Abstract
The mold flux in continuous casting mold experiences a significant temperature gradient ranging from more than 1773 K (1500 °C) to room temperature, and the viscosity of the mold flux would therefore have a non-Arrhenius temperature dependency in such a wide temperature region. Three non-Arrhenius models, including Vogel–Fulcher–Tammann (VFT), Adam and Gibbs (AG), and Avramov (AV), were conducted to describe the relationship between the viscosity and temperature of mold flux in the temperature gradient existing in the casting mold. It found that the results predicted by the VFT and AG models are closer to the measured ones than those by the AV model and that they are much better than the Arrhenius model in characterizing the variation of viscosity of mold flux vs temperature. In addition, the VFT temperature and AG temperature can be considered to be key benchmarks in characterizing the lubrication ability of mold flux beyond the break temperature and glass transition temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Viscosity is one of the most important properties of mold flux as it determines the powder consumption and therefore the lubrication of the shell;[1,2] it also affects the formation of the slag rim in the vicinity of the meniscus,[3] the slag entrapment,[4] as well as the erosion of the nozzle.[5]
Early models for predicting the viscosity of mold flux were developed by using viscosity measurements that spanned relatively small ranges of temperature and viscosity. The data, derived from these restricted ranges of experimental conditions, were generally linear in reciprocal of temperatures; thus, the early models follow Arrhenius formulation strictly.[6–8] But, the mold flux in a continuous casting mold experiences a wider temperature gradient from more than 1773 K (1500 °C) to room temperature.[9,10] For the mold flux on top of the molten steel, its temperature is close to the molten steel, while its temperature decreases to break temperature after the liquid mold flux infiltrates into the gap between the mold wall and the shell; its temperature further decreases to room temperature as the mold flux comes out with the slab from the bottom of the mold. The mold flux viscosity does not show strong Arrhenius dependency over such wide temperature variation.
There are three models developed for description of the non-Arrhenius temperature-dependent viscosity in silicate melts, including Vogel–Fulcher–Tammann (VFT), Adam and Gibbs (AG), and Avramov (AV).
The Vogel–Fulcher–Tammann (VFT) model was proposed by Vogel, Fulcher, and Tammann,[11–13] and it has been widely used to estimate the variation of viscosity of magma,[14,15] glass-forming liquids,[16] polymers,[17] ceramics,[18] ionic liquids,[19] etc. It was expressed as:
where η is the viscosity in Pa s and T is the absolute temperature (K). The variables A VFT, B VFT, and C VFT are adjustable parameters representing the pre-exponential factor, the pseudo-activation energy, and the VFT temperature, respectively.
The Adam–Gibbs model[20] is the result of a generalization and an extension of an earlier work by Gibbs and DiMarzio on the configurational entropy theory; it is expressed as:
where η is the viscosity; T is the absolute temperature; and A AG, B AG, and C AG are also representing the pre-exponential factor, the pseudo-activation energy, and the temperature where the viscosity is infinity. This model has been discussed in the literature of earth science by Bottinga et al.,[21] and more recently, it was adopted to predict viscosity in the silicate system by Thinker et al.,[22] in the glass system by Hrma et al.,[23] and in the polymer system by Xiao et al.[24]
The Avramov model[25] is an entropy-based model to describe the effect of temperature and pressure on structural relaxation time. It assumes that, due to existing disorder, activation energy barriers with different heights occur and that the distribution function for the degree of these barriers depends on the entropy. Thus, viscosity is assumed to be a function of the total entropy of the system that leads to the stretched exponential temperature dependence of equilibrium viscosity:
where A AV is a pre-exponential term, B AV represents a pseudo-activation energy related to the potential energy barriers obstructing the structural rearrangement of the liquid, and C AV is a measure of melt fragility that is an indication of the non-Arrhenius T-dependence of the melt. The AV model also has been used in the glass-forming melt,[26] polyol,[27] coal ash slags,[28] etc.
