Abstract
This study investigates the microstructure across the interconnection zone of IN718/Al/IN718 couples obtained by the Transient Liquid Phase Bonding (TLPB) process at temperatures ranging from 800 °C to 1000 °C. The crystal structure and the chemical composition of the phases formed, including the AlNi intermetallic, were evaluated using TEM-EDS. The evolution of the AlNi layer as a function of bonding time was studied by in situ high energy synchrotron x-ray diffraction. The AlNi lattice parameter increases up to 2.9401 Å and subsequently decreases with the annealing time due to changes in the chemical composition of AlNi. This behavior is related to the formation of two distinct layers of AlNi: Al-rich AlNi and Ni-rich AlNi. The split of the AlNi phase indicates that a chemical partition takes place when Ni and Al atomic concentrations are approximately equal. The growth kinetics of both layers are controlled by diffusion with different growth rate constants. These results contribute to the understanding of the solid-state transformations occurring in a multicomponent and multilayered TLP bond under isothermal conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The operating conditions of some critical metal parts involve prolonged exposition to temperatures up to 0.8Tm exposed to oxidizing environments and from moderate to high stresses.[1] Their efficiency is related to the aptitude of the structural materials to withstand harsh conditions. Inconel 718 Ni-based superalloy, named IN718, is widely used in the aerospace industry.[2,3] It is a precipitation-hardenable alloy with a microstructure consisting of an fcc matrix (γ) and two types of precipitates, γ′(Ni3Al) and γ″(Ni3Nb), that result from ageing heat treatments. This microstructure is strong and stable at high temperatures, making IN718 suitable for applications where creep resistance, fracture toughness, ductility and strength are required.[1] The production of parts made of such complex materials brings along some challenges concerning its processing. Shaping, joining [4,5,6] and cutting [7] processes are usually optimized. In addition, thermal barrier coatings are often applied to extend the lifecycle of superalloy components by decreasing the thermal load and increasing oxidation resistance.[8,9,10] Several aluminides are used as thermal barriers.[11] In particular, the AlNi intermetallic phase is attractive since it exhibits a high melting point (1638 °C) and is stable at room temperature. Its higher thermal conductivity compared to Ni-base superalloys, increases its capacity to withstand thermal cycles.[11] Besides, it has low density (5.86 g/cm3) and high oxidation resistance. Another distinctive feature of this phase is its stable wide range of compositions (45 To 65 at. pct Ni). These characteristics make AlNi a potential candidate to be applied as a high-temperature protective coating. Different techniques are used to promote the formation of an AlNi layer over the surface of superalloys.[11] One of the processes suitable to generate the coating is pack cementation [12], where the superalloy is held at high temperature in contact with an Al-containing mixture to promote the formation of the AlNi phase by diffusion. The coating consists of an interdiffusion zone next to the substrate, and an outer layer of AlNi capable of providing an aluminum flux to form a protective layer of α-Al2O3 on the surface.[11]
Over the last decades, alternative joining methods such as Transient Liquid Phase Bonding (TLPB) have been applied to join Ni-based substrates using filler metals of different compositions.[13,14,15,16,17,18] TLPB is an isothermal reaction-diffusion process where phase formation takes place via liquid-solid interactions between the substrate and the melted filler. Subsequent growth and transformation occur by solid-solid interactions.[19,20] Due to the presence of a liquid phase, TLPB shortens the bonding time producing microstructures similar to those obtained by pure diffusion treatments. Depending on the application, different combinations of substrate and filler metals can be selected. Generally, for repairing mechanical components it is desirable to obtain a joint with similar microstructure and properties of the substrate. Using Ni-based filler metals to joint superalloys, results are promising when similar microstructure and properties than the substrate are to be achieved.[5] On the other hand, combining Ni substrates and Al foil as filler metal, a multilayered bonding results from TLPB, where the formation conditions of AlNi are determined by the bonding temperature and time.[21,22,23] Considering these features, TLPB is a promising technique to promote the formation of an AlNi coating over the surface of a Ni-base superalloy.
