Abstract
The structural roles of alkali and calcium cations are important for understanding the physical and chemical properties of aluminosilicate melts and glasses. Recently, oxygen-17 nuclear magnetic resonance (17O NMR) studies of calcium–sodium aluminosilicate glasses showed that these structural roles are not randomly given, but rather each cation has its own preferential role. However, the relationship between cation type and role preference in calcium aluminosilicate glass is not completely understood. In the present study, the structural roles of lithium, sodium, and potassium cations in selected calcium aluminosilicate glasses are investigated using 17O solid-state NMR experiments. Data from these experiments clearly show that potassium cations have a notably stronger tendency to act as charge compensators within the network structure, compared to sodium and lithium cations. The result of 17O NMR experiment also showed that sodium and lithium cations in part act as network modifier alongside with calcium cations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alkali oxides are important additives for controlling the chemical and physical properties of calcium aluminosilicate melts, which are systems commonly used for slags and fluxes in high-temperature industries. However, the effects of alkali oxide additives on the physical properties of calcium aluminosilicate systems are complicated and cannot be understood from the viewpoint of aluminosilicate anion polymerization alone. For example, the addition of sodium or lithium oxide results in a decrease in viscosity in such melts, while potassium oxide addition has the opposite effect.[1–6] When we focus on the local structure around these alkali cations, two types of structural roles can be observed: network modifier that creates the non-bridging oxygens (NBOs) on SiO4 tetrahedrons, and charge compensators that neutralize the negative charges on bridging oxygens (BOs) between Si4+ and Al3+ (Si-OBO-Al).[7] This classification may be directly linked with the atomic arrangement of these cations, as network modifiers concentrate in breakage regions of the network structure whereas charge compensators prefer to be in the actual network structure. The physico-chemical properties of aluminosilicate glasses and melts strongly depend on the types of network modifiers and charge compensators present, as well as the degree of polymerization.[8,9]
In the previous paper,[2] the authors tried to understand the effect of alkali oxide addition on the polymerization degree of calcium aluminosilicate glasses using silicon-29 (29Si) solid-state nuclear magnetic resonance (SSNMR) spectroscopy, (see Figure 1) and concluded that the addition of potassium oxide resulted in the polymerization of network structure while lithium and sodium oxide additions depolymerized the network structure. The reported 29Si chemical shift range of each Qn species (n: number of bridging oxygen around SiO4) in silicate glasses and minerals are displayed in Figure 1. The chemical shift ranges[10] of each silicon Qn species shift to the less negative side with increasing the number of NBOs in the next nearest neighbors. Considering the 29Si signals of each Qn species shifting to less negative side also by the presence of aluminum atoms in the next nearest neighbors (see Figure 1), it is found that the precise interpretation of the 29Si NMR spectra for the aluminosilicate glasses is difficult without using double-resonance techniques or simulations because of heavy overlapping signals. Therefore, the change in polymerization degree by adding alkali oxides should be reconsidered by another technique. Recently, oxygen-17 (17O) NMR experiments successfully quantified NBO and BO fraction in binary and ternary silicate glasses and these experiments unveiled that the structural role of calcium and sodium is not fully random in these aluminosilicate glasses, and that sodium ions prefer to act as charge compensators in calcium aluminosilicate glasses.[11,12] However, the influence of different alkali cations on these arrangements as well as polymerization degree was not well understood. In the present study, the structural roles of alkali (specifically lithium, sodium, and potassium) and calcium cations in a selected aluminosilicate glass were demonstrated using 17O solid-state NMR spectroscopy. The effect that cation size has on their preferential structural roles is explored in depth. Also, the change in the polymerization degree has been in part reconsidered based on 17O NMR results.
