nd via a one-step process. Curve 3 illustrates the open circuit chronopotentiometry curve obtained in NaCl-KCl-NdCl3 (2 wt pct) melt on a W electrode after potentiostatic electrolysis at −2.0 V for 60 seconds. Therefore, the Na+ reduction plateau disappears, and only one potential plateau B corresponding to the reduction of Nd3+ can be observed.
Open circuit chronopotentiometry curves obtained on a W electrode (S = 0.32 cm2) at 988 K (715 °C) in NaCl-KCl melt after potentiostatic electrolysis at −2.3 V for 10 s (curve 1); in NaCl-KCl-NdCl3 (2 wt pct) melt after potentiostatic electrolysis at −2.3 V for 10 s (curve 2); in NaCl-KCl-NdCl3 (2 wt pct) melt after potentiostatic electrolysis at −2.0 V for 60 s (curve 3), respectively
Based on the above discussion, the potential gap (ΔE) between the reduction of Nd3+ and solvent Na+ ions is about 0.14, which suggests that the extraction of Nd3+ from NaCl-KCl melt on inert electrode is feasible. However, ΔE is not large enough to achieve complete extraction as required for recycling.[3] One way to successfully eliminate Nd3+ ions from the molten salt is to increase ΔE. Therefore, in our paper, we employ two methods to increase ΔE: (i) co-reduction of Cu2+ and Nd3+ via the formation of Cu-Nd intermetallic compounds on an inert W electrode; (ii) electro-reduction of Nd3+ on a Cu electrode.
Reduction Behavior of Nd3+ in NaCl-KCl-CuCl2 Melt on W and Cu Electrodes
Figure 3 illustrates the cyclic voltammograms obtained at a W electrode (S = 0.32 cm2) in NaCl-KCl-1 wt pct CuCl2-2 wt pct NdCl3 melt at different reversal potentials at 988 K (715 °C). Four pairs of well-defined signals A/A′, II/II′, III/III′, and IV/IV′ are detected. In the cathodic sense, the cathode current starts to increase from about −0.33 V, peaking at about −0.40 V (signal A), which is caused by the reduction of Cu2+ to Cu. The corresponding anodic signal A′ is related to the dissolution of Cu. From Figure 1, the oxidation of Nd is at about −1.82 V; therefore, the signals II′, III′, and IV′ at about −1.41, −1.54, and −1.62 V between the oxidation potential of Cu and Nd are ascribed to the dissolution of Cu-Nd intermetallic compounds in the melt.[21] The corresponding cathodic signals II, III, and IV correspond to the formation of Cu-Nd intermetallic compounds, which are formed by the underpotential deposition of Nd3+ on the Cu-coated W electrode, respectively. Moreover, after the signal II′, a weak signal I′ is observed corresponding to the dissolution of a Cu-Nd intermetallic compound. In NaCl-KCl-CuCl2-NdCl3 melts, the formation mechanism of Nd-Cu alloys obtained on the W electrode involves the steps described as follows:
When Cu is used as a working electrode, in NaCl-KCl-1 wt pct CuCl2-2 wt pct NdCl3 melt (Figure 4), in the electrochemical window, apart from the signals A/A′ relating to the formation/dissolution of metal Cu in the melt, only two pairs of well-defined signals II/II′ and III/III′ ascribed to the formation/dissolution of Cu-Nd intermetallic compounds are observed at 988 K (715 °C). The signals corresponding to the formation/dissolution of Nd are not detected, because in the cathodic process the concentration of Nd3+ ions on the surface Cu electrode is close to zero because of the formation of Cu-Nd alloy, and as a result, no free Nd3+ ions exist.
