Abstract
The current study aims to investigate the synthesis and formation mechanism of the carbonate-free strontium titanium oxide nanosized crystals. Monosized strontium titanium oxide nanocrystals with tailored morphology have been successfully synthesized using a novel facile synthesis pathway. The synthesis method is based on an ultrasound-assisted wet chemical processing method. Nanocrystals are characterized and observed by X-ray diffraction, field-emission scanning electron microscopy, and high-resolution transmission electron microscopy techniques. It was found that the ultrasonication accelerates the formation of stoichiometric strontium titanium oxide nanocrystals. Furthermore, the results show that very fine nanocrystals with an average size of about 4.8 nm and a narrow size distribution are obtained when ultrasonication is applied to the reaction mixture. As the most important outcome of this research, carbonate-free strontium titanium oxide nanocrystals are synthesized at a very low temperature of 323 K (50 °C). This temperature is much lower than the temperature required for synthesis of strontium titanium oxide nanocrystals in the similar works. This advantage has been reached, thanks to the applied modifications leading to complete removal of carbon from reaction mixture during synthesis. Moreover, formation mechanism of the nanocrystals synthesized in this work is disclosed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The nanosized crystals frequently exhibit novel electronic, magnetic, optical, chemical, and mechanical properties that cannot be obtained in their bulk forms.[1–3]The development of nanocrystals has been intensively followed, not only for their fundamental scientific interest, but also for many technological applications and device miniaturization.[4,5] The properties of the nanocrystals strongly depend on their composition, structure, morphology and finite size, which in turn, depend on their synthesis method and preparation conditions. Therefore, controllable preparation, ability to reduce the size of the nanocrystals, and control their shape are significant for fundamental research and industrial production.[3] On the other hand, the economic consideration of mass production of nanocrystals is a major issue concerning the research and development.[1]
In recent years, a growing interest can be observed in ceramics based on the mixed oxides,[1–11] mainly for the electronic and optical industries.[7,8] Strontium titanium oxide (strontium titanate; SrTiO3) is a well-known perovskite oxide, which has attracted a great deal of attention due to its excellent dielectric, optical, and catalytic properties.[12–17] It has many potential applications for various microelectronic heterostructures, oxygen sensors, solar cells, multilayer capacitors, dynamic random access memories (DRAM), and thermoelectric devices.[12–17] Therefore, great effort has been devoted to the synthesis of strontium titanium oxide and there are a large number of researches regarding its synthesis in the literature.[12–19] Two major approaches, traditional and advanced, are generally considered for synthesizing strontium titanium oxide powders. Solid phase reaction method is one of the most practical methods in synthesizing strontium titanium oxide powders.[4] In solid phase reaction method, a single-phase strontium titanium oxide is obtained only after calcination of the powder mixture at high temperature 1373 K to 1573 K (1100 °C to 1300 °C). The coarse-grained powder synthesized by this method contains agglomerated particles of different sizes with impure phases due to incomplete reactions.[4,10] These drawbacks always lead to poor performances as well as poor properties, optimization, and reproducibility. Recently, many studies have been performed on advanced synthesis methods such as solvothermal,[12] combustion[13] hydrothermal,[14] polymeric precursor,[15] molten salt,[16] sol–gel,[17] and sonochemical[18–20] methods. A high temperature in the range of 973 K to 1273 K (700 °C to 1000 °C) is required to obtain strontium titanium oxide powders through the above-mentioned methods.[12–17] In some of them,[15,17,19,20] researchers could not obtain pure strontium titanium oxide nanoparticles, and existence of strontium carbonate by-product in the synthesized powders is the main drawback of the mentioned methods. It still remains a challenge for the scientific community to obtain high-quality, homogeneous, stoichiometric strontium titanium oxide nanosized crystals at a low temperature while avoiding unwanted by-products. Therefore, it is desirable to develop a facile, cheap, and low temperature pathway for synthesizing the carbonate-free, stoichiometric, homogeneous strontium titanium oxide nanosized crystals, which can meet the basic requirements for most of the above-mentioned applications. In this work, much effort has been devoted to design and develop a novel synthesis pathway for obtaining carbonate-free strontium titanium oxide nanosized crystals with tailored morphology. We believe that the method developed in this work provides a facile and cost-effective pathway for synthesizing strontium titanium oxide for assembling in nanotechnology.
