Abstract
This article presents the results on speciation of ferric iron generated by the dissolution of chemical reagent hydromolysite (ferric chloride hexahydrate, FeCl3:6H2O) in water at 298.15 K, 313.15 K, and 333.15 K (25 °C, 40 °C, and 60 °C). Experiments were performed with a thermoregulated system up to the equilibrium point, as manifested by solution pH. Solution samples were analyzed in terms of concentration, pH, and electrical conductivity. Measurements of density and refractive index were obtained at different temperatures and iron concentrations. A decrease of pH was observed with the increase in the amount of dissolved iron, indicating that ferric chloride is a strong electrolyte that reacts readily with water. Experimental results were modeled using the hydrogeochemical code PHREEQC in order to obtain solution speciation. Cations and neutral and anion complexes were simultaneously present in the system at the studied conditions according to model simulations, where dominant species included Cl−, FeCl2+, FeCl2 +, FeOHCl 02 , and H+. A decrease in the concentration of Cl− and Fe3+ ions took place with increasing temperature due to the association of Fe-Cl species. Standard equilibrium constants for the formation of FeOHCl 02 obtained in this study were log \( K_{f}^{0} \) = −0.8 ± 0.01 at 298.15 K (25 °C), −0.94 ± 0.02 at 313.15 K (40 °C), and −1.03 ± 0.01 at 333.15 K (60 °C).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In previous research,[1,2] leaching of copper ores was investigated using chloride medium. In this context, the knowledge of chloro-complex ions of copper or iron[3,4,5] is important for the interpretation of results on the extraction of copper in the leaching process. Moreover, solutions with iron in the presence of a chlorinated medium show a low pH. This observation may be beneficial to lower consumption of acid during the leaching operation. In order to explain this phenomenon (lower pH in a medium of Fe-Cl), laboratory tests were designed using hydromolysite (FeCl3:6H2O). The aim is to verify the presence of a new chloro complex FeOHCl 02 that may be formed by the hydrolysis of iron in a chloride medium. In hydrometallurgy, ferric ions in chloride medium are used as oxidants during the on-leaching process for copper sulfides and concentrate, especially of chalcopyrite, CuFeS2.[6,7,8,9,10,11,12]
Previous experimental studies indicated the formation of different iron complexes when ferric chloride is dissolved in water[13] or aqueous solutions of sodium chloride or hydrochloric acid (such as Fe(OH) n± x , Fe(Cl) m± y , Fe(OH) 03 , and FeCl 03 ), considering that ferric chloride is highly soluble in water, 74.39 g FeCl3/100 g water at 273.15 K (0 °C), and 150 g FeCl3/100 g water at 310.15 K (37 °C).[14] Also, ferric hydroxyl-chlorides can be formed, such as FeOHCl+ and Fe(OH)2Cl0. The concentration of these species is strongly dependent on solution composition and temperature. Corresponding aqueous equilibrium reactions with chloride ions are summarized as follows (Eqs. [1] through [6]):[15,16,17,18]
Others reactions that can be considered are as follows:
Liu et al.[19] studied speciation of ferric chloride complexes in hypersaline solutions with the Pitzer’s model and presented the thermodynamic properties for Fe(III) chloride complexes. Lee et al.[20] studied equilibrium concentrations of the system FeCl3-HCl-H2O at 298.15 K (25 °C) and developed a chemical model. These authors estimated the distribution of iron species for FeCl3 and HCl concentrations in the range 0.1 to 2.7 mol/kg H2O. Later, Lee[21] studied the ferrous-ferric-chloride system using Bromley’s equation and estimated the activity coefficients of the complex species. André et al.[22] determined new parameters of Pitzer’s model for the systems FeCl3-H2O and HCl-FeCl3-H2O at 298.15 K (25 °C) at low and high ionic strengths. Majima and Awakura[23,24] determined water activities and mean activity coefficients of solutes, such as HCl and FeCl3 in the HCl-FeCl3-H2O system at 298.15 K (25 °C). Rumyantsev et al.[25] determined and calculated the osmotic coefficients of FeCl3-H2O and FeCl3-MCl n -H2O systems (with M = Na, K, Mg, or Ca).
