Abstract
Iron ore pellets, reduced with hydrogen, were isothermally carburized in CH4-H2-N2 at 823 K, 923 K, and 1023 K (550 °C, 650 °C, and 750 °C). Temperature strongly affected the total carbon concentration after carburization; significant unbound carbon deposited at the highest temperature. For the range of sizes tested (10 to 12 mm), pellet size did not affect carburization. The variability between pellets was much smaller than for industrial pellets; inhomogeneous gas distribution likely affects carburization under large-scale industrial conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Direct-reduced iron (DRI) is used as a low-residual feed material for electric furnace steelmaking, and a source of metallic iron in blast furnace ironmaking. DRI contains carbon as a result of carburization reactions in the lower part of gas-based direct-reduction shafts; the carbon forms by decomposition of methane (CH4). In electric furnace steelmaking, DRI with higher carbon concentrations (around 4 pct by mass), largely present in the form of cementite (Fe3C),[1] promotes carbon transfer to the steel bath, with a beneficial effect on nitrogen removal.[2] However, in the current work, sampling of industrial DRI pellets showed large variability in the carbon concentration in individual pellets, as illustrated by Figure 1—with carbon concentrations ranging from 1 pct to more than 6 pct (average around 4 pct). The likely origin of the variability for industrial DRI is radial variation in gas flow in the direct-reduction shaft, leading to radial differences in degree of reduction and carburization.[3] The aim of the work presented here was to test whether laboratory carburization in simple CH4-H2-N2 gas mixtures would give less-variable carbon concentrations, and to test the effect of temperature on cementite formation. For utilization of DRI in electric furnace steelmaking, carbon as cementite is preferred to unbound carbon (amorphous or graphitic carbon), for better transfer of carbon to the metal bath.[4]
Distribution of carbon concentrations in industrial DRI pellets (produced with the HYL Energiron[1] process)
In this work, no H2S was included in the laboratory gas mixture; in the absence of H2S, cementite would tend to decompose to graphite,[5] but with a higher overall rate of carburization.[6]
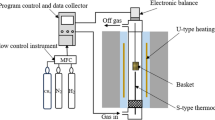
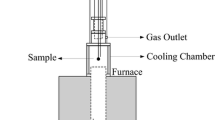
For experimental work, hematite iron ore pellets (average composition in Table I) were obtained from a US Great Lakes mine. The diameter of the pellets was in the range 10 to 13 mm, with mass per pellet in the range 3 to 5 g. Before carburization in CH4-H2-N2 gas mixtures, pellets were reduced in 75 pct H2-25 pct N2 at 1123 K (850 °C), at a gas flow rate of 2 dm3/minute (gas flow rates are given for reference conditions of 297 K (24 °C) and 1 atm). The experimental set-up for both reduction and carburization is schematically presented in Figure 2. A single layer of 8 to 9 pellets was placed on a porous frit in the inner tube of a fused-quartz reactor (inner diameter 50 mm), with reaction gas passing upwards through the layer of pellets. The gas pressure was approximately 1 atm in all cases. The reactor was heated externally with an electrical resistance furnace. Reduction and carburization were performed isothermally (monitoring the temperature immediately above the pellet bed with a K-type thermocouple in a fused-quartz sheath); heating to the reaction temperature was at 30 K/minute and cooling after reaction was at 5 K/minute, both under nitrogen. The gas flow for carburization was 0.3 dm3/minute; gas compositions are summarized in Table II and compared with equilibria in Figure 3.
Equilibrium gas compositions for graphite and cementite formation from gas mixtures containing H2 and CH4, compared with the gas compositions used in this work. Equilibria calculated with FactSage 6.4,[7] assuming unit activities of Fe, C and Fe3C
Phases in the pellets were analyzed by X-ray diffraction (Cu Kα radiation), using Rietveld quantification with X’Pert High Score Plus software (supplied by PANanalytical, Almelo, The Netherlands).