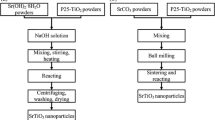
Recently, with development and application of the microelectronics, well-crystallized strontium titanium oxide nanosized crystals with uniform particle size are required. On the other hand, synthesis temperature and purity of strontium titanium oxide are key challenges in the synthesis of this material. Sonochemical synthesis is a facile one-step approach operated at low temperature.[18] There are a few researches studying the synthesis of strontium titanium oxide nanoparticles through sonochemical processing method.[19,20] In those, researchers could not prepare pure strontium titanium oxide nanoparticles, and existence of strontium carbonate by-products in the synthesized powders is the greatest drawback of those methods. The present work aims to develop an innovative low temperature synthesis method for obtaining the carbonate-free strontium titanium oxide nanocrystals. Our new approach is based on an ultrasound-assisted wet chemical processing method. We believe that the method developed in this work provides a simple, fast, and cost-effective route in contrast to other established methods and is suitable for mass production purposes. The flowchart of the new synthesis method is shown in Figure 1. Titanium chloride (>99 pct), strontium chloride (>99 pct), anhydrous sodium hydroxide (>99 pct), and ethanol (99.8 pct) are obtained from Merck. As can be seen in Figure 1, the stoichiometric amounts of strontium chloride and titanium chloride are dissolved in deionized water and ethanol, respectively. These solutions are added into a glass vessel containing sodium hydroxide solution. The pH of the precursor solution is kept at 14, during reaction. The ultrasound irradiation process is carried out using an ultrasonic bath (Soner 220H, 53 kHz, 500 W). The glass vessel containing precursor solution is put into the ultrasonic bath at 323 K (50 °C) for 45 minutes. The sonication was conducted without cooling so that the temperature of the solution increased gradually during sonication. After the reaction is finished, the precipitates are separated, washed, and then dried in an oven at 373 K (100 °C) for 2 hours.
Sample characterizations are carried out using a variety of experimental techniques. Phase identification, crystal structure, and average crystallite size of the nanopowders are characterized using a X-ray diffractometer (Philips; PW3710, 40 kV, 30 mA) with Cu Kα radiation and scanning speed of 5 deg/min over a range of 25 to 70 deg at the room temperature. The average crystallite size of the products is calculated using Scherrer’s formula.[10] FT-IR is known as a sensitive technique for carbonate phase identification. Functional groups in the products are detected using a FT-IR spectrophotometer (Hitachi 3140, Tokyo, Japan). FT-IR spectrum is recorded in the range of 400 to 3600 cm−1 and measured on samples in KBr pellets. For pellet preparation, our synthesized powder and KBr powder are blended together to make the sample, and using water and pressing, the pellet was prepared for the spectroscopy evaluation. The morphological features and microstructure of the products are observed using a field-emission scanning electron microscopy (Hitachi S4160) and a high-resolution transmission electron microscope (ZEISS LIBRA200) working at 150 kV.