Limited thermodynamic information regarding ferric chloride complexes in concentrated aqueous solutions can be obtained from different geochemical databases and experimental results.[18,26,27] Most of the bibliographic information shows results in diluted systems at 298.15 K (25 °C),[21] while complete thermochemical data derived from ferric chloride dissolution in water systems as a function of temperature are unavailable. Table I presents equilibrium constants for the main ferric chloride aqueous complexes compiled at 298.15 K (25 °C).
The aim of this work is to study, from both experimental and theoretical points of view, the chemical equilibrium and speciation of ferric chloride in water at 298.15 K, 313.15 K, and 333.15 K (25 °C, 40 °C, and 60 °C). These temperatures were chosen considering that the industrial process could easily reach this range, for example, using solar energy.
A hydrogeochemical code PHREEQC[26] was used to quantify the speciation in aqueous iron-chloride species at various concentrations and temperatures. The results of model calculations are compared with the experimentally measured values of pH and electrical conductivity (κ). Further, the values of density (ρ) and refractive index (n D) were obtained under concentration and temperature employed in the study.
Materials and methods
Experiments using aqueous ferric chloride solutions were carried out at different concentrations and temperatures, in order to obtain the iron(III) speciation in the Fe(III)-Cl(I)-H2O system.
Materials
Ferric chloride (FeCl3:6H2O) of analytical grade was used (Merck, 99 pct) without any additional purification. Distilled water was passed through a Millipore Co. “Ultrapure Cartridge Kit” to obtain ultrapure water (conductivity ≤0.05 µS/cm).
Methods
In the model, molality was used as a unit of concentration (i.e., mol/kg H2O) in order to avoid comparisons with solution density effects. Solutions of different iron concentrations, ranging from 0.01 to 1.12 mol/kg H2O, were prepared and used in this study. Solutions reached the equilibrium before 14 hours; this was confirmed based on the time required to reach steady values of refractive index and pH.
A known mass of ferric chloride was weighed using a Mettler Toledo Co. Model AX-204 analytical balance with a precision of ±0.07 mg and dissolved in ultrapure water to obtain a solution of required concentration. The solution was continuously stirred for 14 hours. Each solution was maintained inside a double-jacketed glass reactor (100 mL). The temperature of the solution was obtained by control of thermostatic bath. This method was used for measurement of properties at different temperatures of 298.15 K, 313.15 K, and 333.15 K (25 °C, 40 °C, and 60 °C).
When the solutions reached equilibrium, samples were taken for chemical analyses of total iron, ferrous, and chloride to confirm the initial concentration of FeCl3 added to water. Ferrous concentrations were determined to ensure that ferrous species were not present in the solution. Total iron concentration was determined using atomic absorption spectrometry, ferrous concentration by redox titration, and chloride concentration by titration using the Volhard’s method.[28]
Measurements of Properties
Values of pH, electrical conductivities, densities, and refractive indices were measured at different ferric concentrations and temperatures. A portable pH meter Hanna model HI 991003 with a reference electrode of titanium model HI1297D was used for measuring the pH of solutions. The equipment has a precision of ±0.02 pH units. Before each measurement, the equipment is calibrated using buffer solutions of pH 4.01 and 7.01 and checked with a buffer solution of pH 1.68. The electrical conductivities were measured using an Orion model 170 conductivity meter (≤0.5 pct precision). Before each set of measurements, the instrument was calibrated with standard KCl solutions of 12.88, 111.8, and 199 mS/cm depending on the conductivity range. Electrical conductivities and pH values were measured in duplicate. Solution densities were measured using a Mettler Toledo DE50 vibrating tube densimeter (measurement uncertainty: ±5 × 10−5 g/mL). The densimeter was calibrated using air and deionized water before each set of measurements at a given temperature and atmospheric pressure. The refractive indices were measured using a Mettler Toledo RE50 refractometer (measurement uncertainty ±1 × 10−4). Both instruments had a self-contained Peltier system for temperature control (measurement uncertainty ±0.01 K). Density and refractive index measurements were done in triplicate and average values are reported (Table II). Standard deviation values were less than 0.05, 0.02, 0.00006, and 0.0002 for pH, electrical conductivity, density, and refractive index, respectively.
