Abstract
The influence of vanadium additions on transformation kinetics has been investigated in a medium carbon forging steel. Using dilatometry to track transformation during continuous cooling or isothermal transformation, the impact of vanadium on both ferrite-pearlite and bainite has been quantified. Transmission electron microscopy and atom probe tomography have been used to establish whether vanadium was present in solid solution, or as clusters and precipitates. The results show that vanadium in solid solution has a pronounced retarding influence on ferrite-pearlite formation and that, unlike in the case of niobium, this effect can be exploited even during relatively slow cooling. The influence on bainite transformation was found to depend on temperature; an explanation in terms of the effect of vanadium on heterogeneous nucleation is tentatively proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The use of vanadium in engineering steels can be traced to the early 20th century, when its benefits for automotive application were rapidly recognized.[1,2] A century later, vanadium microalloyed ferrite-pearlite steels have become standard for a large variety of high-performance forged components. In the case of automotive crankshafts, for example, addition of 0.1 to 0.15 pct V to a 38MnSi5 (0.38 pct C-1.5 pct Mn-0.6 pct Si wt pct) steel grade raises the yield strength by over 100 MPa. This dramatic increase is due to the interphase precipitation phenomenon, which naturally occurs as ferrite forms from austenite upon cooling,[1] and through which a very fine dispersion of V(C,N) is obtained without the need for any complex heat-treatment. A detailed review of the relative stability of carbides, nitrides, and carbonitrides formed with vanadium, together with relevant thermodynamic data, can be found in Reference 1.
It is thus possible to achieve yield strength (YS) and ultimate tensile strength (UTS) of typically 620 and 900 MPa, respectively, in as-forged parts after natural cooling. In the case of smaller components such as connecting rods, higher percentages of vanadium (0.3 to 0.35 wt pct) have led to the development of one of the highest performance forging steel grades (36MnV4,[3]), with YS and UTS as high as 910 and 1180 MPa, respectively, while retaining a fully ferrite-pearlite microstructure. Nevertheless, mechanical properties usually remain below or at 1000 MPa in most cases, and the loss in ductility which comes with higher tensile strength is not acceptable for all applications.
Quenching and tempering are often used to achieve tensile strength of 1000 MPa and above. Nonetheless, interest is growing both among steel-makers and end users for so-called bainitic steel grades.[4,5,6,7,8,9,10,11] These are designed to achieve a microstructure consisting mainly of bainite, without the need for specific heat-treatment. That is to say, the microstructure is obtained through natural cooling either after hot forging or hot rolling. These grades often exhibit mechanical properties on par with or in excess of those offered by quenched and tempered forging steels.
Because interphase precipitation does not occur during formation of bainite, addition of vanadium to bainitic special steels is not as commonly used as for ferrite-pearlite microstructures. Yet, vanadium is of interest for its influence on hardenability and on bainite formation kinetics. Indeed, the influence of vanadium on hardenability is relatively well established.[12,13,14,15,16,17,18,19,20,21]
On the other hand, seemingly contradictory results have been published, though these can be explained (or are almost to be expected), as the data obtained can depend strongly on the choice of austenitizing temperature.[16,22] With few exceptions, however, these studies have focused on overall measurements of hardenability (Jominy end quench tests, critical diameter, etc.), with relatively little attention paid to the specific action of vanadium on ferrite-pearlite or bainite formation. Moreover, as the effects have been largely investigated after rapid cooling from austenitizing temperatures (as in Reference 20 for example), the applicability to conditions of continuous cooling has not clearly been addressed.
The present study is concerned with quantifying the influence of vanadium on both ferrite-pearlite and bainite formations, under both conditions of continuous cooling and isothermal transformation, in order to establish a relationship between the two.
2 Experimental Work and Calculations
To investigate the influence of vanadium in ‘bainitic’ steel grades, three 20 kg laboratory casts were manufactured using a vacuum induction melting furnace. After solidification and cooling to room temperature, the ingots were re-heated to approximately 1523 K (1250 °C) and hot-forged to bars of 40 mm diameter. After forging, the bars were re-heated to 1273 K (1000 °C) for 45 minutes and air cooled. This heat-treatment leads to a microstructure representative of forged conditions both in terms of grain size and mechanical properties.[23] An additional material was introduced to investigate the influence of grain size on hardenability (the reason for this choice will be made clearer in a later section). This material was taken from industrially produced bars of diameter 35 mm. All chemical compositions (Tables I, II) were determined using optical emission spectrometry, except for carbon content which was measured using combustion (LECO) analysis.
Specimens for dilatometry (3 mm in diameter and 10 mm in length) were manufactured from material taken at mid-radius of the bars. Dilatometry was carried out using a Baehr (now TA Instruments) 805. To build CCT (continuous cooling transformation) and TTT (time temperature transformation) diagrams for the different materials investigated, specimens were heated to 1423 K (1150 °C), maintained at this temperature for 3 minutes and then cooled at different rates using nitrogen gas. As this method employs a faster heating rate (3 K/s) and shorter holding time than conventional furnace heating of larger specimens, this treatment typically leads to identical grain size as that observed on forged components. Isothermal transformation kinetics were investigated using similar austenitizing conditions, followed by quenching at 20 K/s to the holding temperature; the specimens were then maintained at this temperature until the end of transformation or steady state.