Experimental studies performed on TLPB binary diffusion couples showed that the AlNi phase splits into a Ni-rich and an Al-rich region when isothermally annealed.[13] Due to the absence of a grain boundary between both layers, the splitting was suggested as a consequence of a spinodal-like decomposition, where a chemical partition takes place.[24] Additionally, it is well known, that the Ni-rich AlNi undergoes a martensitic transformation under certain cooling conditions and specific Ni concentrations.[24,25,26] The presence of a split line indicates that a composition gradient from Al-rich to Ni-rich occurs in AlNi. Therefore, unexpected variations in the coating’s properties can occur. The existing studies about aluminide coatings are mainly focused on their microstructures evolution, phase characterization, mechanical properties and oxidation behavior.[27,28,29,30] Said studies show that AlNi represents the main phase in the diffusion coatings. Therefore, it is necessary to understand the AlNi growth kinetics and its solid-state transformations when the coating grows from different Ni-based substrates exposed to Al-rich media at elevated temperatures.
Aiming at assessing the feasibility of this approach, we study in detail the effects of time and temperature during TLPB annealing on the formation of AlNi on Ni-base substrates. This research aims to analyze the microstructural, crystallographic and kinetic evolution of the AlNi phase obtained across the bonding zone of an IN718/Al/IN718 couple manufactured by TLPB.
2 Experimental Procedure
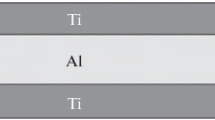
IN718/Al/IN718 bonds were prepared using high purity Al (99.99 at. pct) foils as filler metal (FM) and IN718 as the base material (BM), both polycrystalline. The initial microstructure condition of the IN718 consists of an f.c.c γ matrix with certain amount of δ phase in grain boundary and high amount of γ″, later precipitated after a single ageing stage at 788 °C during 6 hours followed by air cooling. The chemical composition measured by EDS in at. pct is Ni-18.5Cr-17.9Fe-5.3Nb-3.8Mo-1Ti. More details about the microstructure of IN718 can be found elsewhere.[31] Cylindrical slices of 3 and 4 mm thickness were cut from an IN718 rod of 10 mm diameter using a diamond saw. One face of the slices was polished with SiC 600 grit polishing paper to ensure sufficient roughness and promote the wettability to molten Al. Al foils were located between IN718 slices in a sandwich assembly. Joints prepared using the thicker Al foils were preferred as they conducted to thicker connection zones, therefore enabling more accurate identification of the phases.
For the analysis of the phase transformations as a function of time, three different sandwich assemblies using a 250 µm Al foil were isothermally annealed in quartz tubes under Ar atmosphere at 800 °C, 900 °C and 1000 °C, respectively, during different times stages up to 2145 minutes. The bonding thickness evolution was monitored by optical metallographic examination of water quenched samples extracted at intermediate annealing times. The annealing temperatures were selected to ensure the melting of the FM and to speed up the diffusive process. Next, the bonding cross-section view was examined metallographically. The surface containing the interconnection zone was ground and finally polished with a 1 µm diamond paste. The microstructure along the interconnection zone was examined using a Leica DMI 3000M Light Optical Microscope (LOM) and a Philips Scanning Electron Microscope (SEM). The thickness of the layers identified was measured and the average of five measurements per annealing time reported.
In order to identify the crystal structure of the phases present in the interconnection zone, a lamella was extracted from a TLPB couple using a Focused Ion Beam (FIB) attached to a Tescan Lyra 3 XMU SEM. The lamella was analyzed using a FEI Tecnai F20 Transmission Electron Microscope (TEM), equipped with a Schottky field emission gun and an EDAX Apollo EDS unit for chemical analysis with a spatial resolution of 1 nm, operated at 200 kV. Furthermore, selected-area electron diffraction (SAED) patterns were collected from different zone axis from a lamella.
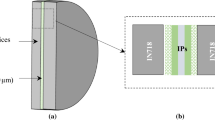
The evolution of the formation of the AlNi phase in the interconnection zone was followed in situ by high-energy synchrotron x-ray diffraction (HEXRD), performed in the Deutsches Elektronen-Synchroton (DESY). Before HEXRD analysis, the sample used was bonded at 700 °C and 240 minutes to ensure the complete transformation of the melted FM and to prevent the presence of liquid phase during the experiment. The in-situ experiment consisted of an annealing treatment at 1000 °C during 120 minutes. The heating rate was 30 °C/s and the cooling rate 43 °C/s from 1000 °C to 347 °C. The couple was placed horizontally in a BÄHR dilatometer under vacuum-controlled atmosphere to avoid surface oxidation. The incident x-ray beam was focused at the sample as shown in Figure 1. This configuration maximizes the beam interaction with the layers, leading to better signal statistics. The set up used was available at the beamline P07-PETRA III.[32] The beam size, energy and wavelength were 1.0x0.1 mm2, 100 kV and 0.124 Å, respectively. The acquisition time was 1 s/frame and the image plate detector was a Perkin Elmer XRD 1621. The sample to detector distance was 1570 mm approximately.