After reduction in hydrogen, the mass loss was 29 to 29.8 pct, indicating full reduction of Fe2O3 to Fe, as did the X-ray pattern (Figure 4). The mass gain after subsequent carburization was used to determine the carbon concentration in pellets. In all but one case (where a small amount of graphite was detected), cementite was the only carbon-containing crystalline phase in the carburized pellets (Figure 4). The analyzed cementite concentration (from Rietveld analysis) was corrected for X-ray invisible amorphous carbon by using the total carbon concentration from mass gain.
The results are presented in Figures 4, 5, 6, and 7. While X-ray diffraction showed that cementite formed for all the combinations of time, temperature and gas composition tested (Figure 4), the total carbon concentration was quite different (Figure 5)—much higher for the highest temperature [1023 K (750 °C)]. Figures 5(b) and (c) also show that for 823 K and 923 K (550 °C and 650 °C), the total carbon (analyzed by mass gain) was nearly equal to the bound carbon (from Fe3C analyzed by X-ray diffraction), reaching on average 5.3 pct C for carburization at 923 K (650 °C) for 1.5 hours. In contrast, a large proportion of the carbon is in unbound form (mainly amorphous carbon) after carburization at the highest temperature of 1023 K (750 °C).
Carbon concentration in pellets carburized at three temperatures for different times. (a) Total carbon does not depend on pellet mass; broken lines give average carbon concentration for each carburization condition. (b) Results of analysis of individual pellets for chemical form of carbon, showing carbon present as Fe3C (crosses) and total carbon (triangles), for different carburization temperatures. (c) The same results as in (b), plotted with respect to time. Broken lines show proposed trends
Based on the analyzed forms of carbon and carburization times, Figure 5(c) presents conjectural trends of the different forms of carbon with time. For carburization at 823 K (550 °C), the results suggest a non-zero incubation time before carburization starts, as generally found for carburization under metal-dusting conditions.[6] For 1023 K (750 °C), formation of amorphous carbon (and of a small proportion of graphite) is assumed to follow cementite formation, since cementite catalyzes deposition of unbound carbon.[6] The concentration of cementite was slightly lower after carburization at 1023 K (750 °C) (5.5 to 5.7 pct bound carbon) than at 923 K (650 °C) (6 to 6.2 pct bound carbon) for the individual pellets analyzed, indicating a role of cementite decomposition to metallic iron and carbon at the highest temperature. (Note that, because of some variability between pellets, the carbon concentrations of individual pellets—for which the forms of carbon were measured and presented in Figures 5(b) and (c)—are different from the averages shown in Figure 5(a).)
In addition to likely changes in the kinetics of carbon deposition and cementite decomposition, temperature also changes the relative driving forces for cementite formation and carbon deposition. As Figure 3 shows, the difference in equilibrium gas compositions for the two reaction products diminishes at higher temperatures; that is, the difference in driving force for formation of cementite and carbon becomes smaller. The strong effect of temperature on cementite formation and the increased proportion of unbound carbon at higher temperature are in line with previous research.[8–10] However, decomposition of cementite played a much smaller role in this work than previously reported for carburization of ore under similar conditions.[10] The results of Zhang and Ostrovski[10] indicate that, for the ore tested in their work (carburization in 55 pct H2-35 pct CH4-10 pct Ar), cementite decomposition was much faster at 923 K (650 °C) than at 1023 K (750 °C), and that at 923 K (650 °C) cementite decomposition became significant at carbon concentrations higher than approximately 4 pct. In contrast, in the present work essentially all the carbon was found to be present as cementite for carbon concentrations up to 6 pct for carburization at 923 K (650 °C) (Figure 5(c)). This difference suggests that other factors need to be studied to fully understand cementite formation and decomposition. The extent of cementite decomposition and the onset of deposition of amorphous carbon are expected to depend on not just temperature, but also the gas composition (CH4:H2 ratio, the presence of sulfur and possibly nitrogen) and pellet characteristics (pore structure after reduction, and presence of surface-active impurities in the ore).
