Abstract
Effect of multiple alloying elements on the glass-forming ability, thermal stability, and crystallization behavior of Zr-based glass-forming alloys were studied in the present work. We investigated the effect of complete or partial substitution of Ti and Ni with similar early and late transition metals, respectively, on the glass-forming ability and crystallization behavior of the Zr50Ti10Cu20Ni10Al10 alloy. Poor correlation was observed between different parameters indicating the glass-forming ability and the critical size of the obtained glassy samples. Importance of the width of the crystallization interval is emphasized. The kinetics of primary crystallization, i.e., the rate of nucleation and rate of growth of the nuclei of primary crystals is very different from that of the eutectic alloys. Thus, it is difficult to estimate the glass-forming ability only on the basis of the empirical parameters not taking into account the crystallization behavior and the crystallization interval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metallic glasses are non-crystalline materials with a disordered arrangement of the constituent atoms. They possess an interesting combination of high strength, good corrosion resistance, and useful magnetic properties. Metallic glasses (or amorphous solids) were first synthesized in thin-film form by vapor deposition[1,2] and later by rapid solidification from the liquid state at high rates of about 105 to 106 K/s.[3,4] Subsequently, bulk metallic glasses (BMGs), in large section sizes, were obtained by slow cooling from the liquid state, as in a casting process.[5] BMGs also show a good combination of physical,[5] mechanical,[6,7,8,9] magnetic,[10,11] and chemical[12] properties and exhibit high strength, hardness, wear resistance, and large elastic deformation.[13] The largest diameter of the glassy rod with a diameter of 80 mm has been produced in a Pd-based alloy.[14]
The glass-forming ability (GFA) of an alloy is an important parameter that helps in identifying appropriate compositions to achieve glass formation in a wide composition range and also in larger diameters. Several criteria have been proposed to estimate and/or establish GFA of alloys and these are based on either thermodynamic parameters, glass transformation temperatures, or modeling studies. There have been successes with some parameters in some alloy systems and it has been shown that not all the criteria proposed satisfactorily explain the GFA of all alloy systems.[15] Table I lists the different criteria that have been proposed and commonly used in evaluating the GFA of alloys.[16,17,18,19,20,21,22,23,24,25,26,27,28]
The GFA of an alloy is determined not only by the composition of the base alloy, purity of the constituent elements, and the atmosphere in which it is cast, but also by the nature of the alloying additions made.[29,30,31] The atomic size difference between the base metal and the alloying element, their chemical interactions, and thermodynamic properties determine the effectiveness of the alloying element in enhancing the GFA of the alloy system.
Zr-based metallic glasses have been investigated for a long time[32] owing to their good GFA and also their mechanical properties.[33] Starting from thin ribbons obtained by rapid solidification processing,[34] Zr-based alloys have been cast as bulk metallic glasses by slow solidification processes up to about 30 mm in diameter,[35] and currently the maximum diameter reported for a Zr-based glassy alloy is 73 mm.[36] A binary Zr50Cu50 alloy (all compositions are given in atomic percent as subscripts) was produced as a BMG with a critical diameter of 2-mm-diameter rod by suction casting.[37] The element Cu was partially replaced by Al to synthesize Zr-Cu-Al glasses with a high GFA and a critical diameter of 8 mm in a Zr50Cu40Al10 alloy.[38] Similarly, a hypoeutectic Zr60Cu30Al10 BMG alloy showed good GFA[39] and high resistance to fatigue even after complete structural relaxation.[38] By additional alloying with Ni, an element further enhancing the GFA of Zr-Cu-Al alloys,[17] Zr60Cu20Ni10Al10 alloy exhibiting a high resistance to structural relaxation-induced embrittlement was produced. The addition of Ti was found to further improve the GFA of these alloys by enhancing the icosahedral short-range order in the Zr62−xTixCu20Ni8Al10 liquid alloys.[40]
During the last decade or so, research on multicomponent alloys has received lot of attention.[41,42,43] These alloys contain either equiatomic proportions of a large number of elements (5 or more), each with a concentration of 5 to 35 at. pct, or multicomponent alloys with one major element and all the others with equal atomic percentages. Such alloys can be called multiprincipal element alloys but are more commonly referred to as high-entropy alloys (HEAs). Bulk metallic glasses have also been produced in such multicomponent alloys, e.g., Ti20Zr20Hf20Ni20Cu20.[44] The process of glass formation, thermal stability, mechanical properties, and corrosion resistance of a number of multicomponent bulk glassy alloys such as Zr55Al10Fe6Co6Ni6Cu6Pd6Ag5 have also been studied recently.[45]
In this publication, we report our results on the synthesis, GFA, and crystallization behavior of a number of glassy alloys based on the Zr50Ti10Cu20Ni10Al10 alloy. Here, Ti and Ni have been replaced with similar early (V, Mo, Nb, Ta) and late (Fe, Ag, Co) transition metals, respectively. We have also evaluated the GFA of these alloys in terms of the known GFA parameters listed in Table I.