3 Results and Discussion
3.1 Phase Identification
The resulting TLPB joints show multiple layers formed symmetrically with respect to the centerline of the interconnection zone. Figure 2(a) shows the micrograph of the TLP bond formed at 1000 °C and 120 minutes. The FIB lamella was extracted from half of its interconnection zone. The joint shows three well-defined zones from BM towards the centerline, as indicated in Figures 2(a) and (b): 1) a thin layer with columnar grains named columnar layer (CL), 2) a monophasic layer constituted by coarse grains (CG) and 3) a zone formed by smaller grains with grain boundary precipitates, labelled as equiaxed grains (EG) layer. The location of the two areas examined by TEM is indicated in Figure 2(a) and enlarged in Figure 2(b). Grains of five different phases were identified and labeled as grains A to E, as shown in Figure 3 together with their SAED patterns.
In all EDS spectra, CuKα and CuKβ peaks were observed. This is expected since the FIB lamella was welded to a Cu grid, and therefore spurious counts may arise from non- collimated electrons or x-rays interacting with the grid. These peaks were disregarded because the alloy under study did not contain any Cu. The chemical composition of the grain A showed that Fe, Cr, Ni are the prevailing elements together with smaller quantities of Nb and Mo. Grain B chemical composition revealed a high amount of Cr and smaller additions of Ni and Fe. The highest concentration of Ni was detected in C grains which also contain Al and minor quantities of Fe. Grain D resulted in a Cr-rich grain with significant amounts of Fe, Ni and Al. Grains such as E are Fe-rich with considerable amounts of Cr and Nb, and smaller quantities of Al, Ni, Mo and Ti.
Several phases were identified, combining the literature and the information from EDS analysis and SAED patterns. The crystallographic information of the candidate phases was obtained from their crystallographic information file (CIF) and compared with the SAED pattern of each grain of Figure 3. It is worth mentioning that the SAED patterns were collected from the larger grains of each phase along the areas analyzed. The indexed SAED patterns with enhanced contrast are shown in Figure 4. Table I summarizes the crystallographic parameters obtained of each grain from the TEM analysis. In all the grains examined, the measured lattice parameter agrees within 10 pct with those of the prototype phases, suggesting an acceptable indexation. However, it must be noted that the prototypes correspond to binary phases whereas the grains analyzed are multicomponent.
Indexed SAED patterns of grains labeled from grains A to E (Fig. 3)
According to the crystal structure and chemical composition, grain A in the CL was identified as a Laves phase grain with a hexagonal cell. The measured lattice parameters are in good agreement with the Laves phase found in superalloys,[1,33] where a general formula includes (Cr, Ni, Fe)2 (Nb, Mo, Ti) composition.[34] The measured diffraction pattern of grain B was indexed in terms of a tetragonal unit cell, as indicated in Figure 4, that agrees with studies of diffusion coatings on Ni-base superalloys, where it was suggested that the Cr-rich layer formed at high temperature next to the superalloy is a σ-phase.[9] According to the previous phase identification concerning grains A and B, Laves and Sigma phases would grow in contact in the CL. This differs from the observations of Leng et al. for the interdiffusion zone of the aluminide coating in the alloy 625, where σ-phase was in contact with the NbMo-rich δ-Ni3Nb phase.[30] However, the phases formed are dependent on the alloy composition and the thermal cycles applied.
The indexation of the SAED pattern of grain C matched with a cubic symmetry prototype. According to the chemical composition, it was identified as a grain of Ni-rich AlNi intermetallic containing Fe and a smaller concentration of Cr in solid solution. The lattice parameters calculated in this work agrees with the literature.[9] The crystal structure of D grain, which grows in contact with AlNi grains in the EG zone, matched with a cubic cell type. The high concentration of Cr in this phase can be associated with the α-Cr bcc. Most of the AlNi grains in the EG zone showed the presence of some grains of α-Cr at their grain boundaries. The bright-field TEM image of Figure 5 shows the AlNi/α-Cr boundary and the SAED pattern collected for the same zone axis on both sides of the interface. The precipitation of the α-Cr phase has been related to the limited solubility of Cr in the AlNi phase.[35] Furthermore, a recent publication describes the precipitation of the α-Cr as being induced by the supersaturation of the AlNi in Cr caused by the dissolution of prior Cr-rich carbides in aluminized bond-coat.[36] In this work, the observed SAED pattern and the chemical composition in each phase show that both AlNi and α-Cr have the same crystal orientation but a different chemical composition, suggesting that both grains form from the same nucleation event. Probably, grains of α-Cr form as coherent or semi-coherent precipitates from the AlNi phase grains.
