Abstract
The oxygen solubility in iron-nickel alloys with niobium was experimentally studied in Fe-40 pct Ni melt at 1823 K (1550 °C). It was shown that the presence of niobium decreases the oxygen solubility in this melt. The equilibrium constant of interaction of niobium with oxygen dissolved in the Fe-40 pct Ni (\( \log K_{{(1)({\text{Fe - }}40\% {\text{Ni}})}} = {-}4.619 \)), the Gibbs energy of this reaction (\( \Delta {\text{G}}_{{(1)({\text{Fe - }}40\% {\text{Ni}})}}^{ \circ } = 161 ,210\,{\text{J}}/{\text{mol}} \)), and the interaction coefficients characterizing these solutions (\( e_{{{\text{Nb}}({\text{Fe - }}40\,{\text{pctNi}})}}^{\text{O}} = {-}0.630 \); \( e_{{{\text{O}}({\text{Fe - }}40\,{\text{pctNi}})}}^{\text{Nb}} = {-}0.105 \); \( e_{{{\text{Nb}}({\text{Fe - }}40\,{\text{pctNi}})}}^{\text{Nb}} = 0.010 \)) were determined. In the wide concentration range, the equilibrium constants and Gibbs energy of interaction of niobium and oxygen dissolved in the Fe-Ni melts and the interaction coefficients for these solutions were calculated for 1823 K (1550 °C). For this temperature, the oxygen solubility in the niobium-containing Fe-Ni melts was also determined. With an increase in the nickel concentration in the alloy the niobium affinity to oxygen rises appreciably. This appears to be associated with a decrease in the bond strength between metal and oxygen in the melt as the nickel concentration increases (\( \gamma_{\text{O(Fe)}}^{ \circ } = 0.0084;\;\gamma_{{{\text{O}}({\text{Ni}})}}^{ \circ } = 0.297 \)).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Fe-Ni-based alloys are widely used in modern technology. The presence of oxygen in these alloys degrades their service characteristics. The thermodynamic properties of oxygen solutions in iron and nickel have been studied previously. The thermodynamic parameters of these solutions have been published.[1,2] However, since no additivity of properties of oxygen solutions in the Fe-Ni melts with respect to pure iron and nickel is observed, it is necessary to study the thermodynamic parameters of oxygen solutions in the iron-nickel alloys to optimize the production of these alloys.
Upon production of iron-nickel alloys, niobium is an alloying element. This element has the higher affinity to oxygen in comparison with iron and nickel. When niobium is added to the nondeoxidized melt, its substantial portion can be oxidized and lost. In the nickel-niobium and iron-nickel alloys with niobium, the oxygen solubility was not studied earlier. For this reason, the investigation of thermodynamics of oxygen solutions in the iron-nickel alloys with niobium is of scientific and commercial interest.
Thermodynamic Consideration
In the iron-nickel melts, the product of interaction of niobium and oxygen dissolved in melt is NbO2 oxide. The reaction
where f Nb and f O are the activity coefficients of Nb and O, respectively, can be represented as a sum of reactions
\( \Delta G_{(2)}^{ \circ } \, = \,773,302\, - \,160.15\,T, \) J/mol[3];
\( \Delta G_{(3)}^{ \circ } \, = \,26,921\, - \,9.8\,T, \) J/mol[4];
where M Nb and M O, and \( \gamma_{\text{Nb(Fe - Ni)}}^{ \circ } \, \) and \( \gamma_{\text{O(Fe - Ni)}}^{ \circ } \) are atomic masses and activity coefficients of Nb and O, respectively.
For Reaction [1], the oxygen concentration in melt being in equilibrium with a given niobium content can be calculated by the following equation; assuming
NbO2 oxide [T m = 2270 K[5] (1997 °C)] is solid at 1823 K (1550 °C), therefore \( a_{{{\text{NbO}}_{2} }} = 1 \).
\( {{e}}_{{\rm{Nb}}\left({{\text{Fe{-}Ni}}} \right)\,}^{{\rm{Nb}}}{\rm{,}}\,\,{e}_{{\rm{O}}\left({{\text{Fe{-}Ni}}} \right)\,}^{{\rm{Nb}}},{e}_{{\rm{O}}\left({{\text{Fe{-}Ni}}} \right)\,}^{\rm{O}}, \) and \( e_{\text{Nb(Fe - Ni)}}^{\text{O}} \) are the interaction coefficients.