2 Experimental Procedure
The multicomponent alloys with the nominal atomic compositions listed in Table II were chosen for the present study. Master alloys were prepared by melting the mixture of pure metals with purities exceeding 99.9 mass. pct in a Ti-getter argon atmosphere. From these ingots, glassy alloy ribbons of around 20 μm in thickness and 2 mm in width were produced by melt spinning on a single copper roller, while bulk samples were cast into a pyramidal-shape mold having 5 mm base. This helped us in determining the maximum diameter at which glasses have formed by visual observation and later confirmed by X-ray diffraction (XRD) studies.
The structure of the as-solidified and crystalized samples was examined by XRD analysis using a Bruker D8 Advanced diffractometer with Cu Kα radiation. The thermal properties of the glassy alloys were measured using a Setaram Labsys differential scanning calorimetry (DSC) instrument under an Ar gas flow. The samples were also heated to the corresponding crystallization temperatures (T x) to study the early stages of the crystallization process.
The GFA of the different alloys was also evaluated in terms of the parameters proposed by different authors[16,17,18,19,20,21,22,23,24,25,26,27,28] and correlations were made between the alloy characteristics and the GFA parameters.
3 Results and Discussion
3.1 Structure and Transformation Temperatures
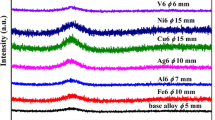
Figure 1(a) presents the XRD patterns, while Table II lists the full width at half maximum (FWHM) and thermal properties of the different multicomponent alloy ribbons studied in this work. All the alloys showed broad and diffuse diffraction peaks, suggesting formation of an amorphous phase. The alloying elements exhibited a strong effect on the FWHM values of the alloys. Analysis of the XRD patterns showed that the FWHM observed was the lowest for the Zr50Ti10Cu20Fe10Al10 alloy (ZHE1). While the FWHM for the base amorphous alloy was 6.1 ± 0.04 deg, complete replacement of Ti by V led to a slight increase in the FWHM value. Substitution of nickel by iron and titanium by vanadium increased the FWHM of the alloys by ~ 0.5 degree. Together, addition of V, Ni, Mo, and Nb slightly decreased the FWHM of the alloy. The largest value of 6.59 ± 0.06 deg for the FWHM was observed for the (ZHE2). But, complete replacement of Ni by Fe reduced this value to a minimum level of 5.8 ± 0.04 deg (ZHE7). In other cases, full or partial replacement of elements didn't have high effect. These results allow us to make some guesses about the stability of the amorphous phases.
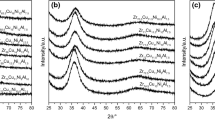
Figure 1(b) presents a HRTEM image and a diffraction pattern of ZHE3 alloy in the as-cast state. A diffuse diffraction ring can be observed in the diffraction pattern. Also, no crystalline planes are detected in the HRTEM image which indicates formation of the amorphous single phase.