Although the VFT, AG, and AV models have been widely used, they have never been adopted to study the rheological property of mold flux. Therefore, in this article, the viscosity of mold fluxes was first measured at the temperatures ranging from 1200 K to 1573 K (927 °C to 1300 °C); then the relationship between viscosity and temperature was established by both the Arrhenius model and the non-Arrhenius VFT, AG, and AV models; finally, the adjusted coefficient of determination (Adj. R 2) for the fitting of each model was compared to conclude which model is better to describe the relationship between viscosity and temperature of mold flux.
The designed mold fluxes in this study are based on a commercial mold flux sample 1 (Table I ) for casting low-carbon steel. Samples 2–6 were prepared by adding reagent grade chemicals of CaCO3, SiO2, Al2O3, MgCO3, Na2CO3, Li2CO3, CaF2, and B2O3 (Supplier: Fine Chemical Engineering and Technology Research and Development Center, Guangdong, China) with different ratios to obtain their target compositions. The sample was first stirred in a blender for 120 minutes to homogenize its chemical composition before the viscosity measurement test.
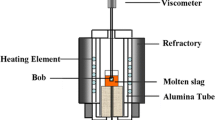
The viscosity measurements were carried out by using a Brookfield DV-II + viscometer (Brookfield Inc., USA), through the rotating cylinder method, which is schematically shown in Figure 1. A calibration measurement was carried out at room temperature by using stand oil with a known viscosity.[29]
During the viscosity measurement test, first, about 250 g of the sample powders were placed in a graphite crucible with a diameter and internal height of 50 and 80 mm, respectively. Second, the crucible was heated to 1773 K (1500 °C) and held for 10 minutes to obtain a homogeneous melt in an electric resistance furnace with MoSi2 as the heating element. Then, the melt was cooled to the target temperature. After that, a bob, which is made of molybdenum with the height of 18 mm and the diameter of 15 mm, was immersed into the liquid slag bath and rotated to obtain the value of viscosity at the target temperature.
The mold fluxes after the viscosity tests were also analyzed by X-ray fluoroscopy (XRF, S4 Pioneer; Bruker AXS; GmbH Karlsruhe, Germany) and Inductively Coupled Plasma Optical Emission (ICP, SPECTRO, Germany). The results are shown in Table I. It could be found that the evaporative loss of mold flux components is relatively small and that the influence caused by the evaporation can be ignored, which is consistent with our previous study.[30,31]
The measured viscosity and the corresponding temperatures were fitted by Arrhenius and non-Arrhenius VFT, AG, and AV models; then the quality of regressions was evaluated by a parameter named Adj. R 2. The Adj. R 2 is a statistical measure on how well the regression line approximates the measured data points, and the ideal Adj. R 2 value of 1 indicates that the regression line fits the data perfectly.[32]
The Adj. R 2 can be written as:
where p is the total number of explanatory variables in the model and n is the sample size. R 2 is the coefficient of determination, which can be computed as:
where SS res is the regression sum of the square measured deviation and SS tot is the total sum of the square of predicted deviation. They can be written as:
where y i are measured values of logη, f i are predicted values of logη, and \( \bar{y} = \frac{1}{n}\sum\limits_{i = 1}^{n} {y_{i} }.\)
The data of viscosity and temperature were fitted by using the Arrhenius, VFT, AG, and AV models and are shown in Figure 2. Among them, Figure 2(a) shows the fitting results of the Arrhenius model, and Figures 2(b) through (d) shows the fitting results of the non-Arrhenius VFT, AG, and AV models, respectively. It can be intuitively seen that the deviation between the measured data and the fitting data is pretty huge in the Arrhenius model as shown in Figure 2(a), especially in the low-temperature zone, which suggests that the Arrhenius model is not good enough for the prediction of the temperature-viscosity property of mold flux. The reason for that is mainly because logη vs T, in the Arrhenius model, is a linear-related function. However, the viscosity of mold flux decreases slowly with the increase of temperature in the high-temperature zone, while it increased sharply with the reduction of temperature in the lower temperature zone, which indicates that logη vs T is obviously nonlinear related in the whole temperature range. Actually, in our previous paper,[7] to study the rheological behavior of F-free mold flux, the whole temperature range was divided into two temperature zone, one is the temperature below break temperature (T < T br) and the other is T > T br. Then, the logη vs T fitted by the Arrhenius model should be separated in two temperature zones.