Modeling
The methodology to study speciation in aqueous multicomponent ionic systems involves identification of dissolved species, definition of equilibrium reactions and main components, and mass balances for each component as a follow-up step. The specific details of the methodology were reported previously.[27,29,30,31,32,33]
Ion activity models, such as Bromley,[21,31] specific interaction,[22,32,33] and extended Debye–Hückel,[27,29,30] among others, were used for aqueous electrolyte solutions. In this work, the extended Debye–Hückel model was used to estimate the activity coefficients (γ i ) of dissolved species.
The main model relationships are as follows:
where TOT X j is the total concentration of a component jth (mol/kg), ν i is the stoichiometric coefficient of component ith, m i is the molal concentration of component ith, K ri is the equilibrium constant of the reaction for the formation of ith species, a j is the activity of a component jth (mol/kg), γi is the activity coefficient of component ith, z i is the charge number of ionic species ith, I is the ionic strength (mol/kg), A and B are Debye–Hückel parameters, å is the ionic hard-core diameter (m), and \( \dot{B} \) is the B-dot parameter of the extended Debye–Hückel model (in kg/mol).
The activity coefficient model (Eq. [12]) has four parameters (A, B, å, and \( \dot{B} \)), including binary electrostatic and short-range ion interactions. This model can be used for electrolyte solution with ionic strengths up to I = 1 m.[27,29,30]
The extended Debye–Hückel model was used by Helgeson and co-workers[30] and introduced in several speciation codes and geochemical software, such as PHREEQC[26] or EQ3/6.[27] The parameter values were obtained from the database thermos.com.V8.R6.230.[34]
The speciation model in this work was validated by measurements of ionic conductivity and pH of tested solutions, the use of the medium range ion activity model, and the selection of the main species reported in thermochemical databases.[19,26,27] Then, the model was calibrated with revised values of equilibrium constants for dissolved iron species. Solubility data for the hydromolysite (FeCl3:6H2O) as a function of pH and temperature were used to obtain the equilibrium constants for FeOHCl 02 by mathematical regressions. Last, the model was validated based on solution electrical conductivity calculations and measurements using independent sets of experimental data, which correspond to a global property of the solution used as indirect determination of iron speciation in the studied solutions.
Results and Discussion
Experimental Results
For different Fe(III) concentrations, Table II presents pH, electrical conductivity, density, and refractive index values at 298.15 K, 313.15 K, and 333.15 K (25 °C, 40 °C, and 60 °C). Ferrous concentrations were ≤0.001 mol/kg H2O. Since iron is predominantly present in the ferric state, species containing iron in ferrous were not considered in the speciation.
A significant increase in conductivity is observed with temperature and ferric chloride concentrations. The conductivity of these solutions is mainly determined by the concentrations of free chloride and hydrogen ions (Cl− and H+), because these ions exhibit the highest mobilities in the study conditions.
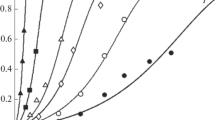
Figure 1 shows experimental results for pH at 298.15 K, 313.15 K, and 333.15 K (25 °C, 40 °C, and 60 °C) in solutions formed by the dissolution of the hydromolysite (FeCl3:6H2O) in deionized water.
These measurements show that solution pH decreases from about 2.2 to 0.81 as Fe(III) concentration increases from 0.01 to 1.12 mol/kg H2O. The increasing acidity can be explained by Eq. [13]:
Equilibrium pH of solutions formed by dissolution of hydromolysite (FeCl3:6H2O) in water as a function of Fe(III) concentration and temperature. Experimental data: 298.15 K, 313.15 K, and 333.15 K (25 °C, 40 °C, and 60 °C). Model predictions at 313.15 K (40 °C): — including Fe(OH)Cl2(aq) species and — without Fe(OH)Cl2(aq) species
A sample of solutions was analyzed using a UV–vis spectrophotometer (Pharo 300, Merck). The electronic spectra of the studied compounds are characteristic of the aqueous-chlorine complex of iron.[35,36] and show d–d transitions of low energy (410 nm); the UV zone shows characteristic bands associated with metal-ligand transitions (210 and 294 nm). This could be explained in terms of the presence of FeOHCl 02 complex.[19,37]
The results presented in Table II show that iron hydrolysis is favored by increasing temperature, manifesting as a decrease in the pH value of the solution in the studied conditions. In addition, solution conductivity increased markedly with temperature. These results were used to calibrate and validate the speciation model.