Similar to grain A, the SAED pattern of the grain E in the EG zone fitted well with the parameters of a hexagonal Laves phase. In this case, the grains were observed in the AlNi grain boundaries and mostly in contact with the α-Cr phase. The precipitation of the α-Cr phase has been observed in the IN718 close to the δ-Ni3Nb phase after long exposures time at 650 °C and 677 °C.[37] In this work, the temperature is near the solvus limit of δ-Ni3Nb phase in IN718 and the chemical composition measured does not correspond to this phase. Nevertheless, it is noteworthy that A as well as E, identified as Laves grains, are Nb-rich and grow close to Cr-rich grains. A detailed study about the precipitation of these phases should be done to analyze if the mechanisms involved are similar to those described by Bi et al.[37]
The microstructure formed along the joint under the experimental conditions investigated is different from that of the IN718 base metal, suggesting that its properties will not be suitable for high temperature service. Nevertheless, the procedure developed may be suitable to produce diffusion coatings on the superalloy.
3.2 In Situ High Energy Synchrotron X-Ray Diffraction
The initial phases of the interconnection zone of the sample used for the in-situ high-energy synchrotron x-ray experiments was examined by XRD at room temperature. Figure 6 shows the diffraction pattern obtained, indicating the Al3Ni2, γ and Al-rich AlNi peak positions. The {hkl} reflections match well with Al3Ni2 intermetallic phase and there is no appreciable amount of γ. This indicates that the x-ray beam was correctly positioned in the interconnection zone. Peaks corresponding to AlNi are hardly distinguishable, probably due to its small volume fraction. The EDS chemical composition profile along the interconnection zone in a similar sample bonded at 700 °C and 240 minutes is shown in Figure 7. The concentration of the main elements, i.e. 70Al-18Ni-6Cr-6Fe, suggests that the Al3Ni2 phase has formed in the bonding zone at this temperature, in good agreement with the XRD analysis (Figure 6). This phase identification is consistent with the phase formation found at 800 °C in similar systems.[38] Considering that the Al3Ni2 phase is stable from room temperature up to 1133 °C, it is feasible to found this intermetallic in the initial sample.
The evolution of the x-ray diffraction spectrum from the interconnection zone as a function of annealing time at 1000 °C is shown in Figure 8. A set of peaks was found to match well with the AlNi phase. These are identified and marked along a selected range of 2θ by green lines. A detailed examination of the spectrum shows that the AlNi peaks positions shift towards smaller 2θ with respect to the green lines until 40 minutes of annealing. From that time on, the same peaks return to the reference green line until the end of the annealing. On the other hand, the second set of peaks, marked by red lines, remain in the same position throughout the annealing. These peaks could correspond to the TCP phases identified in section 3.1. However, since several phases may be present with varied morphologies and perhaps preferential orientations, the peaks could not be unequivocally indexed. These observations verify that the shifts observed in the green set are the result of changes in the phase and not due to experimental errors.
Since the AlNi phase forms under the presence of a composition gradient between the FM and the BM which is typical of the diffusion couple, further diffusion of Ni into the interconnection zone will result in changes of the AlNi lattice parameter. The changes in the peak positions suggest that there is an increase in the lattice parameter up to 40 minutes and a subsequent decrease. Figure 9 shows the evolution of the lattice parameter of the AlNi phase as a function of annealing time for different {hkl} reflections and the mean value at each time analyzed.