Figure 2 presents a typical DSC trace, while Table II also lists the characteristic temperatures of the different alloy ribbons studied. Analysis of the DSC results showed that the multicomponent alloy Zr50(Ti,V,Mo,Nb)10Cu20(Ni,Fe)10Al10 (ZHE3) has the maximum interval of supercooled liquid region (Table II). Full replacement of titanium by vanadium led to a substantial increase in both T g (from 679 K to 741 K) and T x (from 702 K to 755 K). On the other hand, partial replacement increased only T x, but did not change T g (except for ZHE2), thereby increasing the interval of the supercooled liquid region from 23 K to 59 K. Replacement of nickel by cobalt or iron led to a slight decrease in both T g and T x, but a slight increase in T l. Partial replacement of titanium and nickel by vanadium and iron, respectively, led to an increase in the interval of the supercooled liquid region. These changes in the characteristic temperatures lead to different GFA values of the alloys, to be described later.
The above results can be rationalized as follows. The thermal stability of the glass and its transformation behavior are determined by the T g and T x temperatures. By looking at the effect of the different solute elements on these parameters, described above, it becomes clear that the transformation temperatures are significantly increased when the structure or the atomic size (or both) of the alloying element is significantly different from the element it is replacing. On the other hand, when the structure is similar and the atomic size is also similar, then the alloying element will not have any measurable effect on the transformation temperatures.
Replacement of nickel for iron (ZHE1 and ZHE7 alloys) increases the intervals of the supercooled liquid and the crystallization interval, that is, the thermal stability of the supercooled liquid increases, but at the same time the critical cooling rate necessary for obtaining the amorphous state increases, since we move away from the eutectic point. The replacement of titanium with vanadium (ZHE1 and ZHE5) and nickel by cobalt (ZHE1 and ZHE6) reduces the thermal stability of the supercooled liquid, and also deteriorates formation of the amorphous phase during casting due to the increase in the crystallization interval. Partial replacement of titanium and nickel perfectly stabilizes the supercooled liquid; however, in all alloys except for ZHE3 the crystallization interval increases dramatically, which makes it difficult to obtain BMGs in these alloys.
3.2 Crystallization Process
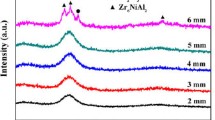
All the alloys were subjected to a short crystallization treatment of a 10-minute anneal at the crystallization temperature, T x, and the resulting XRD patterns are presented in Figure 3. The XRD patterns of the alloys with the highest Ti content (ZHE1, ZHE6, and ZHE7) are very similar to the as-solidified alloys, but with minor differences. For example, a trace amount of the ZrO2 phase is present owing to surface oxidation. Further, the broad maxima are slightly sharper than in the as-solidified condition (Figure 3). The FWHM of the diffraction peaks have decreased to 4.50 (ZHE1), 4.79 (ZHE6), and 5.05 (ZHE7) degrees indicating that nanocrystallization has occurred in these alloys. A similar effect was found earlier in Cu-Zr-Ti alloys. While the primary crystals during crystallization of glassy Cu-Zr alloys are usually large,[46] they are much finer (i.e., nanocrystallization of the primary phase occurs) with the addition of Ti.[47]
All alloys with low titanium content (ZHE2, ZHE3, and ZHE4) exhibit a similar crystallization process. Crystals of Zr2Cu (I4/mmm) and ZrAl3 (Fm\( \bar{3} \)m) phases grow from the amorphous matrix at the first stage of crystallization process, likely by a eutectic reaction. In the titanium-free alloy (ZHE5), the crystallization process started with the formation of the Zr2Ni (I4/mcm) phase. Based on these results, we can say that even though titanium decreases the thermal stability of these alloys (decreasing T x), it also causes nanocrystallization. Also, despite the fact that the heating of samples was carried out under an argon atmosphere, the surface was oxidized and the ZrO2 peaks are present in the XRD patterns. Table III shows the crystallization and melting enthalpies of the studied alloys. Correlations between the obtained values are shown in Figure 4.