From Table II, it can be observed that the values on the density and refractive index present a linear relationship with respect to the ferric concentrations (with average correlation coefficients of 0.9955 and 0.9990, respectively) for the three temperatures tested. Moreover, for a fixed ferric concentration, density and refractive index values decrease when the increase in temperature.
Modeling Results
Speciation modeling was carried out using the extended Debye–Hückel thermodynamic equation described in a previous article.[29] Table III summarizes the species considered and their equilibrium constant values at 298.15 K, 313.15 K, and 333.15 K (25 °C, 40 °C, and 60 °C). Calculation results are presented in Figures 1, 2, and 3 for pH, aqueous species distribution, and solution conductivity, respectively. Table III presents model species and equilibrium constants at the studied temperatures. Species were selected from literature[18,26,27] considering consistent values of their K values as a function of temperature. The complex FeOHCl+ was not considered in the model, because this species exhibits low stability at the studied conditions and consistent thermochemical properties are not available in the published literature.[15,16] The complex FeOHCl 02 was included in the model to represent the solution hydrolysis and pH decrease when FeCl3:6H2O was dissolved (Table II).
Model calibration
This work considered the equilibrium constant approach to model the speciation, where equilibrium constants represent the key parameters. Table III corresponds to “speciation tableau” and represents the aqueous speciation model for the Fe(III)-Cl(I)-H2O system, where reactions are represented as stoichiometric combinations of components.
The neutral FeOHCl 02 species must be incorporated in the model to fit the solution pHs obtained along ferric chloride concentrations. Figure 1 presents these results, and shows that, if the FeOHCl 02 is not included, the model cannot predict the pH. If the presence of this complex is considered, then the pH calculations are in agreement with the measurement data for the Fe(III)-Cl(I)-H2O system in the concentration and temperature ranges studied.
Using the following set of mass balance equations, the system can be evaluated for each component:
To solve the mass balance equations, three concentrations of the selected components must be measured or fixed in advance: these are [H] t , [Cl] t , and [Fe(III)] t .
Equations [10] through [12] summarize the equilibrium speciation model used in this work, which corresponds to a nonlinear set of algebraic equations derived from the ionic activities of dissolved species, equilibrium constants, and the mass balance equations of each solution component. The hydrogeochemical code PHREEQC was used to solve the model equations. This program implements a multidimensional Newton–Raphson numerical algorithm and allows the calculation of speciation for the aqueous multicomponent system formed by minerals, dissolved metals, and aqueous complexes. The key values for the aqueous neutral species FeOHCl 02 were obtained by fitting the model to experimental measuments of pH at different Fe(III) concentrations. The mathematical regression was defined by minimizing the sum of squares of the differences between the experimental and calculated data presented in Figure 1. The K values obtained for FeOHCl 02 were log K 0 f = –0.8 ± 0.01 at 298.15 K (25 °C), –0.94 ± 0.02 at 313.15 K (40 °C), and –1.03 ± 0.01 at 333.15 K (60 °C).
Model calculations
Figure 2 shows the equilibrium calculation results at three different ferric concentrations and temperatures. A decrease in the concentration of free ions except for H+ was observed as temperature increased due to the increased stability of FeCl2 + and FeOHCl 02 species with temperature. For example, at 313.15 K (40 °C) and [Fe] t = 0.75 mol/kg H2O, ferric species distribute as 0.01 pct < Fe(OH) +2 , 0.3 pct FeOH2+, 0.5 pct FeCl4 –, 1.9 pct FeCl 03 , 3.8 pct Fe3+, 16.1 pct FeOHCl 02 , 37.1 pct FeCl2+, and 40.3 pct FeCl2 +. Chloride species distribute as 0.6 pct HCl0, 0.7 pct FeCl4 –, 1.9 pct FeCl 03 , 10.7 pct FeOHCl 02 , 12.3 pct FeCl2+, 26.8 pct FeCl2 +, and 47 pct Cl–. Hydrogen species distribute as 10.6 pct HCl and 89.4 pct H+. Relative distributions of Fe-Cl complexes show similar results as presented by Lee et al.[20] and Liu et al.[19] Clearly, when the Fe(III) concentration increases, there is an increase in the quantities of different ferric species (Figure 2).