The [pctO] term in the right side of Eq. [6a] because of its smallness can be written using the ratio \( \left( {K_{(1)} /[{\text{pctNb}}]} \right)^{1/2} \) if is assumed in Eq. [1a] that f Nb ≈ 1 and f O ≈ 1. Such a substitution does not give a substantial error.[3] Then Eq. [6a] becomes
or, in general terms,
Experimental
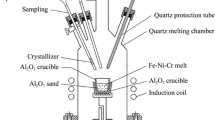
The experiments were carried out in an induction furnace fed by a 400-kHz high-frequency generator with a power of 10 KVA. The scheme of experimental apparatus is shown in Figure 1. The charge contained 99.99 pct-pure carbonyl iron (OOO “Sintez-PKZh”, Russia), 99.99 pct-pure electrolytic nickel (OAO “GMK “Noril’skiy Nikel”, Russia), and 99.9 pct-pure aluminothermal niobium (ZAO “SpetsMetalMaster”, Russia). The charge mass was 100 g. This specimen with the composition corresponding to that of alloy under investigation was placed into an Al2O3 crucible; in turn, this crucible was put into another outer protective alumina crucible. Then, the charge was placed into the furnace and was melted in the Ar-H2 atmosphere.
Hydrogen and argon were preliminarily purified from oxygen, water vapor, sulfides, organic compounds, and mechanical and other impurities. The consumptions of argon and hydrogen were 150 and 50 mL/min, respectively. When the specimen was completely melted, the hydrogen supply was stopped and metal was held under the argon atmosphere (150 mL/min) at 1823 K (1550 °C). Niobium was added into the melt without breaking the seal of furnace and, then, the melt was held at the given temperature under the argon atmosphere until the equilibrium was achieved. The temperature was measured by the Pt-6 pctRh/Pt-30 pctRh thermocouple.
In the course of preliminary experiments by sampling (every 5 minutes) and analyzing the niobium and oxygen concentrations, it was found that the system achieves the equilibrium in 18 to 20 minutes after niobium was added.[6] In these experiments, to be sure that the equilibrium is achieved, the holding time was about 30 minutes. Then, the melt was sampled and chemically analyzed. Samples were taken by a quartz tube with an internal diameter of 6 mm, which was equipped with copper crystallizer. Since metal was melted in an induction field, its surface was dome-shaped. For this reason, NbO2 film was arranged along the perimeter of crucible wall, i.e., the central part of melt surface was film-free. Samples were taken through this film-free surface. 70-mm-long specimens had a diameter of 6 mm.
The oxygen concentration in the melt was determined with an accuracy of ±5 × 10−5 mass pct using a Leco TC-600 Gas Analyzer. Niobium and nickel were analyzed with an accuracy of ±0.001 mass pct using an Ultima 2 Horiba Jobin-Yvon ICP optical emission spectrometer.
Results and Discussion
The results obtained are given in Table I and Figure 2. The dashed line (Figure 2) shows the oxygen solubility in the Fe-40 pct Ni at 1823 K (1550 °C) [O](Fe-40 pctNi) = 0.144 pct, which was calculated by the method published earlier.[7]
The experimental data were processed according to Eq. [7] by the methods of regression analysis using a Quattro Pro program. The following coefficients for this equation were obtained (determination coefficient R 2 = 0.91);
In Eq. [7] \( A = 1/2\log K_{(1)} ;\;B = {-}1/2\left[ {e_{{{\text{Nb}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{Nb}} + 2e_{{{\text{O}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{Nb}} } \right] \); \( C = {-}1/2\left[ {2e_{{{\text{O}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{O}} + e_{{{\text{Nb}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{O}} } \right]\left( {K_{(1)} } \right)^{1/2} \). Based on the experimental data, this allows the calculation of the interaction coefficients and the equilibrium constant for Eq. [1].
Since the Fe-Ni melts are characterized by a slight deviation from ideality,[8] in the first approximation, it can assumed for the calculation of the \( \varepsilon_{{i({\text{Fe - Ni}})}}^{j} \) and \( e_{{i({\text{Fe - Ni}})}}^{j} \) interaction coefficients that[9]
With the knowledge of the values \( e_{\text{O(Fe)}}^{\text{O}} \) = −0.2[1] and \( e_{\text{O(Ni)}}^{\text{O}} \) = 0[2] at 1823 K (1550 °C) (Table II), and using Eq. [9], \( e_{{{\text{O}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{O}} \) = −0.1248. Taking into account the values of the coefficients in Eq. [8], and the \( e_{{{\text{O}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{O}} \) value, it was determined for the Fe-40 pct Ni alloy at 1823 K (1550 °C) that \( e_{{{\text{Nb}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{O}} \) = −0.630; \( e_{{{\text{O}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{Nb}} \) = −0.105; \( e_{{{\text{Nb}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{Nb}} \) = 0.010; \( \log K_{{(1)({\text{Fe - }}40{\text{pctNi}})}} \) = −4.619; \( K_{{(1)({\text{Fe - }}40{\text{pctNi}})}} \) = 2.404 × 10−5; \( \Delta G_{{(1)({\text{Fe - }}40{\text{pctNi}})}}^{ \circ } = 161,210\,{\text{J}}/{\text{mol}} \).