29Si MAS NMR spectra of the calcium aluminosilicate glasses with different types of alkali oxides.[2] Reported chemical shift ranges[10] of each Qn species are displayed as black bars. Q4(mAl) means Q4 species with different number of aluminum cation next to nearest neighbors: m represents the number of aluminum atoms around a SiO4 tetrahedron. The nominal compositions of the glass samples for 29Si MAS NMR experiments were same as the present work (see Table I). Then the samples were labeled according to the present study and represented in this figure
Experimental
Sample Preparation
Initial compositions of the samples are listed in the Table I. A ternary calcium aluminosilicate (CAS) glass was used as the fundamental glass composition. The other samples are labeled as CASx, with x denoting the type of alkali oxide (L: lithium oxide (Li2O); N: sodium oxide (Na2O); K: potassium oxide (K2O)). The alumina (Al2O3) and alkali oxide concentration and molar ratio of calcium oxide (CaO) to silica (SiO2) (CaO/SiO2) of the samples are 12.6 ± 0.8 mol pct, 10.8 mol pct, and 0.71, respectively. The nominal concentration of NBOs (listed in Table I) was estimated using Eq. [1]:[13]
where X O, X Si, and X Al represent the atomic proportions (pct) of each element. This equation is based on the fact that silicon and aluminum cations have four-fold coordination within the glasses. Previous studies determined that the aluminum cations in glasses of similar compositions had mostly four-fold coordination, as determined using 27Al MAS NMR spectroscopy.[14] As shown in the Table I, the nominal concentration of non-bridging oxygen (NBO/total-O) increased with the addition of alkali oxides, and the value of NBO/total-O is comparable for all alkali oxide-containing systems.
Since oxygen-17 (17O) is an isotope with a low natural abundance (0.037 pct), it is difficult to detect 17O NMR signals for samples that contain a natural abundance of 17O. Subsequently, 17O abundance in our glass samples was enriched by using oxygen-17-enriched silica as a raw material in the preparation of glass samples. 17O-enriched silica was synthesized from silicon tetrachloride (SiCl4) and oxygen-17-enriched water (H 172 O) using Eq. [2], which is based on literature protocols.[15]
A mixture of 17O-enriched silica (17O/total-O ≈ 40 pct) powder was mixed with reagent powders of calcium carbonate (CaCO3), alkali carbonates, and alumina (Al2O3) in an alumina mortar. The mixtures were wrapped in Pt foil and placed in an electric furnace. The samples were melted at 1873 K (1600 °C) for 90 minutes under an Ar atmosphere. After the melting process, the melts were quenched on a copper plate to obtain vitreous samples. The quenched samples were crushed into powders, which were used for the 17O solid-state NMR experiments.
17O Solid-State NMR Measurements
17O magic angle spinning (MAS) NMR spectra and a triple-quantum magic angle spinning (3QMAS) NMR spectrum were obtained using JEOL ECA 700 (16.4 T) and Varian Inova 500 (11.7 T) spectrometers at Larmor frequencies of 94.9 and 67.8 MHz, respectively. For single pulse experiments, a JEOL 4.0 mm MAS probe was used with a small (solids 18°) tip angle at a spinning speed of 18 kHz. Pulse recycle delays were long enough to allow full relaxation of 17O spins. 17O chemical shifts were referenced to pure water.
The additional structural details of each oxygen site were obtained by the 17O triple-quantum MAS (3QMAS)[16] NMR experiment. In the present study, a z-filter type pulse sequence was applied.[17] Pulse durations for 3QMAS were 3 µs (excitation), 1.1 µs (conversion), and 14 µs (z-filter). The 3QMAS spectrum was collected using a Varian/Chemagnetics 3.2 mm MAS probe at a spinning rate of 20 kHz. The isotropic chemical shift δ CS/ppm and the quadrupolar parameter P q/MHz can be calculated from the signal position in two dimensions according to the following Eqs. [3] and [4][18]
where δ 1 and δ 2 are the peak positions in the F1 and the F2 dimensions, respectively. S is the spin number (5/2 for 17O) and ν 0 is the resonance frequency (67.8 MHz) of 17O nuclei at 11.7 T. Normally, δ CS can be correlated with bond length while P q is a measure of polyhedral distortion.[19]