Despite the differences in the lattice parameters measured for the various reflexes accounted, the trend is the same. A steep increase in the mean lattice of the AlNi phase takes place up to 40 minutes approximately, where the maximum is reached. For longer holding times, a slow decrease in the lattice parameter is observed. The change in the lattice parameter of the AlNi phase can be correlated with its chemical composition. Experimental studies at different temperatures show that the AlNi lattice parameter exhibits a maximum when the stoichiometric composition is reached, and it decreases for both Al-rich and Ni-rich sides.[39,40] This suggests that the largest lattice parameter measured in this work corresponds to the stoichiometric composition. Considering that the initial composition of the interconnection zone is rich in Al, as shown in Figure 7, it can be assumed that during the early stages of annealing the AlNi phase grows as Al-rich AlNi in the interconnection zone. After longer annealing times, as Ni and other alloying elements from the BM diffuse into the interconnection zone, the AlNi phase becomes richer in Ni. The shift in the peak positions of the AlNi phase was also related to the chemical composition of this phase in discrete XRD experiments on diffusion coating research.[38] The lattice parameters measured in this work are larger than those reported for binary Al-Ni couples. The maximum of the mean lattice parameter in this study, 2.9401 Å, resulted larger than the maximum, from 2.920 to 2.930 Å, for the binary AlNi phase at 1000 °C.[40] This difference may arise from the multicomponent nature of the AlNi phase examined in this research that contains significant amounts of alloying elements supplied by the BM. For instance, Fe, Ti and Co can provide solid-solution strengthening [41] and Fe can occupy the position of Ni atoms the sublattice.[42]
Figure 10 shows the interconnection zone after the in-situ HEXRD experiment. The microstructure along the interconnection zone is similar to that obtained after the regular TLPB shown in Figure 2. The three distinctive layers are marked. The variation in the lattice parameter with the composition of the AlNi phase was correlated with the change in chemical composition across a similar interconnection zone measured by EDS. Figure 11 shows the optical microscopy image overlapped to the SEM micrograph and the EDS scan (yellow dotted line) across the interconnection zone of a similar sample obtained at 1000ºC after 60 minutes. The position where the chemical composition of the AlNi changes from Ni-rich to Al-rich (≈ 47 at. pct of Ni) is indicated by a dotted line in the graph. A similar microstructure was observed in a multicomponent system when the split of the AlNi phase takes place.[38] In binary couples, the Al-rich and Ni-rich portions of AlNi also appear, but as two smooth layers.[24]
Micrograph and concentration profile of a couple bonded at 1000 °C and 60 min showing the same microstructure and layer thicknesses of the sample of Fig. 10
The variation of the AlNi lattice parameter as a function of the annealing time (Figure 9) is consistent with the progressive diffusion of Ni into the Al-rich AlNi that grows during the first stage of the annealing. The raise in Ni concentration causes an increase in its lattice parameter until a maximum is reached. Beyond this peak, further diffusion of Ni towards AlNi involves a decrease in the lattice parameter. In the microstructure, this is reflected as the split of the AlNi phase into two layers: Al-rich and Ni-rich AlNi. The split of the AlNi phase occurs in the Al–Ni binary system nearly at the equiatomic composition (Ni-50Al) under different conditions of time, temperature and bonding process.[21,24,39] In this work, the split line in Figure 11 was detected when the AlNi phase reaches Ni47-Al47 approximately, similar to the concentration measured by other authors.[35,38] This chemical partition is characteristic of the AlNi intermetallic and could be similar to spinodal decomposition.
3.3 Growth Kinetics of the AlNi Phase
The growth kinetics of the layers formed can be represented by the power law, Eq. [1][13]:
where x is the thickness of the layer, t is the annealing time, n is the exponential factor and k is the growth rate constant. The exponential factor indicates the controlling mechanism of the layer growth. Values approaching 0.5 are associated with growth controlled by volume diffusion, while values near 1 are related to a reaction mechanism.[21,23,43] The experimental data were fitted using the logarithmic form of Eq. [1]:
To study the growth kinetics of the AlNi phase, the Al-rich AlNi and the Ni-rich AlNi were analyzed separately. The kinetics of the Al-rich AlNi was evaluated considering the thickness of the EG zone since this zone is mainly constituted by Al-rich AlNi grains. On the other hand, the kinetics of the Ni-rich AlNi was studied throughout the thickness evolution of the CG zone which is constituted by coarse grains of Ni-rich AlNi. Figure 12 shows the microstructure of the interconnection zone at 1000 °C after 1710 minutes, exemplifying the layer thickness measurement procedure.
Figure 13 plots the thickness of the different layers as function of annealing time at 800 °C, 900 °C and 1000 °C, and Table 2 lists the linear fitting parameters. In all cases, the first readings of the layer’s thicknesses were done after the annealing time was long enough to generate layers of about 5 microns, to reduce the measurement error.
The n-values listed in Table 2 close to 0.5, suggest that the growth of the AlNi layers is controlled by volume diffusion along the range of temperatures investigated, but there are differences in the growth rates. Parabolic growth behavior of the Ni-rich AlNi phase was also observed during the annealing of pack-aluminized Ni-Cr-rich wires at 825 °C, 900 °C and 1000 °C.[35] Moreover, the values of the exponential factor found in this work for the AlNi phase are in agreement with those reported in the literature for binary Ni/Al/Ni couples bonded at 720 °C [21, 23], where growth was controlled by diffusion.