Model simulations indicate that cations, anions, and neutral complexes were present in the concentrated Fe(III)-Cl(I)-H2O system. Dominant species in the studied conditions were Cl–, FeCl2+, FeCl2 +, and H+, which indicate that this solution presents a low buffer capacity due to the existence of iron chloride complexes and the relatively low concentration of FeOHCl 02 and HCl0 species.
Model validation
The inclusion of the complex FeOHCl 02 into the speciation model permitted its validation, according to the measurements and model calculations of pH and solution conductivity presented by Figures 1 and 3, respectively.
The formation of this species generates hydrolysis due to the water and ion associations, generating the increase in H+ concentration (Eq. [13]). A comparison between conductivity measurements and model calculations was also used to validate the thermodynamic speciation model.[29,38] The conductivity of the solution was calculated as
where κ is the solution conductivity (mS/cm), F is the Faraday’s constant (96,487 coulomb/mol), R is the ideal gas constant (8.3173 J/mol/K), T is absolute temperature (K), C is the molar concentration (kmol/m3), and D ef is the effective diffusivity of the ionic species (m2/s).
The use of Eq. [17] requires knowledge of ion diffusivities and their respective concentrations in the aqueous solution. Ionic concentrations were calculated with the speciation model (Eqs. [10] through [12]), and the effective diffusivity (D ef, i ) was determined by mathematical regression from multicomponent solution measurements in this work.
The ionic conductivity of solution was related to the effective diffusivity of chloride ion (most mobile species) according to
Then, the effective diffusivity ratios were approximated by a standard diffusivity ratio at 298.15 K (25 °C), which is known for diluted solutions, as follows:
For the case of the complex ions, the standard diffusivity (D 0) was calculated according to the methodology proposed by Anderko and Lenka[38] for multielectrolyte solutions as follows:
Table IV shows the standard diffusivities of ionic species at 298.15 K (25 °C), calculated for iron complexes using Eq. [20], and the electrical conductivities of the free ions reported by Haynes.[39]
Finally, the effective diffusivity of chloride ions was corrected for studied solutions (due to iron concentration and changes in density and viscosity) using an empirical relationship developed by the authors, which was then fitted to experimental data for the studied systems. The proposed relationship is
where D Cl – is the chloride ion diffusivity in the supporting electrolyte, m Fe is the total dissolved iron concentration, and m ref corresponds to an empirical parameter for chloride ion diffusivity.
Measurements and calculations of the electrical conductivity for various solution compositions are shown in Table II and Figure 3. Electrical conductivity increases with dissolved iron chloride concentration in solution and temperature. The speciation model simulations explain this phenomenon, which indicates that the concentration of hydrogen ions (H+) increases with temperature together with the mobilities of ionic species. The ionic association degree increases with temperature; i.e., the relative amounts of Fe-Cl complexes increase. This is also evident by the decrease in the ionic strength of solution from 1.61 mol/kg H2O at 298.15 K (25 °C) to 1.2 mol/kg H2O at 333.15 K (60 °C).
Figure 3 shows that there is good agreement between experimental values and the values predicted by the speciation model developed by the authors. The standard deviations between experimental and calculated values were about 0.1 to 5 pct, depending on solution concentration. Greater differences were observed in more concentrated solutions (for pH < 0.7), due to the increase in the ionic strength (I > 1.5 mol/kg H2O) and the deviations predicted by the extended Debye–Hückel model used.