Results
17O MAS NMR Spectra of Alkali and Calcium Aluminosilicate Glasses
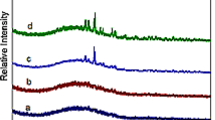
Figure 2 shows the 17O solid-state NMR spectra of the glasses at 16.4 T. The 17O spectrum of the CAS glass shows two main peaks at approximately 110 and 50 ppm. The peak at approximately 110 ppm is close to the NBO signal detected in calcium aluminosilicate glasses.[20–22] Therefore, this peak can be assigned to NBOs coordinated to several Ca cations (Ca-ONBO-T, T: tetrahedral-coordinated Si(or Al)) while the peaks near 50 ppm can be attributed to signals from BOs.[20–22]
17O solid-state NMR spectra of the CASL, CASN, and CASK glasses also bear these two main peaks, indicating that Ca-ONBO-T is the major component of the NBO species in the alkali oxide-containing glasses, a tendency that agrees well with previous work on calcium–sodium aluminosilicate glasses.[11,12] It should be also noted that other signals are present near 80 ppm when Li2O or Na2O are added to the CAS glass. The area around 80 ppm is between the chemical shifts of Ca-ONBO-T and Na-ONBO-T (or Li-ONBO-T), as previously reported in the literature.[23,24] In multicomponent silicate glasses, the non-bridging oxygen is coordinated by one silicon cation and three or four modifier cations with a variety of possible mixing ratios.[25] Therefore, the broad signals around 80 ppm can be attributed to NBOs coordinated with calcium and lithium cations ((Ca, Li)-ONBO-T) in the CASL glass, and from the NBOs coordinated with calcium and sodium cations ((Ca, Na)-ONBO-T) in the CASN glass.[23] These results indicate that lithium and sodium cations can exist in the breakage region alongside calcium cations as network modifiers (i.e., there is cation mixing in breakage regions). By contrast, the creation of another signal around 80 ppm was not observed when K2O was added to the CAS glass, implying that potassium cations rarely exist in the breakage regions but instead exist within the network structure as charge compensators to BOs (i.e., Si-OBO-Al). The 17O MAS NMR results clearly show that the potassium cation has notably stronger preference to be located nearer to the BOs (i.e., Si-OBO-Al), compared to sodium and lithium cations. However, direct evidence of the changes in the type of charge compensators caused by adding potassium oxides was not found in the 17O MAS NMR spectra.
17O 3Q MAS NMR Spectrum of the CASK Glass
In order to obtain more detailed structural information of the alkali and calcium aluminosilicate glasses, a 17O 3QMAS NMR spectrum of the CASK glass (which has the clearest preference in the role of potassium cations) was also collected. Figure 3 shows this spectrum in two dimensions, which shows three main signals labeled (a), (b), and (c). Structurally relevant parameters are listed in Table II. The parameters of the signal (a) (δ CS: 115 ppm, P q: 2.4 MHz) are close to that of the typical NBO signal found in calcium aluminosilicate glasses,[20] supporting the 17O MAS NMR results that suggest that the NBO signal is mainly connected with calcium cations. The P q value of the signal (b) (3.3 MHz) is in the typical range of BOs between silicon and aluminum cations (Si-OBO-Al, 3.3-3.5 MHz).[20,26] However, the δ CS (54 ppm) of signal (b) differs from that of the Si-OBO-Al species in calcium aluminosilicate glass (e.g., 61 ppm[20]). This indicates that another type of Si-OBO-Al is created by the addition of potassium oxide. Namely, the charge compensator cations of Si-OBO-Al were changed from calcium to potassium cations by the addition of potassium oxide. It was difficult to determine the precise peak position of signal (c) because of signal overlap; however, the P q value of signal (c) (≈4.5 MHz) was clearly higher than that of Si-OBO-Al species. This indicates that signal (c) can be assigned to BO between two silicon cations (Si-OBO-Si).[26] One other possible bridging oxygen species of Al-OBO-Al, which is expected to have a smaller P q value (approximately 1.7 MHz[26]), cannot be detected in the present spectrum, indicating that the amount of this Al-OBO-Al species is small. As a result, the presence of Al-OBO-Al was ignored in the present study.