Since the growth of the layers is controlled by volume diffusion, a growth rate constant k* was calculated for each layer after considering n = 0.5, (see Table 2). It is shown that the Al-rich layer grows faster than the Ni-rich layer at 1000 °C. Figure 13 shows that the Al-rich AlNi layer reaches an appreciable thickness after just few minutes of annealing. Then its width decreases when the Ni-rich AlNi appears, as shown in Figure 14. This observation agrees with the kinetics observed in binary [13] and alloyed AlNi [35], indicating that it is typical of the AlNi phase.
The kinetics of growth of the AlNi layers can be closely related to the predominant defects in the lattice. In the Al-rich AlNi, the structural Ni vacancies dominate, while in the Ni-rich AlNi the Ni antisites prevail. Exhaustive studies about the diffusion in the AlNi phase, including experimental results and calculations, show a marked decrease of the interdiffusion and intrinsic diffusion coefficient for the equiatomic composition at 1000 °C.[44] Moreover, the intrinsic diffusion coefficients of Al and Ni over the AlNi phase shows that Al atoms diffuse faster in the Al-rich side, whereas Ni atoms are the quicker diffusing species in the Ni-rich AlNi.[44] This could explain in part the slower growth rate of the Ni-rich AlNi. As shown in Figure 14, the Al-rich AlNi layer is observed to form first. It grows until its chemical composition reaches the split line concentration approximately up to 190 minutes, and then it becomes the source of Al for other layers growing such as the Ni-rich AlNi. The rate at which Ni atoms cross the split line, where the diffusion gets slower, would be slow. Besides, Ni atoms forming the Ni-rich AlNi must diffuse from the IN718 through the growing CL, involving a long diffusion path, as shown schematically in Figure 15. These restrictions to the volume diffusion justify the slow growth rate of the Ni-rich AlNi layer, characterized by a smaller k*. At 800 °C and 900 °C, the Ni-rich AlNi was not observed for similar annealing times, highlighting that the growth of this layer is delayed compared to the Al-rich AlNi.
It is worth mentioning that although the Al-rich AlNi thickness may have been overestimated due to the heterogeneity of the EG layer and the defects in the centerline of the joint, its growth is markedly faster than the Ni-rich side. On the contrary, the Ni-rich AlNi layer is smooth and free of precipitates, allowing a more accurate measurement of its thickness. Therefore, the growth constant calculated can be considered representative.
As expected for a thermally activated processes, the growth rate of the Al-rich AlNi at 1000 °C is greater than the rate at 900 and 800 °C. As shown in Figure 16, the Al-rich AlNi growth rate constant obeys the Arrhenius function as Eq. [3]:
where k* is the growth rate constant, k0 the preexponential factor, R the molar gas constant, Q is the activation energy and T is the temperature in K.
The activation energy and the pre-exponential factor obtained are Q = 246 kJ/mol and k0 = 1.85x10-2 m2/s, respectively. Since the controlling mechanism was found to be volume diffusion, the activation energy will be more closely related to atoms diffusing than to reaction at the interfaces. The activation energy for the Al-rich AlNi layer is comparable with the values reported for the Ni-rich AlNi phase in aluminized Ni-Cr wires [35] and 47 pct higher than the Al-rich AlNi layer in Ni/Al/Ni couples.[13] The extra energy required to grow the AlNi phase in multicomponent couples could be caused by differences in the controlling mechanisms and changes in the intrinsic diffusivities, probably affected by the presence of alloying elements. Concentrations up to 10 pct Fe and 7 pct Cr were measured in the Al-rich AlNi layer (Figure 11). Moreover, in the IN718/Al/IN718 TLPB couples, other layers and precipitates demand Ni or Al contents for its formation, such as Laves phases, representing competitive processes that decrease the mass transport across the bulk. Due to the sluggish growth of the Ni-rich AlNi layer at lower temperatures, it was not possible to estimate the activation energy associated under those conditions.
This investigation shows that the formation of both Al-rich and Ni-rich AlNi layers is controlled by the same mechanism, but they grow at different rates. These remarks are in good agreement with the data reported in binary [13, 21] and alloyed AlNi phase.[35] The diffusion path through the layers and the crystal features of the alloyed AlNi phase directly affect the diffusion rate of the atoms and hence, the rate at which the layers form.