The model was validated comparing the calculated and experimental results; i.e., the species distribution and its concentrations are in agreement with the Fe(III) in aqueous solutions in the 298.15 K to 333.15 K (25 °C to 60 °C) temperature range. The average parameters of Eq. [21] in the aqueous solution are D Cl – = 2.03·10−9 (m2/s) and m ref 0.5 ± 0.05 (mol/kg) at 298.15 K (25 °C), D Cl – = 2.91·10−9 (m2/s) and m ref 0.45 ± 0.05 (mol/kg) at 313.15 K (40 °C), and D Cl – = 4.34·10−9 (m2/s) and m ref = 0.38 ± 0.05 (mol/kg) at 333.15 K (60 °C).
Figure 4 shows the effective diffusivity of Cl– in Fe(III)-Cl(I)-H2O solutions at 298.15 K, 313.15 K, and 333.15 K (25 °C, 40 °C, and 60 °C), as a function of total dissolved iron concentration. An increase in Cl– diffusivity was observed with an increase in solution temperature, and a reverse trend was observed with the dissolved iron concentration. In the range of 0 to 1.12 mol/kg H2O Fe(III), the diffusivities diminished by 88, 91, and 94 pct at 298.15 K, 313.15 K, and 333.15 K (25 °C, 40 °C, and 60 °C), respectively.
The model developed here includes electrostatic (long-range) binary interactions between cations and anions and short-range interactions evaluated as a function of solution ionic strength. This model could not be extended to a higher concentration range due to the difficulties in pH measurements for high acidity solutions. Additional multi-ion interactions, which are significant at high concentrations, were not included in this model and require further work and the use of a wider range of ion activity models, such as Pitzer and co-workers’ model.[32,40] Also, the inclusion of other ionic and neutral iron-chloride complexes, such as Fe(OH)Cl+ or Fe(OH)2Cl0, Fe(OH)Cl –3 , requires further experimental studies.
Conclusions
The experimental data and thermodynamic model described in this work allow the prediction of the speciation in the Fe(III)-Cl(I)-H2O system at 298.15 K, 313.15 K, and 333.15 K (25 °C, 40 °C, and 60 °C). Calculations are in fair agreement with experimental observations of pH and electrical conductivity throughout the studied concentration ranges and temperatures.
Free acidity increases with the amount of dissolved iron in solution, indicating the existence of hydrolysis between dissolved ferric and chloride species to form the dissolved complex FeOHCl 02 . Electrical conductivity of studied solutions increased with both temperature and the amount of dissolved ferric chloride concentrations, indicating that more mobile ions H+ and Cl– were present.
The properties, densities, and refractive indices change linearly with the ferric concentration. For a fixed ferric concentration, the values of density and refractive index show a decrease with the increase of temperature. Model simulations indicate that cations, anions, and neutral complexes are present; the dominant species in the studied conditions were Cl–, FeCl2+, FeCl2 +, FeOHCl 02 , and H+. A decrease in the concentration of Fe3+ and Cl– was observed as the temperature increased, due to the stability increase of Fe-Cl complexes. These ion associations result in lower values for ionic strength as the temperature is increased.
The agreement obtained by comparison of experimental and calculated results shows that the model has been qualitatively and quantitatively validated; i.e., the predicted species distribution and solution concentrations are correct for aqueous solutions of Fe(III) and Cl(I) in the ranges of temperature 298.15 K to 333.15 K (25 °C to 60 °C) and ferric iron concentration 0.01 to 1.12 mol/kg H2O. Finally, the following standard equilibrium constants for FeOHCl 02 were obtained by using the thermochemical equilibrium model developed in this work: log K 0 f = −0.8 ± 0.01 at 298.15 K (25 °C), −0.94 ± 0.02 at 313.15 K (40 °C), and −1.03 ± 0.01 at 333.15 K (60 °C).