Based on the data obtained, the interaction coefficients were calculated for different compositions of Fe-Ni alloys (Table II). The \( \log {\text{K}}_{(1)}^{\exp } \) values for each experiments (Table I) were calculated according to Eq. [1a] using the estimated interaction coefficients.
According to Eq. [5], for the Fe-40 pct Ni alloy
At 1823 K (1550 °C) \( \Delta G_{{(5) ( {\text{Fe - 40\% Ni)}}}}^{ \circ } = - 2 2 3, 2 6 1\,{\text{J}}/{\text{mol}} \). The molecular masses for the Fe-Ni melts were estimated according to the equation
and the activity coefficient \( \gamma_{{i({\text{Fe - Ni}})}}^{ \circ } \) was determined using the formula[10]
where ε are the molar interaction coefficients.
To calculate the \( \gamma_{{{\text{O}}({\text{Fe - Ni}})}}^{ \circ } \) activity coefficient, \( \gamma_{{{\text{O}}({\text{Fe}})}}^{ \circ } \) = 0.0084,[1] \( \gamma_{{{\text{O}}({\text{Ni}})}}^{ \circ } \) = 0.297[2] (Table II), \( \varepsilon_{{{\text{O}}({\text{Fe}})}}^{\text{Ni}} = 0.538 \),[11] and \( \varepsilon_{{{\text{O}}({\text{Ni}})}}^{\text{Fe}} = - 5.667 \)[11] were used. The obtained results are given in Table II.
After the calculation of \( \Delta G_{{(2)({\text{Fe - }}40{\text{pctNi}})}}^{ \circ } \) = 481,349 J/mol and \( \Delta G_{{(3)({\text{Fe - }}40{\text{pctNi}})}}^{ \circ } \) = 9,056 J/mol for 1823 K (1550 °C), with the knowledge of \( \Delta G_{{(1)({\text{Fe - }}40{\text{pctNi}})}}^{ \circ } \) and \( \Delta G_{{(5)({\text{Fe - }}40{\text{pctNi}})}}^{ \circ } \), one can determine the Gibbs energy for Eq. [4], \( \Delta G_{{(4)({\text{Fe - }}40{\text{pctNi}})}}^{ \circ } \) = −105,934 J/mol. This allows the calculation of \( \gamma_{{{\text{Nb}}({\text{Fe - }}40{\text{pctNi}})}}^{ \circ } \) according to the equation
At 1823 K (1550 °C) \( \gamma_{{{\text{Nb}}({\text{Fe - }}40{\text{pctNi}})}}^{ \circ } \) = 0.150. Using \( \gamma_{{{\text{Nb}}({\text{Fe}})}}^{ \circ } \) = 0.171[3] (Table II) and \( \gamma_{{{\text{Nb}}({\text{Fe - }}40{\text{pctNi}})}}^{ \circ } \), the activity coefficient for the Fe-Ni alloys was estimated according to Eq. [10] (Table II). In these calculations, the interaction coefficients \( \varepsilon_{{{\text{Nb}}({\text{Fe}})}}^{\text{Ni}} \) = −3.91[12] and \( \varepsilon_{{{\text{Nb}}({\text{Ni}})}}^{\text{Fe}} \) = 4.29[13] were used.
Based on the results obtained, the Gibbs energies for Reaction [1] and the equilibrium constants of this reaction (\( \Delta G_{(1)}^{ \circ } \), \( K_{(1)} \)) were determined for the Fe-Ni alloys (Table II). The equilibrium constants of Reaction [1] as a function of the nickel content are given in Figure 3. As is seen, the value of the equilibrium constant decreases considerably as the nickel concentration in melt rises. This may be attributed to a decrease in the bond strength between metal and oxygen weakens as the nickel concentration in melt increases (\( \gamma_{{{\text{O}}({\text{Fe}})}}^{ \circ } \) = 0.0084; \( \gamma_{{{\text{O}}({\text{Ni}})}}^{ \circ } \) = 0.297).
Taking into account the equilibrium constants for Reaction [1] and the interaction coefficients (Table II), as applied to the alloys with different compositions at 1823 K (1550 °C), the oxygen solubilities in the melts can be described by the following equations:
The dependence of the equilibrium oxygen content on the nickel and niobium concentrations in the Fe-Ni melts, which was calculated by Eqs. [11a] through [11f] is shown in Table III and Figure 4. It follows from the data obtained, the affinity of niobium to oxygen increases considerably with the nickel concentration. The curves of oxygen solubility in the iron-nickel melts pass through minimum and its position shifts to the higher niobium contents with an increase in the nickel content. As for pure nickel, no minimum was observed in the range of niobium contents under consideration.