Discussion
Structural Role of Alkali Cations in the Calcium Aluminosilicate Glass
In aluminosilicate glasses, the alkali and calcium cations neutralize the negative charges on the NBOs and the BOs. Following Stebbins’ estimation[27], the negative charges are relatively concentrated on NBOs: the amount of negative charge on NBO is −1, based on its formal valence of −2 and a contribution of +4/4 from Si4+ in four coordination. On the other hand, the Si-OBO-Al has the smaller residual charge of −0.25: it is based on formal valence of oxygen ion (−2) with contributions of +4/4 from Si4+ and +3/4 from Al3+ in four coordination. Here, the ionic potential (Z/r, Z: valence, r: cationic radius) can be a measure of charge density at the cationic surface. Considering the coordination numbers of calcium, lithium, sodium, and potassium cations are reported as 7[28], 4[29], 6[30], and 9[31], respectively, in silicate glasses, the effective cationic radius of cations, r, are in the order of potassium (1.55 Å) > calcium (1.06 Å) ≈ sodium (1.02 Å) > lithium (0.59 Å) according to Shannon’s[32] report. The ionic potential of calcium and alkali cations can be calculated using Shannon’s values, and are in the order of calcium (1.89) > lithium (1.69) > sodium (0.98) > potassium (0.65). This indicates that cations with lower ionic potential tend to compensate the smaller negative charge of the BOs (i.e., Si-OBO-Al) while cations with higher ionic potential prefer to link with NBOs, which are more negatively charged than BOs. Therefore, potassium cations with the lower ionic potential prefer to be charge compensators, compared to sodium and lithium cations. This tendency agrees well with the structural preference of calcium and magnesium cations in aluminosilicate glasses, reported in the literatures.[33,34]
Non-bridging Oxygen Concentration
Concentration of NBOs is one of the most important features for evaluating the physical properties of glass structures. The area fraction of the non-bridging oxygen signal in the MAS NMR spectra of the CAS and CASK glasses (Figure 2) was roughly obtained by signal integration. The observed NBO concentration in the CAS and the CASK glasses was 28.9 and 31.7 pct, respectively. Those values were relatively close to the nominal values: 27.2 pct for the CAS and 33.9 pct for the CASK. Consequently, a clear depolymerization of the network structure was observed after the addition of potassium oxide. This goes against previously published results from 29Si MAS NMR analysis conducted on similar glasses,[2] which described network structure polymerization after the addition of potassium oxide. This disagreement is based on the misunderstanding of 29Si MAS NMR spectra collected from aluminosilicate glasses; it is difficult to determine the non-bridging oxygen concentration in aluminosilicate systems using 29Si MAS NMR data alone because the curve fitting is too arbitrary of a process for this style of NMR.
For lithium- and sodium-containing systems (CASL and CASN), the peak positions of the (Ca, Li)-ONBO-T and (Ca, Na)-ONBO-T are still ambiguous in the 17O MAS NMR spectra, and it was difficult to quantify those area fractions in the present study. Application of NMR double-resonance techniques[35] is necessary in order to quantify overlapped signals, and is planned as future work related to this study.
Conclusion
The conventional view of alkali and alkaline-earth aluminosilicate glasses is that the structural roles of these cations are randomly distributed according to the chemical composition. The structural roles of alkali and calcium cations in selected calcium aluminosilicate glasses were demonstrated by 17O MAS NMR spectroscopy to not be fully random. It was found that alkali cations with the lower ionic potential tend to act as charge compensators for Si-OBO-Al while the alkali cations with higher ionic potential tends to link with NBOs. This structural preference can link with the physical properties of the alkali and calcium aluminosilicate melts (e.g., viscosity).
References
H. Y. Chang, T. F. Lee and T. Ejima: Trans. Iron Steel Inst. Jpn., 1987, vol. 27, pp. 797-804.
S. Sukenaga, N. Saito, K. Kawakami and K. Nakashima: ISIJ Int., 2006, vol. 46, pp. 352-58.
W. H. Kim, I. Sohn and D. J. Min: Steel Res. Int., 2010, vol. 81, pp. 735-41.
I. Sohn and D. J. Min: Steel Res. Int., 2012, vol. 83, pp. 611-30.
G. H. Zhang and K. C. Chou: Metal. Mater. Trans. B, 2012, vol. 43, pp. 841-48.
T. Higo, S. Sukenaga, K. Kanehashi, H. Shibata, T. Osugi, N. Saito and K. Nakashima: ISIJ Int., 2014, vol. 54, pp. 2039-44.
L. Cormier, D. R. Neuville and G. Calas: J. Am. Ceram. Soc., 2005, vol. 88, pp. 2292-99.
M. J. Toplis and D. B. Dingwell: Geochim. Cosmochim. Acta, 2004, vol. 68, pp. 5169-88.
G. Urbain, Y. Bottinga and P. Richet: Geochim. Cosmochim. Acta, 1982, vol. 46, pp. 1061-72.
K. J. D. MacKenzie and M. E. Smith: Multinuclear solid-state NMR of inorganic materials. 1st ed. (Pergamon, Oxford, 2002).
S. K. Lee and S. Sung: Chem. Geol., 2008, vol. 256, pp. 326-33.
A. Pedone, E. Gambuzzi and M. C. Menziani: J. Phys. Chem. C, 2012, vol. 116, pp. 14599-609.
B. O. Mysen and P. Richet: Silicate Glasses and Melts: Properties and Structure. 1st ed. (Elsevier, Amsterdam, 2005).
S. Sukenaga, T. Nagahisa, K. Kanehashi, N. Saito and K. Nakashima: ISIJ Int., 2011, vol. 51, pp. 333-35.
A.E. Geissberger and P.J. Bray: J. Non-Cryst. Solids, 1983, vol. 54, pp. 121-37.