3.3 Glass-Forming Ability
It was mentioned in the Introduction that a large number of criteria were developed to predict the GFA of alloys. Since the present series of alloys can also be considered multicomponent (high-entropy) alloys, let us first see if the GFA of these alloys can be rationalized in terms of the criteria developed for HEAs.
Majority of the HEAs are expected to form solid solution phases.[41,42,43] Depending on the atomic size difference, δ, and the thermodynamic properties (entropy of mixing, ∆S mix, and enthalpy of mixing, ∆H mix) of the constituent elements, it was reported[48] that a solid solution phase forms when δ is small and the value of ∆H mix is near zero (not negative enough to form intermetallic compounds). On the other hand, large values of δ and more negative ∆H mix values were noted to promote glass formation. These arguments were further extended by Yang and Zhang,[49] who introduced the Ω parameter to determine if solid solutions form in these multicomponent alloys or not.
Calculation of Ω also makes use of the parameters T m, the melting temperature of the n-element alloy, ∆S mix, and ∆H mix, and the Ω parameter is defined as
where \( T_{\text{m}} = \mathop \sum \limits_{i = 1}^{n} c_{\text{i}} (T_{\text{m}} )_{\text{i}} \), where (T m)i is the melting point of the ith component of the alloy, \( \Delta S_{\text{mix}} = - \,R\mathop \sum \limits_{i = 1}^{n} (c_{\text{i}} \ln c_{\text{i}} ) \) is the entropy of mixing of an n-element regular solution, and \( \Delta H_{mix} = \mathop \sum \limits_{i = 1,i \ne j}^{n}\Omega _{ij} c_{i} c_{j} \) is the enthalpy of mixing for the multicomponent alloy system with n elements, where \( \Omega _{\text{ij}} = 4\Delta H_{\text{AB}}^{\text{mix}}. \)
The prediction of a solid solution or a bulk metallic glass was made by considering both the δ and Ω values. It was shown that a solid solution forms if the δ values are small (≤ 6.6 pct) and Ω is ≥ 1.1. On the other hand, BMGs are expected to form if the value of δ is larger and the Ω value is smaller. These thermodynamic parameters have been calculated by most authors on the basis of a regular solution model, even though such an assumption was questioned recently.[50]
The results of our calculations of δ and Ω for the different alloys studied are shown in Table IV. A few points are of interest. Firstly, all the alloys have the same δ value, 9.9 pct, which is > 6.6 pct. Secondly, the value of ∆H mix is negative, but quite small. Thirdly, the value of ∆S mix is high. Lastly, the calculated weighted average melting temperature of the alloys is significantly different from the measured liquidus temperature, owing to the formation of low-temperature eutectics in the real alloys. This leads to a large difference in the Ω value for the different alloys, but is still < 1.1. Thus, according to Reference 49, these values clearly suggest that all alloys should form BMGs. But, the values are not able to indicate which alloy is a better glass former than the other(s). Therefore, we have analyzed our results in terms of the different GFA parameters described for BMGs.
As mentioned earlier, a number of different criteria were proposed to estimate/predict the GFA of alloys. These approaches achieved limited success in the sense that not all criteria could correctly predict the GFA of all the glasses studied in all the systems.[15] In spite of this, these criteria have been helpful in providing a reasonable estimate of the GFA in several instances. Table V lists the values of these different criteria for all the alloys studied in this investigation along with the values for some base alloys.