According to hot spot theory,[18] in the sonochemical processing method, very high temperatures [>5000 K (4727 °C)] and pressures of roughly 1000 atm are obtained upon the collapse of a bubble. These extreme conditions can drive a variety of chemical reactions to fabricate nanoscale materials. Nevertheless, such high temperatures and pressures can activate existing carbon atoms, carbon species, and dissolved CO2 gas (in the precursor solution) for chemical reactions to form carbonate by-products (carbonate phases). This event is the reason behind the fact that other researchers[19,20] were not successful in synthesizing carbonate-free perovskite materials. Therefore, in order to synthesize carbonate-free strontium titanium oxide nanocrystals, the above-described method (explained in the experimental section) must be modified. To reach this aim, the next steps are followed: (1) removal of the carbon species and dissolved CO2 gas from the deionized water, (2) preventing the formation of carbonates during sonication (reaction), and (3) dissolution of (possible) formed carbonate by-products from the synthesized nanopowders. Details of these steps are shown in Figure 1.
Figure 2 shows XRD pattern of the as-prepared strontium titanium oxide nanocrystals obtained in this work. The XRD pattern reveals that the sample is well crystalline and all the main diffraction peaks correspond to the peaks of strontium titanium oxide (JCPDS Table no. 35-0734). Moreover, X-ray diffraction pattern presented in Figure 2(a) proves that our method leads to obtainment of carbonate-free strontium titanium oxide nanocrystals with perovskite symmetry. The contaminations (carbonate by-products) have been eliminated using a sealed system (closed container shown in Figure 1). The synthesized strontium titanium oxide nanostructures are characterized by well-resolved peaks shown and labeled in Figure 2(a). The highest intensity peak (which is found at 2θ = 32.37 deg) corresponds to (1 1 0) plane of strontium titanium oxide perovskite structure, confirming that the particles are preferably orientated along (1 1 0) plane. The XRD characteristics of the synthesized nanopowders are consistent with those of other reports in the literature.[12–17] As shown in Figure 2(b), peak splitting has occurred at approximately 2θ = 57.44 deg, which is an indication of the tetragonal phase. Considering the prominent peak and using the Scherrer formula given in Reference 10 we estimate the average crystallite size of the synthesized powders. The crystallite size of the powders is found to be 4.8 nm. These results indicate that very fine strontium titanium oxide nanocrystals are synthesized by our established method. There are several methods to synthesize the strontium titanium oxide powders,[12–20] but no method could produce carbonate-free strontium titanium oxide particles with tailored morphology as ours.
Chemical bonds vary widely in their sensitivity to probing by infrared techniques. Thus, the potential utility of FT-IR spectrophotometry is a function of the chemical bond of interest, rather than being applicable. Peak position is most commonly exploited for qualitative identification because each chemical functional group displays peaks at a unique set of characteristic frequencies. This provides a fingerprint that can be used to identify chemical groups as a generic probe.[10] Moreover, FT-IR is known as a sensitive technique for carbonate phase identification. This characterization method is used here in order to prove that the powders synthesized in this work are carbonate-free. Figure 3 shows the FT-IR spectrum of the synthesized nanopowders. The FT-IR spectrum shows the existence of absorption bands at around 557, 1004, 1354, 1593, and 3419 cm−1. The absorption bands at 3419 and 1593 cm−1 are assigned to O-H stretching and bending vibrations of water, respectively.[21] The absorption bands at 1004 and 1354 cm−1 can be considered as the bending vibrations of the alcohol group (C-OH functional groups)[21] implying the adsorption of small amounts of alcohol on the surface of nanostructures. These absorptions are seen in the spectrum due to the use of ethanol in the final stage of washing the as-synthesized nanocrystals. Strong and wide band located between 800 and 500 cm−1 can be ascribed to stretching vibrations of strontium titanium oxide and confirms the formation of strontium titanium oxide. This band is a compilation of two absorption bands including a band at 745 cm−1 which is assigned to stretching vibrations of SrO and a band at 557 cm−1 that corresponds to stretching vibrations of TiO2.[13] Moreover, since the characteristic bands of the carbonates (which is usually seen at 867, 1067, 1440 cm−1)[22] have not been detected in the FT-IR spectrum, it can be stated that the synthesized nanopowders are carbonate-free. This result is in good agreement with the XRD results.