Similar to the observations on binary couples, when the AlNi phase grows in this specific multi-component system, the split line is detected for approximately equal atomic concentrations of Al and Ni. The presence of the split line indicates the AlNi phase is divided and it occurs under different conditions of time, temperature and bonding process.[24,35,38,39]
Even though the changes in the interdiffusion coefficient along the AlNi phase would explain the growth kinetics of the layers, this does not fully explain the splitting occurred. The splitting observed in AlNi for equal atomic concentrations of Al and Ni, suggests that a decomposition proceeded without the need for nucleation, perhaps under conditions similar to those found under a chemical spinodal. Rigorous calculations of free energy should be done to study in detail the split phenomena of this phase in binary and multicomponent systems under the processing conditions.
4 Summary and Conclusions
A comprehensive study of the development of the microstructure along the interconnection zone of IN718/Al/IN718 TLP bonds was carried out. The following conclusions were drawn:
-
The SAED patterns of the different phases formed were consistently indexed. It was found that the AlNi phase covers most of the interconnection zone, together with minor phases like Laves, Sigma and α-Cr. The α-Cr grains showed an identical crystal orientation than the adjacent AlNi grains.
-
The in-situ HEXRD experiment allowed to follow the change of the AlNi lattice parameter as a function of the annealing time. It was found that the lattice parameter increases quickly to reach a maximum of 2.9401 Å after 40 minutes and then decreases slowly with a further increase of the annealing time. This trend is related to the split of the alloyed AlNi into two layers: one Al-rich and other Ni-rich probably formed as a consequence of a chemical spinodal. At the split line, the concentration of Al and Ni equals at approximately 47 at. pct.
-
The analysis of the growth kinetics of Al-rich and Ni-rich AlNi layers shows that the exhibit not only different chemical composition, but also different growth rate. Although growth is diffusion-controlled in both cases, the Al-rich AlNi forms faster. On the other hand, the growth of the Ni-rich AlNi involves a progressive reduction of the width of the Al-rich layer.
References
Joseph R. Davis: ASM specialty handbook: heat-resistant materials, ASM International, 408 Materials Park, OH, 1997, pp. 221–54.
M. Hacksteiner: Master Thesis TU-Graz, Graz, 2013, pp. 7–14.
A. Jones: Superalloy 718: Metall. Appl., Proc. Int. Symp. Metall. Appl. Superalloy 718, 1989, p. 307.
J.C. Lippold, S.D. Kiser, J.N. DuPont, Welding Metallurgy and Weldability of Nickel-Base Alloys (Wiley, Hoboken, New Jersey, 2011), pp. 281–324
M. Pouranvari, A. Ekrami, A.H. Kokabi, J. Alloys Compd. 723, 84–91 (2017)
G. Zhang, R.S. Chandel, H.P. Seow, Sci. Technol. Weld. Joining 6, 235–239 (2001)
G. Singh, M.K. Gupta, M. Mia, V.S. Sharma, Int. J. Adv. Manuf. Technol. 97, 481–494 (2018)
S.A. Azarmehr, K. Shirvani, M. Schütze, M. Galetz, Surf. Coat. Technol. 321, 455–463 (2017)
W. Leng, R. Pillai, P. Huczkowski, D. Naumenko, W.J. Quadakkers, Surf. Coat. Technol. 354, 268–280 (2018)
X. Gong, H. Peng, Y. Ma, H. Guo, S. Gong, J. Alloys Compd. 672, 36–44 (2016)
S. Gialanella, A. Malandruccolo, Aerospace Alloys (Springer, Switzerland, 2020), pp. 387–439
R. Bianco, R.A. Rapp, Pack Cementation Diffusion Coatings (Springer, Dordrecht, 1996), pp. 236–260
A. Urrutia, S. Tumminello, S.F. Aricó, S. Sommadossi, Calphad 44, 108–113 (2014)