It is clear from the values listed in Table V that the ZHE3 alloy shows the highest GFA criteria/indicators (see also Figure 5) though its critical sample thickness is not high. Additionally, the alloys with the best GFA (including the base alloys) also show the largest value of the width of the supercooled liquid region (∆T x). However, no correlation was found between the best glass formers and the T rg values. Alloys which show larger values of T rg did not exhibit the best predicted GFA according to other criteria. Similarly, the GFA predicted by the criteria and the critical diameter also do not show any direct correlation. For example, ZHE2 has a critical diameter of 0.5 mm and ZHE4 1.0 mm, while ZHE6 and ZHE7 have critical diameters of 2.0 mm. In this context, it may be important to remember that several intrinsic and extrinsic parameters could actually limit the critical diameter of the fully glassy rod.[51]
Also, none of the parameters of the GFA, except for Reference 28, takes into account the width of the crystallization interval. And the ability to stabilize the supercooled liquid and to suppress the primary crystallization required for glass formation is best ensured only for the eutectic alloys. The kinetics of primary crystallization, i.e., the rate of nucleation and rate of growth of nuclei of primary crystals[46,52] is very different from that of the eutectic alloys. In this connection, we can speak about the impossibility of estimating the glass-forming ability only on the basis of the entropy of the alloy and the size factor of the atoms. The parameters of the GFA should have mutual correlation only for the eutectic alloys. Therefore, it is not necessary to expect correlations with the critical diameter from alloys with different types of crystallization,[53] which is observed.
Recently, Blyskun et al.[24] analyzed the relationship between the critical diameter and GFA of four different Zr-based alloys and came to the conclusion that satisfying correlations were achieved between GFA and γ, γ m, β (α), and ζ, even though they had proposed another criterion ω m, which showed a slightly better correlation with the GFA than the other parameters. But, these authors had also shown that ∆T x showed the weakest correlation with the GFA. There are also several other instances in the literature for such a behavior.[5]
Since the different GFA indicators are showing a good correlation with the ∆T x values and the compositions for the best glass formers were identified, it should be possible to theoretically calculate the maximum diameter that could be expected to be fully glassy. This is possible because the authors of some of the criteria provided equations relating the critical diameter and the critical cooling rate to the GFA parameter. Using these equations, the values of α, β, and γ, and the critical cooling rate required to obtain 1-, 10-, and 100-mm-diameter rods are calculated and the results are presented in Table VI.[5] Interpolating the GFA parameters that were obtained for our alloys, it becomes clear that it should be possible to produce the ZHE3 alloy as a fully glassy rod with a diameter of at least 5 mm.
4 Conclusions
Metallic glassy samples with the nominal multicomponent composition Zr50Ti10Cu20Ni10Al10, Zr50V10Cu20Ni10Al10, Zr50Ti10Cu20Fe10Al10, Zr50Ti10Cu20Co10Al10, Zr50(Ti, V)10Cu20(Ni, Fe)10Al10, Zr50(Ti, V, Mo, Nb)10Cu20(Ni, Fe)10Al10, Zr50(Ti, V, Mo, Nb, Ta)10Cu20(Ni, Fe, Ag, Co)10Al10 were produced. Partial replacement of titanium and nickel by V, Fe, Mo, Nb, Ta, Ag, and Co leads to an increased interval of the supercooled liquid region up to 59 K. Alloys with high Ti content have lower thermal stability but very low crystal growth rate. A similar effect is caused by Co addition. Although δ and Ω parameters showed that all of the multicomponent glassy alloys are in the region of BMGs, the GFA of the multicomponent alloys is not so high; likely owing to relatively large solidification interval. The main problem with finding the multicomponent alloys of high GFA is the difficulty to find the alloy composition located near the quaternary eutectic points with a small solidification interval.
References
W. Buckel and R. Hilsch: Z. Phys., 1952,vol. 132, pp. 420-422.
W. Buckel: Z. Phys, 1954, vol. 138, pp. 136-150
W. Klement, R.H. Willens and P. Duwez: Nature, 1960, vol. 187, pp. 869-870
H.H. Liebermann: Rapidly Solidified Alloys: Processes, Structures, Properties, Applications, 1st ed., Marcel Dekker, New York, NY, 1993.
C. Suryanarayana and A. Inoue (2018) Bulk Metallic Glasses, CRC Press: Boca Raton.
C.A. Schuh, T.C. Huffnagel and U. Ramamurty: Acta Mater., 2007, vol. 55, pp.4067-4109.