The niobium concentration corresponding to the minimum oxygen concentrations can be estimated by the following equation[14]:
where m and n are the stoichiometric coefficients in R m O n oxide. For NbO2, this equation takes the form
The niobium concentrations calculated by Eq. [12a] for the points of minimum and the corresponding oxygen contents are given below:
Ni (pct) | 0 | 20 | 40 | 60 | 80 |
\( [{\text{pctNb]}}^{{\prime }} \) | 1.279 | 1.602 | 2.174 | 3.459 | 9.000 |
[pctO]min | 0.0148 | 0.0121 | 0.0055 | 0.0016 | 3.4 × 10−4 |
Conclusions
-
1.
The oxygen solubility in the niobium-containing Fe-Ni melts was experimentally measured in the Fe-40 pct Ni alloy at 1823 K (1550 °C). It was shown that the presence of niobium decreases the oxygen solubility in this melt. The equilibrium constant of interaction of niobium with oxygen dissolved in the Fe-40 pct Ni (\( \log K_{{(1)({\text{Fe - }}40{\text{pctNi}})}} \) = −4.619), the Gibbs energy of this reaction (\( \Delta G_{{(1)({\text{Fe - }}40{\text{pctNi}})}}^{ \circ } = 1 6 1, 2 10\,{\text{J}}/{\text{mol}} \)), and the interaction coefficients characterizing these solutions (\( e_{{{\text{Nb}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{O}} = - 0.630 \); \( e_{{{\text{O}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{Nb}} = - 0.105 \); \( e_{{{\text{Nb}}({\text{Fe - }}40{\text{pctNi}})}}^{\text{Nb}} \) = 0.010) were determined.
-
2.
The Gibbs energy of interaction of niobium and oxygen dissolved in the Fe-Ni melts, the equilibrium constants of this reaction, and the interaction coefficients for these solutions were calculated for a wide range of compositions at 1823 K (1550 °C). For this temperature, the oxygen solubility in the niobium-containing Fe-Ni melts was also determined.
-
3.
With an increase in the nickel concentration in these melts, the niobium affinity to oxygen rises appreciably. This appears to be associated with a decrease in the bond strength between metal and oxygen in the melt as the nickel concentration increases (\( \gamma_{{{\text{O}}({\text{Fe}})}}^{ \circ } \) = 0.0084; \( \gamma_{{{\text{O}}({\text{Ni}})}}^{ \circ } \) = 0.297).
References
K. Narita and T. Onoye: Steelmaking Data Sourcebook, Gordon & Breach Science, New York, 1988, p. 137.
G.K. Sigworth, J.F. Elliott, G. Vaughn, and G.H. Geiger: Met.Soc.CIM, Ann. V (1977), 104.
I.S. Kulikov: Raskislenie Metallov (Deoxidation of Metals). Metallurgiya, Moscow, (1975), 344.
E.T. Turkdogan: Physical Chemistry of High Temperature Technology. Academic Press, New York, (1980), 344.
I.S. Kulikov: Termodinamika Oksidov (Thermodynamics of Oxides). Metallurgiya, Moscow, (1975), 504.
V. Dashevskii, A.A. Aleksandrov, and L.I. Leontev: Dokl. Akad. Nauk, 452 (2013), 2, 172.
V. Dashevskii, N.N. Makarova, K.V. Grigorovich, and V.I. Kashin: Dokl. Akad. Nauk, 357 (1997), 6, 789.
R. Hultgren, P.D. Desai, D.T. Hawkins, M. Gleiser, and K.K. Kelley: Selected Values of the Thermodynamic Properties of Binary Alloys. American Society of Metals, Metals Park, OH, 1973, 1435.
V.Ya. Dashevskii: Fizikokhemicheskie osnovy raskisleniya zhelezonikelevykh splavov (Physicochemical Basis of Deoxidation of Iron-Nickel Melts), Fizmatlit, Moscow, (2011), 152.
M.G. Frohberg, M. Wang: Z. Metallkd, 81 (1990), 7, 513.
T. Chiang, Y.A. Chang: Metall. Trans., 7B (1976), 453.
Yu.P. Snitko, Yu.N. Surovoy, and N.P. Lyakishev: Dokl. Akad. Nauk, 286 (1983), 5, 1154.
L.N. Belyanchikov: Elektrometallurgiya, (2009), 2, 29.
V.Ya. Dashevskii, A.A. Aleksandrov, and L.I. Leont’ev: Izv. Vysch. Uchebn. Zaved., Chern. Metall., (2014), 5, 33.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted July 10, 2014.
Rights and permissions
About this article
Cite this article
Dashevskii, V., Aleksandrov, A., Kanevskii, A. et al. Deoxidation Equilibrium of Niobium in the Iron-Nickel Melts. Metall Mater Trans B 46, 220–225 (2015). https://doi.org/10.1007/s11663-014-0214-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0214-9