FE-SEM micrographs showing the morphology and particle size of the synthesized strontium titanium oxide nanopowders are shown in Figure 4. The synthesized product shows a more monosized spherical shape than the other preparation methods. The powders appear to be agglomerated caused primarily by the processes that occur during the drying stage of the as-synthesized nanopowders. Moreover, small particles embedded in each agglomerated cluster correspond to the strontium titanium oxide nanocrystals. The synthesized strontium titanium oxide shows spherical morphology with the size in the range of 6 to 15 nm. All the SEM micrographs exhibit particles greater than the average crystallite size determined by the analysis of XRD, suggesting an internal structure of the particles. The morphological properties and size distribution characterization of the prepared powders indicate that the products consist of somewhat regularly shaped and relatively spherical particles with a narrow size distribution. The above results indicated that with the help of ultrasound irradiation, carbonate-free strontium titanium oxide particles with tailored morphology can be synthesized using our established method. Moreover, the utilization of ultrasonication accelerates the homogeneous precipitation process and can also be beneficial in controlling the size and shape of the synthesized nanocrystals.
The possible formation mechanism of the synthesized nanocrystals is related to radicals involved in the reaction because of the bubble’s collapse. Application of ultrasound to reaction mixture involves the use of acoustic cavitation. This cavitation involves the nucleation, growth, and collapse of the gas of vapor-filled microbubbles formed from acoustical wave-induced compression/rarefaction in a body of the reaction mixture. The implosion of the microscopic bubbles in the reaction mixture generates energy, which induces chemical and mechanical effects.[23] This collapse leads to localization, a transient high temperature and pressures, resulting in an oxidative environment due to the generation of highly reactive species, including hydroxyl radical (·OH).[24,25] These radicals are formed in the reaction mixture due to the use of deionized water and alcohol as precursors. On the other hand, this oxidative environment provides suitable conditions for the formation of the oxide nanocrystal. This mechanism, which will be discussed in more detail later, governs mostly sonochemical reactions conducted in aqueous solutions. The main advantage of the present method is its capability to synthesize very fine strontium titanium oxide nanocrystals, which are free from any carbonate by-product at a very low temperature of 323 K (50 °C). The reaction pattern for the formation of the strontium titanium oxide nanocrystals is as follows:
where (aq) denotes a salt dissolved in deionized water and (s) denotes the synthesized precipitates. SrTiO3 is the main product of the above reaction pattern exhibiting the perovskite symmetry. Our approach has several advantages over other reported methods.[12–20] In contrast to other sonochemical processes,[19,20] in this work, for the first time, carbonate-free strontium titanium oxide nanocrystals are prepared. Moreover, it is interesting that the strontium titanium oxide nanocrystals are synthesized at a lower temperature. Other researchers[20,22] have synthesized strontium titanium oxide nanocrystals by ultrasonication at a temperature above 363 K (90 °C) while their products contain some carbonate by-products. In this work, carbonate-free strontium titanium oxide nanocrystals are synthesized at 323 K (50 °C). This temperature is also much lower than the temperature required for other processing methods.[12–17] It seems that the removal of the carbon species and dissolved CO2 gas from deionized water and other applied modifications lead to formation of the carbonate-free strontium titanium oxide nanocrystals at lower temperature in contrast to the previous reports.