Compared with the Arrhenius model in Figure 2(a), the fitting data match the measured ones much better in the VFT, AG, and AV models, as shown in Figures 2(b) through (d). To evaluate those non-Arrhenius models, the Adj. R 2 of each model was obtained and shown in Figure 3. It can be found in Figure 3 that the Adj. R 2 values for VFT, AG, and AV models are much higher than those of the Arrhenius model, which further confirms that the viscosity of mold flux is non-Arrhenius. Also, the Adj. R 2 of VFT and AG models varies around 0.92 to 0.96, which is relatively uniform compared with that of the AV model ranging from 0.85 to 0.96. Furthermore, the Adj. R 2 values of the VFT and AG models are pretty close. Therefore, the VFT and AG models are more accurate for the description of the relationship between viscosity and temperature of mold flux in a wide temperature range.
The values of these parameters used in the Arrhenius and non-Arrhenius models could be obtained in the fitting process, and they are listed in Table II. First, it indicates that those parameters used in the Arrhenius, VFT, AG, and AV models varied with the change of mold flux composition, which suggests that the parameters are composition related. The details about the effect of the mold flux component on the parameters of Arrhenius and non-Arrhenius models will be discussed in a later paper. Second, the values of the parameters for the VFT and AG models are close as the physic meaning of those parameters in both the VFT and AG models are the same. The B VFT in the VFT model and the B AG in the AG model are corresponding to the pseudo-activation energy that is thought to represent a potential energy barrier for the obstruction of the structural rearrangement of the mold flux melt. Although both C VFT and C AG are the temperature (K) at which viscosity becomes infinite, which indicates that the mold flux will lose flowability and cannot lubricate the shell well when the temperature is below them. Therefore, the C VFT and C AG can be considered to be another key benchmark for characterizing the lubrication ability of mold flux below the break temperature (T br) and glass transition temperature (T g). In fact, it is meaningful to use the C VFT and C AG to describe the mold flux lubrication ability as they can be obtained directly and accurately from the VFT and AG models based on the viscosity and temperature data from the measurement, although it often produces an error in the determination of T br and T g due to the arbitrary estimation on the slope change of the viscosity–temperature curve.
The current communication presents an application of both Arrhenius and non-Arrhenius VFT, AG, and AV models to describe the relationship between viscosity and temperature. The main conclusions are summarized as follows:
-
1.
The logη vs T of mold flux was obviously nonlinear related at a temperature ranging from 1200 K to 1573 K (927 °C to 1300 °C), which indicates that the Arrhenius model is not good enough for the description of temperature-dependent viscosity of mold flux in the wider temperature range.
-
2.
The Adj. R 2 of VFT and AG models was around 0.92 to 0.96, which is relatively higher than that of the AV model ranging from 0.85 to 0.96. So, the VFT and AG are more accurate than the Arrhenius model in characterizing the relationship between viscosity and temperature.
-
3.
The values of the parameters used in the VFT and AG models are close. And C VFT and C AG can be considered to be key benchmarks for characterizing the lubrication ability of mold flux.