O.A. Ojo, N.L. Richards, M.C. Chaturvedi, Sci. Technol. Wel. Joining 9, 532–540 (2004)
M. Pouranvari, A. Ekrami, A.H. Kokabi, J. Alloys Compd. 563, 143–149 (2013)
B. Zhang, G. Sheng, Y. Jiao, Z. Gao, X. Gong, H. Fan, J. Zhong, J. Alloys Compd. 695, 3202–3210 (2017)
A. Ghasemi, M. Pouranvari, Mater. Des. 182, 108008 (2019)
A. Doroudi, A. Shamsipur, H. Omidvar, M. Vatanara, J. Manuf. Process. 38, 235–243 (2019)
W.F. Gale, D.A. Butts, Sci. Technol. Weld. Joining 9, 283–300 (2004)
G.O. Cook, C.D. Sorensen, J. Mater. Sci. 46, 5305–5323 (2011)
I. Kwiecien, P. Bobrowski, A. Wierzbicka-Miernik, L. Litynska-Dobrzynska, J. Wojewoda-Budka, Nanomaterials 9, 134 (2019)
G.A. López, S. Sommadossi, P. Zieba, W. Gust, E.J. Mittemeijer, Mater. Chem. Phys. 78, 459–463 (2003)
S. Tumminello, S. Sommadossi, Defect Diffus. Forum 323, 465–470 (2012)
M. Poliserpi, R. Buzolin, R. Boeri, C. Poletti, S. Sommadossi, Metall. Mater. Trans. B 51, 916–924 (2020)
S. Chakravorty, C.M. Wayman, Metall. Mater. Trans. A 7, 555–568 (1967)
Y.K. Au, C.M. Wayman, Scr. Metall. 6, 1209–1214 (1972)
R.S. Dutta, A. Arya, C. Yusufali, B. Vishwanadh, R. Tewari, G.K. Dey, Surf. Coat. Technol. 235, 741–747 (2013)
S. Wöllmer, S. Zaefferer, M. Göken, T. Mack, U. Glatzel, Surf. Coat. Technol. 167, 83–96 (2003)
X. Gong, Y. Yang, Y. Ma, H. Peng, H. Guo, Mater. Sci. Eng. A 673, 39–46 (2016)
W. Leng, R. Pillai, D. Naumenko, T. Galiullin, W.J. Quadakkers, Corros. Sci. 167, 108494 (2020)
D. Huber, M. Hacksteiner, C. Poletti, F. Warchomicka, M. Stockinger, MATEC 14, 10002 (2014)
P. Barriobero-Vila, J. Gussone, K. Kelm, J. Haubrich, A. Stark, N. Schell, G. Requena, Mater. Sci. Eng. A 717, 134–143 (2018)
A.S. Wilson, Mater. Sci. Technol. 33, 1108–1118 (2017)
J. J. Schirra, R. H. Caless, R. W. Hatala: Superalloys 718, 625 Var. Deriv., 1991, pp. 375–388.
A. P. y Puente, D. C. Dunand: Intermetallics, 2018, vol. 101, pp. 108–115.
R. Pillai, A. Jalowicka, T. Galiullin, D. Naumenko, M. Ernsberger, R. Herzog, W.J. Quadakkers, Calphadoupling 65, 340–345 (2019)
Z.N. Bi, J.X. Dong, M.C. Zhang, L. Zheng, X.S. Xie, Int. J. Miner. Metall. Mater. 17, 312–317 (2010)
E. Pauletti, A.S.C.M. d’Oliveira, J. Vac. Sci. Technol. A 36, 041504 (2018)
A. Kodentsov, Diffus. Found. 13, 56–97 (2017)
T. Hughes, E.P. Lautenschlager, J.B. Cohen, J.O. Brittain, J. Appl. Phys. 42, 3705–3716 (1971)
R. Darolia, JOM 43, 44–49 (1991)
M. Rudy, G. Sauthoff, Mater. Sci. Eng. 81, 525–530 (1986)
V.I. Dybkov, Reaction Diffusion and Solid-State Chemical Kinetics (IPMS publications, Kyiv, 2010), pp. 1–71
A. Paul, A.A. Kodentsov, F.J.J. Van Loo, J. Alloys Compd. 403, 147–153 (2005)
Acknowledgments
Authors wish to express their gratitude to CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas). The Deutsches Elektronen-Synchrotron (DESY) is acknowledged for the provision of synchrotron radiation facilities in the framework of the proposal I-20170438 and the cooperation with the Institute of Physics of Materials CAS was realized in the frame of Strategy 21 “Top research in the public interest” of Czech Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted October 7, 2020; accepted January 14, 2021.
Rights and permissions
About this article
Cite this article
Poliserpi, M., Barriobero-Vila, P., Requena, G. et al. TEM and Synchrotron X-ray Study of the Evolution of Phases Formed During Bonding of IN718/Al/IN718 Couples by TLPB. Metall Mater Trans A 52, 1382–1394 (2021). https://doi.org/10.1007/s11661-021-06159-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-021-06159-y