Reduction Behavior of Nd3+ in NaCl-KCl Melt on Cu Electrodes
To further study the Cu-Nd intermetallic compound formation, we investigated the cyclic voltammograms obtained at 988 K (715 °C) in NaCl-KCl-4 wt pct NdCl3 on a Cu wire (dotted line) and plate (solid line) electrodes, respectively (see Figure 5). In the solid line, four pairs of signals I/I′, II/II′, III/III′, and IV/IV′ are detected. They correspond to the formation/dissolution of four different Cu-Nd intermetallic compounds. In the dotted line, for the Cu wire electrode, the intensity of signal I′ become much weaker, because the formation rate of the Cu-Nd intermetallic compound (signal I) is slow in NaCl-KCl-NdCl3 melt system. In NaCl-KCl-NdCl3 melts, the formation mechanism corresponding to the formation of Nd-Cu alloys obtained on Cu electrode involves one step described as follows:
Figure 6 elucidates open circuit chronopotentiometry curves of NaCl-KCl-NdCl3 (4 wt pct) melt obtained on Cu wire (curve 1) and plate (curve 2) electrodes at 988 K (715 °C). In this method, potentiostatic electrolysis was employed to deposit Nd on a Cu electrode. The deposited Nd diffuses into the Cu electrode and the electrode potential gradually shifted to more positive values. During this process, a potential plateau is observed when the composition of the electrode surface is within the range of a two-phase coexisting state. In curve 1, apart from potential plateau A, corresponding to abandon the potential of Cu wire, three plateaus (plateaus 2 to 4) are observed. In curve 2, apart from plateaus A, 2 to 4, a new plateau (plateau 1) is detected. The results are in good agreement with those obtained in the anodic direction of cyclic voltammograms in Figure 5. Plateaus 1, 2, 3, and 4 are related to the two-phase coexisting states of: (1) intermetallic compound I and Cu; (2) intermetallic compounds II and I; (3) intermetallic compounds III and II; (4) intermetallic compounds IV and III, respectively. According to the subsequent XRD patterns of Nd-Cu alloys, the two-phase coexisting states of plateaus 1 to 4 can be identified as:
Open circuit chronopotentiometry curves obtained in NaCl-KCl-NdCl3 (4 wt pct) melt at 988 K (715 °C) on a Cu wire electrode (curve 1) (S = 0.32 cm2) after potentiostatic electrolysis at −2.0 V for 30 s, and on a Cu plate electrode (curve 2) (S = 1.00 cm2) after potentiostatic electrolysis at −2.0 V for 10 s, respectively
Preparation of Cu-Nd Alloys on Cu Electrode
Cu-Nd alloys were prepared via galvanostatic electrolysis on a Cu electrode in NaCl-KCl-NdCl3 melt at 988 K (715 °C). Figure 7 displays XRD pattern of the surface of deposits obtained on Cu electrodes at 988 K (715 °C) under galvanostatic electrolysis at −0.5 A cm−2 for 2 hours in NaCl-KCl melt containing NdCl3 2 wt pct (A) and 4 wt pct (B), respectively. The observed diffraction peaks were identified as Cu5Nd phase. The diffraction peaks of Cu matrix were not detected. It indicates that the whole surface of working electrode is coated with Cu5Nd alloy film, and the X-ray does not reach the Cu substrate.[22] The intensity of those Cu5Nd diffraction peaks is rather weak, which is due to: (i) the existence of crystal defects in Cu5Nd alloy during formation; (ii) the attached Cu5Nd alloy film being very thin on the Cu electrode.
Figure 8 shows SEM micrograph of a cross section of a Cu electrode at 988 K (715 °C) after galvanostatic electrolysis at −0.5 A cm−2 for 2 hours in NaCl-KCl melt containing NdCl3 4 wt pct. Alloy films are observed, which are divided into three obvious layers. The innermost alloy layer is rather compact with a smooth surface. To examine the distribution of the copper and neodymium elements, the EDS quantitative analysis was employed. The EDS results of the points labeled 001 and 002 taken from the innermost alloy layer (see Figure 8, B-1) indicate that the layer is composed of Cu and Nd.
To further study the Cu-Nd alloy formation, we performed a series of electrolysis at 1143 K (870 °C). Figure 9 illustrates SEM micrograph of a cross section of Cu electrodes after galvanostatic electrolysis in NaCl-KCl-NdCl3 4 wt pct melt at −0.5 A cm−2 for 4 hours (sample C) and 6 hours (sample D), respectively. The alloy film in sample C is divided into three obvious alloy layers, while in sample D, only two obvious alloy layers are present. After galvanostatic electrolysis at −0.5 A cm−2 for 6 hours in NaCl-KCl-NdCl3 4 wt pct melt, an interesting phenomenon occurs. At the bottom of the crucible, a spherical alloy (sample E) was obtained. Figure 10 shows the XRD pattern of alloy obtained at the bottom of the crucible. The Cu, Cu5Nd, Cu4Nd, and Cu2Nd phases are identified. Moreover, there are some unknown diffraction peaks that could not be identified by current XRD database, which are thought to be related to other Cu-Nd intermetallic compounds. Therefore, the SEM with EDS quantitative analysis was adopted to analyze the phase constitution of alloy. Figure 11 displays the SEM images and EDS quantitative analysis data of sample E obtained at the bottom of the crucible. The surface of the alloy is rough (see SEM images). And some ribbon-like precipitates are observed. The EDS results of the points labeled 001 and 003 taken from sample E indicate that the layer is composed of Cu and Nd, with the atom percentage ratios Cu/Nd at about 1.7 and 0.78, respectively. Therefore, the precipitated Cu-Nd intermetallic compound is thought to be Cu2Nd and CuNd phases, when the measurement error is taken into account. Since the current XRD database lacks the JCPDS of CuNd phase, therefore, the unknown diffraction peaks in XRD patterns (in Figure 10) might be related to the CuNd phase. Liquid compounds easily sink to the bottom of the crucible and keep the surface of the cathode free of diffusion layer; therefore, it can enhance the extraction rate.[3,17]
Conclusion
On a W electrode, in NaCl-KCl melt, the reduction of Nd3+ ions is via a one-step process: Nd3+ + 3e− → Nd; with the assistance of CuCl2, Nd3+ ions were reduced at more positive potentials. On a Cu electrode, four Cu-Nd intermetallic compounds were detected. Cu-Nd alloys were prepared on Cu electrode at 988 K (715 °C). Only Cu5Nd phase was identified by XRD. SEM with EDS results suggest that the alloy film is divided into three obvious layers. At 1143 K (870 °C), the obtained alloy film was much thicker. With the increase of electrolysis time, a globate Cu-Nd alloy was obtained at the bottom of the crucible. Cu, Cu5Nd, Cu4Nd, Cu2Nd, and CuNd phases are identified by XRD and EDS quantitative analysis. The liquid alloy formation keeps the surface of the cathode free of diffusion layer, which facilitates the extraction of Nd from the melt.