While there was some pellet-to-pellet variability of carbon concentration (Figure 5(a)), this did not show any clear trend with pellet mass, and was much smaller than the variability in industrial pellets (Figure 1). The absence of an effect of pellet size on carbon concentration indicates that pore diffusion of the methane was not rate-controlling during carburization. To further assess this, the core of a pellet [carburized at 823 K (550 °C) for 4 hours] was analyzed, and compared with the outer shell of the same pellet. For analysis by X-ray diffraction, the pellet core (diameter 3.5 mm) was removed by milling, after immobilizing the pellet by embedding its lower part in cold-setting resin, and milling away the upper half of the pellet. The remaining shell (outer diameter 6.5 mm) was also crushed for X-ray diffraction. Comparison of the X-ray diffraction patterns (Figure 6(a)) shows no difference in proportions of cementite (Fe3C) and ferrite (Fe) between the core and shell (outer) regions. Uniform carburization throughout the pellets is consistent with previous conclusions that the interfacial reaction of methane is rate-determining.[10]
While there is no apparent difference in carbon concentration between the core and shell region, there is some difference in the appearance of the porosity which develops during reduction (Figure 6(b)): the pores in the core were generally somewhat coarser, indicating a role of sintering of wüstite on the pore structure after full reduction. For the sizes of pellets used here, reduction of iron oxide in hydrogen would follow shrinking-core kinetics.[11] This means that the core would have remained as wüstite for longer than the shell (which reduced more rapidly to metallic iron), allowing more time for the coarser pore structure of the core to develop. However, it should be emphasized that the difference in pore structure after reduction was not associated with any significant difference in carbon concentration after reduction. It is concluded that the variability in carbon content of industrial pellets likely results from inhomogeneous gas distribution, and is not caused by the inherent kinetics of carburization.
References
A.A. Manenti: AISTech 2015 proceedings, Association for Iron and Steel Technology, Warrendale. 2015, pp. 333–44.
A.G. Shamilov and A.M. Nemenov: Metallurgist, 2010, vol. 54, pp. 210-225.
D. Dalle Nogare, A. Zugliano, A. Primavera, T. Melchiori, and P. Canu: STEELSIM 2013, Proceedings of the 5th International Conference, Czech Metallurgical Society, Ostrava, Czech Republic, 2013. Available at http://www.hutnickaspol.cz/dokumenty[1126]-[cz]-conference-proceedings.
F. Memoli: AISTech 2015 proceedings, Association for Iron and Steel Technology, Warrendale, PA, 2015, pp. 1928–45.
H.J. Grabke, D. Moszynski, E. M. Müller-Lorenz and A. Schneider: Surf. Interface Anal., 2002, vol. 34, pp. 369–374.
H.J. Grabke: Materials and Corrosion, 2003, vol. 54, pp. 736-746.
C.W. Bale, P. Chartrand, S.A. Decterov, G. Eriksson, K. Hack, R. Ben Mahfoud, J. Melançon, A.D. Pelton and S. Petersen: Calphad, 2002, vol. 62, pp. 189-228.
F.B. Benique, J.C. D’Abreu, M.M. Otaviano and H.M. Kohler: Tecnologia em Metalurgia, Materiais e Mineracao, 2011, pp. 379–88.
H. Nakagawa, T. Murayama and Y. Ono: Tetsu to Hagané, 1996, vol. 82, pp. 261-266.
J. Zhang and O. Ostrovski: ISIJ International, 2001, vol. 41, pp. 333-339.
E.T. Turkdogan and J.V. Vinters: Metallurgical Transactions A, 1971, vol. 2, pp. 3175-3188.
Financial and technical support by the industrial members of the Center for Iron and Steelmaking Research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 29, 2015.
Rights and permissions
About this article
Cite this article
He, Y., Pistorius, P.C. Laboratory Carburization of Direct-Reduced Iron in CH4-H2-N2 Gas Mixtures, and Comparison with Industrial Samples. Metall Mater Trans B 47, 1538–1541 (2016). https://doi.org/10.1007/s11663-016-0619-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0619-8