L. Frydman and J.S. Harwood: J. Am. Chem. Soc., 1995, vol. 117, pp. 5367-68.
J.P. Amoureux, C. Fernandez, and S. Steuernagel: J. Magn. Reson., Ser A, 1996, vol. 123, pp. 116–18.
J. P. Amoureux, C. Huguenard, F. Engelke and F. Taulelle: Chem. Phys. Lett., 2002, vol. 356, pp. 497-504.
K. Kanehashi, K. Shimoda and K. Saito: Tetsu-to-Hagané, 2009, vol. 95, pp. 321-30.
J. F. Stebbins and Z. Xu: Nature, 1997, vol. 390, pp. 60-62.
J. F. Stebbins, J. V. Oglesby and S. K. Lee: Chem. Geol., 2001, vol. 174, pp. 63-75.
J.F. Stebbins, E.V. Dubinsky, K. Kanehashi and K.E. Kelsey: Geochim. Cosmochim. Acta, 2008, vol. 72, pp. 910-25.
S. K. Lee and J. F. Stebbins: J. Phys. Chem. B, 2003, vol. 107, pp. 3141-48.
H. Maekawa, P. Florian, D. Massiot, H. Kiyono and M. Nakamura: J. Phys. Chem., 1996, vol. 100, pp. 5525-32.
P. Florian, K. E. Vermillion, P. J. Grandinetti, I. Farnan and J. F. Stebbins: J. Am. Chem. Soc., 1996, vol. 118, pp. 3493-97.
S.K. Lee and J.F. Stebbins: J. Non-Cryst. Solids, 2000, vol. 270, pp. 260-64.
J. F. Stebbins, J. S. Wu and L. M. Thompson: Chem. Geol., 2013, vol. 346, pp. 34-46.
K. Shimoda, Y. Tobu, K. Kanehashi, K. Saito and T. Nemoto: Solid State Nucl. Magn. Reson., 2006, vol. 30, pp. 198-202.
H. Uhlig, M. J. Hoffmann, H. P. Lamparter, F. Aldinger, R. Bellissent and S. Steeb: J. Am. Ceram. Soc., 1996, vol. 79, pp. 2833-38.
X. Y. Xue and J. F. Stebbins: Phys. Chem. Miner., 1993, vol. 20, pp. 297-307.
W. E. Jackson, G. E. Brown and C. W. Ponader: J. Non-Cryst. Solids, 1987, vol. 93, pp. 311-22.
R. D. Shannon: Acta Crystallogr. Sect. A, 1976, vol. 32, pp. 751-67.
D. R. Neuville, L. Cormier, V. Montouillout, P. Florian, F. Millot, J. C. Rifflet and D. Massiot: Am. Mineral., 2008, vol. 93, pp. 1721-31.
K. E. Kelsey, J. R. Allwardt and J. F. Stebbins: J. Non-Cryst. Solids, 2008, vol. 354, pp. 4644-53.
X. Y. Xue: Solid State Nucl. Magn. Reson., 2010, vol. 38, pp. 62-73.
Acknowledgments
The authors (H.S., K.N., N.S., and S.S.) are grateful for the financial support from the Cooperative Research Program of “Network Joint Research Center for Materials and Devices”, IMRAM Tohoku University. This work was financially supported in part by a Grant-in-Aid for Scientific Research (C) Grant (No. 25420792) from the Japan Society for the Promotion of Science (JSPS) and research fund by IMRAM, Tohoku University. The authors would like to acknowledge and thank Dr. Takafumi Takahashi and Mr. Tatsuya Nishiura (Nippon Steel & Sumitomo Metal Corporation) for technical support in NMR measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 18, 2015.
Rights and permissions
About this article
Cite this article
Sukenaga, S., Kanehashi, K., Shibata, H. et al. Structural Role of Alkali Cations in Calcium Aluminosilicate Glasses as Examined Using Oxygen-17 Solid-State Nuclear Magnetic Resonance Spectroscopy. Metall Mater Trans B 47, 2177–2181 (2016). https://doi.org/10.1007/s11663-016-0689-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0689-7