Abbreviations
- a :
-
Activity (a i = m i ·γ i ), mol/kg
- å :
-
Ionic hard-core diameter, m
- A :
-
Debye–Hückel parameter, kg1/2/mol1/2
- B :
-
Debye–Hückel parameter
- \( \dot{B} \) :
-
B-dot parameter of the extended Debye–Hückel model, kg/mol
- C :
-
Molar concentration, kmol/m3
- D ef :
-
Effective diffusivity of the ionic species, m2/s
- F :
-
Faraday’s constant, 96,487 coulomb/mol
- I :
-
Ionic strength, mol/kg
- K :
-
Equilibrium constant of formation reaction on a molal basis
- m :
-
Molal concentration, mol/kg
- m ref :
-
Empirical reference concentration parameter, mol/kg
- NC :
-
Number of solution components
- NI :
-
Number of ionic species in the solution
- R:
-
Ideal gas constant, 8.3173 J/mol/K
- T :
-
Absolute temperature, K
- TOT X :
-
Total concentration, mol/kg
- X :
-
Concentration of a component, mol/kg
- z :
-
Charge number of ionic species
- γ :
-
Activity coefficient
- κ :
-
Solution conductivity, mS/cm
- ν :
-
Stoichiometric coefficient
- ρ :
-
Density
- f :
-
Formation
- i :
-
Species subindex
- j :
-
Component subindex
- sp:
-
Species
- ef:
-
Effective
- r:
-
Reaction
- Fe:
-
Dissolved iron
- Cl:
-
dissolved chloride
- 0:
-
Thermodynamic standard state
References
1. P. Hernández, M. Taboada, O. Herreros, C. Torres, and Y. Ghorbani: Hydrometallurgy, 2015, vol. 157, pp. 325–32.
2. C. Torres, M. Taboada, T. Graber, O. Herreros, Y. Ghorbani, and H. Watling: Miner. Eng., 2015, vol. 71, pp. 139–45.
3. N. Welham, K. Malatt, and S. Vukcevic: Hydrometallurgy, 2000, vol. 57 (3), pp. 209–23.
4. R. Winand: Hydrometallurgy, 1991, vol. 27 (3), pp. 285–316.
5. U. Strahm, R.C. Patel, and E. Matijevic: J. Phys. Chem., 1979, vol. 83 (13), pp. 1689–95.
6. J. Dutrizac: Metall. Trans. B, 1981, vol. 12B, pp. 371–78.
7. M. O’Malley and K. Liddell: Metall. Trans. B, 1987, vol. 18B, pp. 505–10.
E. Godočíkov, P. Baláž, and E. Boldižárová: Hydrometallurgy, 2002, vol. 65 (1), pp. 83–93
9. M.F.C. Carneiro and V.A. Leão: Revista Escola de Minas, 2005, vol. 58 (3), pp. 231–35.
G. Harris, C. White, and G. Demopoulos: in Iron Control Technologies, J.E. Dutrizac and P.A. Riveros, eds., Proc. 3rd Int. Symp. on Iron Control in Hydrometallurgy, 36th Annual CIM Hydrometallurgical Meeting, Montreal, 2006.
11. M. Al-Harahsheh, S. Kingman, and A. Al-Harahsheh: Hydrometallurgy, 2008, vol. 91 (1), pp. 89–97.
12. H. Watling: Hydrometallurgy, 2014, vol. 146, pp. 96–110.
13. D. Yang and W.-Y. Xu: Spectrosc. Spect. Analysis, 2011, vol. 31 (10), pp. 2742–46.
W.F. Linke and A. Seidell: Solubilities, Inorganic and Metal-Organic Compounds, 4th ed., American Chemical Society, Washington, DC, 1965, vols. 1–2.
15. R. Koren and B. Perlmutter-Hayman: Inorg. Chem., 1972, vol. 11 (12), pp. 3055–59.
16. R.H. Byrne and D.R. Kester: J. Solut. Chem., 1981, vol. 10 (1), pp. 51–67.
17. R.H. Byrne, W. Yao, Y.-R. Luo, and B. Wang: Mar. Chem., 2005, vol. 97 (1), pp. 34–48.
R.J. Lemire, U. Berner, C. Musikas, D.A. Palmer, P. Taylor, O. Tochiyama, and J. Perrone: Chemical Thermodynamics of Iron-Part 1-Chemical Thermodynamics Volume 13a, Data Bank, 2013.