M.W. Chen: Annual Rev. Mater. Res., 2008, vol. 38, pp. 445-469.
C. Suryanarayana: Mater. Today, 2012, vol. 15, pp. 486-498.
T.C. Huffnagel, C.A. Schuh and M.L. Falk: Acta Mater., 2016, vol. 109, 375-393.
A. Inoue, A. Takeuchi and T. Zhang: Metall. Mater. Trans. A, 1998, vol. 29A, pp. 1779-1793.
G. Herzer: Acta Mater., 2013, vol. 61, pp. 718-734.
J.R. Scully, A. Gebert and J.H. Payer: J. Mater. Res., 2007, vol. 22, pp. 302-313.
D.V. Louzguine-Luzgin and A. Inoue: Handbook of Magnetic Materials, Elsevier, Vol. 21, 2013, pp. 131-171.
N. Nishiyama, K. Takenaka, H. Miura, N. Saidoh, Y. Zeng, and A. Inoue: Intermetallics, 2012, vol.30, pp. 19-24.
C. Suryanarayana, I. Seki and A. Inoue: J. Non-Cryst. Solids, 2009, vol. 355, pp. 355-360.
D. Turnbull: Contemp. Phys., 1969, vol. 10, pp. 473-488.
A. Inoue: Mater. Trans. JIM, 1995, vol. 36, pp. 866-875.
K. Mondal and B.S. Murty: J. Non-Cryst. Solids, 2005, vol. 351, pp. 1366-1371.
Z.Z. Yuan, S.L. Bao, Y. Lu, D.P. Zhang and L. Yao: J. Alloy. Compd., 2008, vol. 459, pp. 251-260.
Z.P. Lu and C.T. Liu, Acta Mater., 2002, vol. 50, pp. 3501-3512
X.H. Du, J.C. Huang, C.T. Liu and Z.P. Lu: J. Appl. Phys., 2007, vol. 101, pp. 086108-1-086108-3.
Q.J. Chen, J. Shen, D. Zhang, H.B. Fan, J.F. Sun and D.G. McCartney: Mater. Sci. Eng. A, 2006, vol. 433, pp. 155-160.
Z.L. Long, H.Q. Wei, Y.H. Ding, P. Zhang, G.Q. Xie and A. Inoue: J. Alloy. Compd., 2009, vol. 475, pp. 207-219.
P. Blyskun, P. Maj, M. Kowalczyk, J. Latuch and T. Kulik: J. Alloy. Compd., 2015, vol. 625, pp. 13-17.
G.J. Fan, H. Choo and P.K. Liaw: J. Non-Cryst. Solids, 2007, vol. 353, pp. 102-107.
X.H. Du and J.C. Huang: Chinese Phys., 2008, vol. B17, pp. 249-254.
A. Hrubý: Czech. J. Phys., 1972, vol. B22, pp. 1187-1193.
N. Nishiyama, A. Inoue: Mater. Trans., 2008, vol.43, pp.1913-1917.
Z.P. Lu and C.T. Liu: J. Mater. Sci., 2004, vol. 39, pp. 3965-3974.
W.H. Wang: Prog. Mater. Sci., 2007, vol. 52, pp. 540-596.
D. V. Louzguine-Luzgin, N. Chen, A. Yu. Churymov, L. V. Louzguina-Luzgina, V. I. Polkin, L. Battezzati and A. R. Yavari: J. Mater. Sci., 2015, vol. 50, pp. 1783-1793.
Inoue, T. Zhang and T. Masumoto: Mater. Trans., JIM, 1990, vol. 31, pp. 177-183.
D. V. Louzguine-Luzgin, S.V. Ketov, Z. Wang, M.J. Miyama, A.A. Tsarkov and A.Yu. Churyumov: Mater. Sci. Eng. A, 2014, vol. 616, pp. 288–296.