References
1. T. Kajitani, Y. Kato, K. Harada, K. Saito, K. Harashima, and W. Yamada: ISIJ Int., 2008, vol. 48, pp. 1215-24.
2. E. Takeuchi and J.K. Brimacombe: Metall. Trans. B, 1984, vol. 15, pp. 493-509.
B. Thomas and J. Sengupta: JOM, vol. 58, pp. 16–18.
4. Y. Chung and A.W. Cramb: Metall. Mater. Trans. B, 2000, vol. 31, pp. 957-71.
5. H. Harmuth and G. Xia: ISIJ Int., 2015, vol. 55, pp. 775-80.
6. G. Kim and I.L. Sohn: Metall. Mater. Trans. B, 2013, vol. 45, pp. 86-95.
7. L. Zhou, W. Wang, and K. Zhou: ISIJ Int., 2015, vol. 55, pp. 1916-24.
8. Z. Zhang, G. Wen, P. Tang, and S. Sridhar: ISIJ Int., 2008, vol. 48, pp. 739-46.
A. Cramb: AISI/DOE Technology Roadmap Program, 2003.
10. Y. Meng and B. Thomas: Metall. Mater. Trans. B, 2003, vol. 34, pp. 685-705.
D.H. Vogel Das: Physikalische Zeitschrift, 1921, vol. 22, pp. 645–46.
12. G.S. Fulcher: J. Am. Ceram. Soc., 1925, vol. 8, pp. 339-55.
13. G. Tammann and W. Hesse: Z. Anorg. Allg. Chem., 1926, vol. 156, pp. 245-57.
14. D. Giordano and D. Dingwell: Earth Planet. Sci. Let., 2003, vol. 208, pp. 337-49.
15. D. Giordano, J. Russell, and D. Dingwell: Earth Planet. Sci. Let., 2008, vol. 271, pp. 123-34.
16. J. Rault: J. Non-Cryst. Solids, 2000, vol. 271, pp. 177-17.
17. H. Lu and W. Huang: Smart Mater. Struct. 2013, vol. 22, pp. 1-8.
18. S. Salema, S. Jazayeri, F. Bondioli, A. Allahverdi, and M. Shirvani: Thermochim. Acta, 2011, vol. 521, pp. 191-96.
19. B. Mokhtarani, A. Sharifi, H. Mortaheb, M. Mirzaei, M. Mafi, and F. Sadeghian: J. Chem. Thermodyn., 2009, vol. 41, pp. 323-29.
20. G. Adam and J. Gibbs: J. Chem. Phys., 1965, vol. 43, pp. 139-46.
21. Y. Bottinga and Daniel F. Weill: Amer. J. Sci., 1972, vol. 272, pp. 438-75.
22. D. Thinker, C. Lesher, G. Baxter, T. Uchida, and Y. Wang: Am. Mineral., 2004, vol. 89, pp. 1701-708.
23. P. Hrma: J. Non-Cryst. Solids, 2008, vol. 354, pp. 3389-99.
24. R. Xiao and T. Nguyen: Soft Matter., 2013, vol. 9, pp. 9455-64.
25. I. Avramov: J. Non-Cryst. Solids, 1998, vol. 238, pp. 6-10.
A. Puzenko, P. Ben, and M. Paluch: J. Chem. Phys., 2007, vol. 127, pp. 094503 (1)–(4).
27. M. Longinotti, J. Gonzalez, and H. Corti: Cryobiology, 2014, vol. 69, pp. 84-90.
28. T. Nentwig, A. Kondratiev, E. Yazhenskikh, K. Hack, and M. Müller: Energ. Fuel., 2013, vol. 27, pp. 6469-76.
29. L. Zhou, W. Wang, B. Lu, and G. Wen: Metall. Mater. Int., 2015, vol. 21, pp. 126-33.
30. L. Zhou, W. Wang, F. Ma, J. Li, J. Wei, H. Matsuur, and F. Tsukihashi: Metall. Mater. Trans. B, 2012, vol. 43, pp. 354-62.
L. Zhou, W. Wang, D. Huang, J. Wei, and J. Li: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 925-26.
32. E. Kurnianto, A. Shinjo, and D. Suga: Asian Austral. J. Anim. Sci., 1999, vol. 12, pp. 331-35.
This work was financially supported by the National Science Foundation of China (51504294, 51322405), and the Opening Foundation of the State Key Laboratory of Advanced Metallurgy (KF14-10) is great acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 18, 2015.
Rights and permissions
About this article
Cite this article
Zhou, L., Wang, W. Application of Non-Arrhenius Models to the Viscosity of Mold Flux. Metall Mater Trans B 47, 1548–1552 (2016). https://doi.org/10.1007/s11663-016-0651-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0651-8