References
M. Salvatores: Nucl. Eng. Des., 2005, vol. 235, pp. 805–16.
M. Salvatores and G. Palmiotti: Prog. Part. Nucl. Phys., 2011, vol. 66, pp. 144–66.
P. Taxil, L. Massot, C. Nourry, M. Gibilaro, P. Chamelot, and L. Cassayre: J. Fluor. Chem., 2009, vol. 130, pp. 94–101.
D. Hudry, I. Bardez, A. Rakhmatullin, C. Bessada, F. Bart, S. Jobic, and P. Deniard: J. Nucl. Mater., 2008, vol. 381, pp. 284–89.
T.R. Griffiths, V.A. Volkovich, S.M. Yakimov, I. May, C.A. Sharrad, and J.M. Charnock: J. Alloy. Compd., 2006, vol. 418, pp. 116–21.
K. Fukasawa, A. Uehara, T. Nagai, T. Fujii, and H. Yamana: J. Nucl. Mater., 2011, vol. 414, pp. 265–69.
J. Serp, P. Chamelot, S. Fourcaudot, R.J.M. Konings, R. Malmbeck, C. Pernel, J.C. Poignet, J. Rebizant, and J.P. Glatz: Electrochim. Acta, 2006, vol. 51, pp. 4024–32.
A. Novoselova and V. Smolenski: Russ. J. Electrochem., 2013, vol. 49, pp. 931–37.
G. DeCórdoba, A. Laplace, O. Conocar, J. Lacquement, and C. Caravaca: Electrochim. Acta, 2008, vol. 54, pp. 280–88.
K. Fukasawa, A. Uehara, T. Nagai, T. Fujii, and H. Yamana: J. Alloy. Compd., 2011, vol. 509, pp. 5112–18.
P. Masset, R.J.M. Konings, R. Malmbeck, J. Serp, and J.P. Glatz: J. Nucl. Mater., 2005, vol. 344, pp. 173–79.
Y.D. Yan, Y.L. Xu, M.L. Zhang, Y. Xue, W. Han, Y. Huang, Q. Chen, and Z.J. Zhang: J. Nucl. Mater., 2013, vol. 433, pp. 152–59.
A. Novoselova and V. Smolenski: Electrochim. Acta, 2013, vol. 87, pp. 657–62.
K. Yasuda, S. Kobayashi, T. Nohira, and R. Hagiwara: Electrochim. Acta, 2013, vol. 92, pp. 349–55.
C. Hamel, P. Chamelot, and P. Taxil: Electrochim. Acta, 2004, vol. 49, pp. 4467–76.
E. Stefanidaki, C. Hasiotis, and C. Kontoyannis: Electrochim. Acta, 2001, vol. 46, pp. 2665–70.
C. Nourry, L. Massot, P. Chamelot, and P. Taxil: J. Appl. Electrochem., 2009, vol. 39, pp. 927–33.
M. Gibilaro, L. Massot, P. Chamelot, and P. Taxil: J. Nucl. Mater., 2008, vol. 382, pp. 39–45.
P. Chamelot, L. Massot, C. Hamel, C. Nourry, and P. Taxil: J. Nucl. Mater., 2007, vol. 360, pp. 64–74.
Y. Castrillejo, M.R. Bermejo, E. Barrado, A.M. Martínez, and P. Díaz Arocas: J. Electroanal. Chem., 2003, vol. 545, pp. 141–57.
M. Gibilaro, L. Massot, P. Chamelot, and P. Taxil: Electrochim. Acta, 2009, vol. 54, pp. 5300–06.
H. Konishi, T. Nohira, and Y. Ito: Electrochim. Acta, 2002, vol. 47, pp. 3533–39.
Acknowledgments
The work was financially supported by the Key Laboratory of Superlight Materials and Surface Technology (Harbin Engineering University), Ministry of Education, the National Natural Science Foundation of China (91326113, 91226201, and 51574097), the Science Foundation of Heilongjiang Province (LC2016018), the Fundamental Research Funds for the Central Universities (HEUCF2016012), the Foundation for University Key Teacher of Heilongjiang Province of China and Harbin Engineering University (1253G016 and HEUCFQ1415), and the Scientific Research and Special Foundation Heilongjiang Postdoctoral Science Foundation (LBH-Q15019, LBH-Q15020, and LBH-TZ0411).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Manuscript submitted November 9, 2015.
Rights and permissions
About this article
Cite this article
Zhang, ZL., Zhou, LZ., Ji, DB. et al. Electrochemical Extraction of Nd from NaCl-KCl Melt by Formation of Cu-Nd Alloys. Metall Mater Trans B 48, 2535–2542 (2017). https://doi.org/10.1007/s11663-017-1017-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-017-1017-6