[12–17,19,20,22] In analyzing these results, it can be said that the strontium carbonate is thermodynamically more stable than strontium titanium oxide; therefore, in the presence of free carbon, carbon species and dissolved CO2 gas, formation of the strontium carbonate is preferred, especially in the ultrasound-assisted synthesis due to its critical conditions. During sonication, ultrasonic waves radiate through the precursor solution. This causes alternating high and low pressure in the solution and also leads to the formation, growth, and implosive collapse of bubbles in the reaction mixture. The collapse of bubbles with short lifetime produces intense local heating and high reaction mixture. According to the hot spot theory,[18] very high temperatures [>5000 K (4727 °C)] and pressures of roughly 1000 atm are obtained upon the collapse of a bubble. These conditions greatly accelerate the rate of the Reaction [1] without need for any additional external heating. Nevertheless, such high temperatures and pressures can also activate existing carbon atoms, carbon species, and dissolved CO2 gas to form carbonate by-products. This fact is the reason that other researchers were not successful in synthesizing carbonate-free strontium titanium oxide nanocrystals by the sonochemical processing method.[19,20] In the present work, in order to prevent the unwanted reactions, we have tried to remove the carbon species and dissolved CO2 gas from the precursor solution before ultrasound irradiation. We have also tried to prevent the formation of the carbonate phases during sonication by use of a closed container (see the Figure 1). Moreover, it can be discussed that the formation of strontium carbonate or other carbonate by-products postpones the formation of strontium titanium oxide. It is known that strontium titanium oxide is formed after the decomposition of strontium carbonate. Therefore, with an increase in the amount of carbonate phases in the reaction mixture, their decomposition needs more thermal energy and longer time. As a result, the synthesis of strontium titanium oxide is completed at a higher temperature and after a longer synthesis time. On the other hand, since the above-mentioned collapses (of the bubbles) occur in less than a nanosecond, very high cooling rates (>1010 K/s) are also obtained due to self-quenching effect of the reaction mixture.[26] Such high cooling rate prevents the growth of the forming nanocrystals; therefore, very fine spherical or close-to-spherical strontium titanium oxide nanocrystals are synthesized. Figure 5 shows the TEM micrographs at different magnifications and SAED pattern of the strontium titanium oxide nanopowders synthesized in this work. These micrographs show strontium titanium oxide nanocrystals and nanometric agglomerates, and prove the nanocrystalline structure of the synthesized strontium titanium oxide. Moreover, the crystallite size of particles is in good agreement with the data obtained from the Scherrer equation. The selected-area electron diffraction (SAED) pattern exhibits hazy circles at the center, which indicates the powders are nanocrystalline. This pattern also shows the presence of concentric rings that confirms the polycrystallinity of the agglomerates. The sonochemical processing method used in this work leads to the homogeneous precipitation of non-agglomerated strontium titanium oxide nanocrystals at the end of the synthesis process. Then the precipitates are separated, washed, and then dried in an oven at 373 K (100 °C) for 2 hours. As can be seen in Figure 6(a), the dried nanopowders are seen as agglomerates composed by strontium titanium oxide nanocrystals. However, they are loose agglomerates and can be dispersed in aqueous medium without any need for grinding them. Moreover, the better clear HRTEM micrographs presented in Figure 6(b) clearly shows the contacted areas of the synthesized nanocrystals indicating the nanocrystals have been agglomerated together with weak forces during drying stage of the as-synthesized precipitates.
Monosized barium strontium titanate nanocrystals also have been successfully synthesized by the sonochemical synthesis pathway developed in this work (see Figures 7 and 8). So our established method is sure to be extended to synthesize other perovskite-type oxides with the general formula ABO3, which is simple, fast, convenient, and efficient.
The work aimed at investigating the synthesis and formation mechanism of the carbonate-free strontium titanium oxide nanosized crystals. The results indicated that with the help of the ultrasound irradiation, carbonate-free strontium titanium oxide nanocrystals with tailored morphology can be synthesized using our developed method. Moreover, the utilization of ultrasonication accelerated the homogeneous precipitation process and could also be beneficial in controlling the size and shape of the synthesized nanosized crystals. The methodology developed in this work provides a simple, fast, and cost-effective pathway for synthesizing the carbonate-free strontium titanium oxide for assembling in nanotechnology. These advantages have been reached, thanks to the applied modifications leading to complete removal of carbon from reaction mixture during synthesis.