R. Ray, B.C. Giessen and N.J. Grant: Scripta Metall., 1968, vol. 2, pp. 357-359.
Q.S. Zhang, W. Zhang and A. Inoue: Mater. Trans., 2007, vol. 48, pp. 3031-3033.
H.B. Lou, X.D. Wang, F. Xu, S.Q. Ding, Q.P. Cao, K. Hono and J.Z. Jiang: Appl. Phys. Lett., 2011, vol. 99, p. 051910.
J. Eckert, J. Das, K.B. Kim, F. Baier, M.B. Tang, W.H. Wang and Z.F. Zhang: Intermetallics, 2006, vol. 14, pp. 876-881.
Y. Yokoyama, T. Yamasaki, P.K. Liaw and A. Inoue: Acta Mater., 2008, vol. 56, pp. 6097-6108.
Q.S. Zhang, W. Zhang, D.V. Louzguine-Luzgin and A. Inoue: Mater. Sci. Forum, 2010, vol. 654-656, pp. 1042-1045.
T. H. Kim, A. K. Gangopadhyay, L. Q. Xing, G. W. Lee, Y. T. Shen, K. F. Kelton, A. I. Goldman, R. W. Hyers and J. R. Rogers: Appl. Phys. Lett., 2005, vol. 87, p. 251924.
B.S. Murty, J.W. Yeh, and S. Ranganathan: High Entropy Alloys, Butterworth-Heinemann, London, 2014.
M.C. Gao, J.-W. Yeh, P.K. Liaw, and Y. Zhang: High Entropy Alloys: Fundamentals and Applications, Springer, New York, 2016.
B. Cantor: Entropy, 2014, vol. 16, pp. 4749-4768.
L. Ma, L. Wang, T. Zhang and A. Inoue: Mater. Trans., 2002, vol. 43, pp. 277-280.
A. Inoue, Z. Wang, D.V. Louzguine-Luzgin, Y. Han, F.L. Kong, E. Shalaan and F. Al-Marzouki: J. Alloy. Compd., 2015, vol. 638, pp. 197-203.
D. V. Louzguine-Luzgin, G. Xie, W. Zhang and A. Inoue: Mater. Sci. Eng. A,2007, vol. A465, pp. 146-152.
D. V. Louzguine and A. Inoue: J. Mater. Res., 2002, vol. 17, pp. 2112-2120.
Y. Zhang, Y.J. Zhou, J.P. Lin, G. L. Chen and P.K. Liaw: Adv. Eng. Mater., 2008, vol. 10, pp. 534-538.
X. Yang and Y. Zhang: Mater. Chem. Phys., 2012, vol. 132, pp. 233-238.
I.A. Tomilin and S.D. Kaloshkin: Mater. Sci. Technol., 2015, vol. 31, pp. 1231-1234.
D.V. Louzguine-Luzgin, D.B. Miracle and A. Inoue: Adv. Eng. Mater., 2008, vol. 10, pp. 1008-1015.
A.S. Aronin, G.E. Abrosimova, A.F. Gurov, Yu.V. Kir’yanov, and V.V. Molokanov: Mater. Sci. Eng. A, 2001, vol. 304–306, pp. 375–79.
R.V. Sundeev, A.M. Glezer, and A.V. Shalimova: Mater. Lett., 2016, vol. 175, pp. 72–74.
Acknowledgments
This work was supported by the World Premier International Research Center Initiative (WPI), MEXT, Japan and by the Ministry of Education Science of the Russian Federation in the framework of Increase Competitiveness Program of NUST«MISiS» (No. К2-2014-013 and K2-2016-071).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 8, 2017.
Rights and permissions
About this article
Cite this article
Bazlov, A.I., Tsarkov, A.A., Ketov, S.V. et al. Effect of Multiple Alloying Elements on the Glass-Forming Ability, Thermal Stability, and Crystallization Behavior of Zr-Based Alloys. Metall Mater Trans A 49, 644–651 (2018). https://doi.org/10.1007/s11661-017-4441-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-017-4441-y