19. W. Liu, B. Etschmann, J. Brugger, L. Spiccia, G. Foran, and B. McInnes: Chem. Geol., 2006, vol. 231 (4), pp. 326–49.
20. M.-S. Lee, J.-G. Ahn, and Y.-J. Oh: Mater. Trans., 2003, vol. 44 (5), pp. 957–61.
21. M.-S. Lee: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 173–79.
22. L. André, C. Christov, A. Lassin, and M. Azaroual: Proc. Earth Planetary Sci., 2013, vol. 7, pp. 14–18.
23. H. Majima and Y. Awakura: Metall. Trans. B, 1985, vol. 16B, pp. 433–39.
24. H. Majima and Y. Awakura: Metall. Trans. B, 1986, vol. 17B, pp. 621–27.
25. A.V. Rumyantsev, S. Hagemann, and H.C. Moog: Z. Phys. Chemie/Int. J Res. Phys. Chem. Chem. Phys. 2004, vol. 218 (9/2004), pp. 1089–1127.
D.L. Parkhurst and C. Appelo: Description of Input and Examples for PHREEQC Version 3-A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations, Book 6, ch. A43, 497 p. http://pubs.usgs.gov/tm/06/a43; 2328–7055; US Geological Survey, 2013.
T.W. Wolery and R.L. Jarek: Sandia National Laboratories, U.S. Department of Energy Report, 2003, p. 376.
A.I. Vogel: Vogel’s Textbook of Quantitative Inorganic Analysis, Including Elementary Instrumental Analysis, 4th ed., 1978.
29. J. Casas, G. Crisóstomo, and L. Cifuentes: Hydrometallurgy, 2005, vol. 80 (4), pp. 254–64.
30. H.C. Helgeson, D.H. Kirkham, and G.C. Flowers: Am. J. Sci., 1981, vol. 281 (10), pp. 1249–1516.
31. J.F. Zemaitis, D.M. Clark, M. Rafal, and N.C. Scrivner: Handbook of Aqueous Electrolyte Thermodynamics: Theory & Application. Design Institute for Physical Property Data-AIChE, John Wiley & Sons, New York, NY, 1986.
32. K.S. Pitzer: Activity Coefficients in Electrolyte Solutions, CRC Press, Boca Raton, FL, 1991.
33. I. Grenthe and A. Plyasunov: Pure Appl. Chem., 1997, vol. 69 (5), pp. 951–58.
J. Johnson, G. Anderson, and D. Parkhurst: Database ‘thermo.com.V8.R6.230,’ Rev. 1.11. Lawrence Livermore National Laboratory, Livermore, CA, 2000.
35. D. Sutton: Espectros Electrónicos de los Complejos de los Metales de Transición, Reverté, Barcelona, 1975.
36. D.F. Shiver and P.W. Atkins: Inorganic Chemistry, 3rd ed., Oxford University Press, New York, NY, 2001.
37. S. Nappini, A. Matruglio, D. Naumenko, S. Dal Zilio, F. Bondino, M. Lazzarino, and E. Magnano: Nanoscale, 2017, vol. 9 (13), pp. 4456–66.
38. A. Anderko and M.M. Lencka: Ind. Eng. Chem. Res., 1997, vol. 36 (5), pp. 1932–43.
39. W.M. Haynes: “Ionic Conductivity and Diffusion at Infinite Dilution,” CRC Handbook of Chemistry & Physics, 96th ed., CRC Press/Taylor and Francis, Boca Raton, FL, 2015.
40. F.J. Millero and D. Pierrot: Geochim. Cosmochim. Acta, 2007, vol. 71 (20), pp. 4825–33.
A. Stefánsson, K. Lemke, and T. Seward: Proc. 15th Int. Conf. on the Properties of Water and Steam, Berlin, 2008.
Acknowledgments
The authors acknowledge CORFO for financing CSIRO Chile Supports, Fondecyt Project No. 1140169, and Departamento de Ingeniería Química y Procesos de Minerales of Universidad de Antofagasta.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted October 4, 2016.
Rights and permissions
About this article
Cite this article
Jamett, N.E., Hernández, P.C., Casas, J.M. et al. Speciation in the Fe(III)-Cl(I)-H2O System at 298.15 K, 313.15 K, and 333.15 K (25 °C, 40 °C, and 60 °C). Metall Mater Trans B 49, 451–459 (2018). https://doi.org/10.1007/s11663-017-1010-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-017-1010-0