References
R. Ashiri, A. Nemati, M. Sasani Ghamsari, S. Sanjabi, and M. Aalipour: Mater. Res. Bull., 2011, vol. 46, pp. 2291–95.
F. Davar, M.R. Loghman-Estarki, and R.Ashiri: J. Ind. Eng. Chem., DOI: 10.16/j.jiec.2014.05.002.
[3] R. Ashiri, A. Moghtada, A. Shahrouzianfar and R. Ajami: J. Am. Ceram. Soc., 2014, vol. 97, pp. 2027-31.
[4] T.X. Wang and W.W. Chen: Mater. Lett., 2008, vol. 62, pp. 2865-7.
[5] F. Davar, M.R. Loghman-Estarki, M. Salavati -Niasari and R. Ashiri: Int. J. Appl. Ceram. Technol., 2014, vol. 11, pp. 637-44.
R. Ashiri, A. Nemati and M. Sasani Ghamsari: Ceram. Int., 2014, vol. 40, pp. 8613-9.
R. Ashiri, A. Nemati, M. Sasani Ghamsari, and H. Adelkhani: J. Non-Cryst. Sol., 2009, vol. 355, pp. 2480–84.
[8] R. Ashiri: Metall. Mater. Trans., 2014, vol. 45B, pp. 1472-83.
[9] R. Ashiri: Metall Mat Trans., 2014, vol. 45A, pp. 4138-54.
[10] R. Ashiri: Metall Mat Trans, 2012, vol. 43A, pp. 4414-26.
R. Ashiri, A. Nemati, M. Sasani Ghamsari, and M.M. Dastgahi: J. Mater. Sci.: Mater. Electron., DOI: 10.1007/s10854-014-2312-5.
[12] J.Q. Zheng, Y.J. Zhu, J.S. Xu and B.Q. Lu: Mater. Lett., 2013, vol. 100, pp. 62-5.
[13] C.N. George, J.K. Thomas and R. Jose: J. Alloy. Compd., 2009, vol. 48, pp. 711-5.
[14] Sh. Zhang and J. Liu: Mater. Sci. Eng., 2004, vol. 110B, pp. 11-7.
[15] L.F.D. Silva and L.J.Q. Maia: Mater. Chem. Phys., 2011, vol. 125, pp. 168-73.
[16] H.L. Li and Z. N. Du: Mater. Lett., 2010, vol. 64, pp. 431-4.
[17] H. Cui, M. Zayat and D. Levy: J. Non-Cryst. Solid., 2007, vol. 353, pp. 1011-6.
[18] T.J. Mason and J.P. Lolimer: Applied Sonochemistry. Wiley-VCH Verlag, U.K., 2002.
[19] M. Xu and Y.N. Lu: J. Am. Ceram. Soc., 2006, vol. 89, pp. 3631-4.
[20] M. Xu and Y.N. Lu: Powder. Technol., 2006, vol. 161, pp. 185-9.
[21] R. Ashiri: Vib. Spec., 2013, vol. 66, pp. 24-9.
[22] E. Bacha and Ph. Deniard: Thin. Solid. Films., 2011, vol. 519, pp. 5816-9.
[23] Y.G. Adewuyi: Ind. Eng. Chem. Res., 2001, vol. 40, pp. 4681-715.
[24] K.S. Suslick: Science, 1990, vol. 247, pp. 1439-45.
[25] E.B. Flint and K.S. Suslick: Science, 1991, vol. 253, pp. 1397-9.
R. Ashiri, R. Ajami, and A. Moghtada: Int. J. Appl. Ceram. Technol., DOI: 10.1111/ijac.12315.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 30, 2014.
Rights and permissions
About this article
Cite this article
Ashiri, R., Moghtada, A. Carbonate-Free Strontium Titanium Oxide Nanosized Crystals with Tailored Morphology: Facile Synthesis, Characterization, and Formation Mechanism. Metall Mater Trans B 45, 1979–1986 (2014). https://doi.org/10.1007/s11663-014-0213-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0213-x