Metallography was carried out using standard preparation methods, with 4 pct Nital as an etchant. Prior austenite grain size (PAGS) was determined as per ASTM E112, using a hot picric acid aqueous solution (Bechet-beaujard,[24]). In some cases, PAGS was also determined using thermal etching.[25] This method involves heating a polished specimen in vacuum up to the austenitizing temperature, then quenching it to room temperature. This type of heat-treatments was also carried out in a Baehr 805 dilatometer. Finally, PAGS was also identified, in some cases, by reconstruction of austenite domains from electron backscattered diffraction (EBSD) maps acquired on the quenched specimens.[26]
Scanning electron microscopy (SEM) observation was carried out on a JEOL J8M-6500 field emission gun SEM (SEM-FEG) operating at 7 kV. Before etching, samples were polished using colloidal silica suspension. For transmission electron microscopy (TEM), thin foils were prepared using a twin jet electrolytic polishing apparatus, in an electrolyte of 5 pct perchloric acid in acetic acid. Samples were examined in a JEOL 2010F microscope operating at 200 kV. TEM specimens were observed in conventional transmission electron microscopy mode (CTEM), and in high-angle annular dark field mode (HAADF). The latter is better adapted to the detection of small precipitates, as the contrast is then proportional to Z 2 (where Z is the atomic mass), with little crystallographic contrast. As a consequence, vanadium, but also titanium carbonitrides, appear darker than the surrounding matrix and can be easily identified, even for precipitates of size 10 to 30 nm (Figure 1). Crystallographic contrast can be added to the HAADF images by tilting the beam towards the detector, thus helping to identify features on which precipitates were observed (the imaging mode is then referred to as ADF). Qualitative chemical analyses were performed on the matrix and on precipitates using Energy Dispersive Spectroscopy (EDS).
Quantitative estimation of the fraction of microalloying elements present as precipitates or in solution was carried out using an electrolytic dissolution technique as follows: a specimen of approximately 1 g was dissolved electrolytically and precipitates were collected on a 200-nm polycarbonate membrane filter; the filter and its content were then dissolved in a mixture of hydrochloric, nitric, perchloric, and hydrofluoric acids; the resulting solution was diluted and analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES). With accurate weighing of the specimens before and after dissolution, and with the known chemical bulk composition, it was then possible to estimate the fraction of the element to be analyzed that was present in solid solution in the metal, and that which was present as precipitates. The above technique will be referred to hereafter as ED-ICP analysis (electrolytic dissolution-inductive coupled plasma).[27]
Specimens for Atom Probe Tomography (APT) were prepared as thin needles, with an apex radius of curvature of less than 100 nm. This was accomplished using a standard electrochemical etching technique, with a mixture of 2 pct perchloric acid in 98 pct 2-butoxyethanol, and a voltage of 10 to 20 V. Analyses were conducted using a wide angle energy compensated atom probe (ECOWATAP) in voltage mode, under standard analytical conditions (80 K (−193 °C), 19 pct pulse fraction, 30 kHz pulse repetition rate, and 0.01 atom per pulse detection rate). Several samples and thus several million atoms were collected for each metallurgical condition tested, ensuring that the collected data were reasonably representative, as well as a precision in measured compositions better than 0.05 at. pct. An example standard mass spectrum is shown in Figure 2, which illustrates the mass resolution obtained with this instrument (quantified as \(m/ \Delta m=1500 \) at 10 pct of the maximum). A notable result of this analysis is that vanadium (yellow) is clearly distinguishable from chromium (green), even at composition levels of a few tenths of atomic percent. In the example given, the measured V content is 0.21 ± 0.01 at. pct, in perfect agreement with the bulk measured content (0.22 at. pct).
Finally, thermodynamic calculations were carried out using the commercial software package Thermocalc with the TCFE3 database.
3 Quantifying the Vanadium Fraction in Solution After Austenitizing
Both CCT and TTT diagrams were determined using austenitizing at 1423 K (1150 °C) for 3 minutes (heating rate 3 K/s, see earlier for justification of temperature). Equilibrium calculations suggest that of all three grades, only the 400 V material was likely to exhibit a small fraction of vanadium present as precipitates after austenitizing at 1423 K (1150 °C). Indeed, as shown in Figure 3, V is mostly in solution in this steel grade at 1423 K (1150 °C), which means that only a small amount of V has precipitated. Following the same logic, both 200 V (and even more likely 0 V) were predicted to have no significant quantity of vanadium precipitates stable at this temperature.
To test these hypothesis, ED-ICP measurements were carried out on heat-treated 40-mm diameter bars [1273 K (1000 °C)/45 minutes, air cooling, representative of forged components]. From these measurements (of the weight of vanadium present as precipitates), the weight fraction of vanadium remaining in solid solution was calculated. The results, shown in Figure 3, are in good agreement with the calculated values, and confirm that only the 400 V material contains a small fraction of precipitated vanadium (<5 pct of the total vanadium available). Additional ED-ICP measurements were carried out on a dilatometry specimen of the 400 V material heated at 1423 K (1150 °C) for 3 minutes, and quenched at 50 K/s, which yielded similar results (Figure 3).
The possible presence of precipitates was further investigated for both 0 and 400 V, treated in the same condition (austenitizing 1423 K (1150 °C)/3 minutes, quenching), using transmission electron microscopy (see example for 400 V in Figure 4). For the 0 V material, only MnS and oxides were identified. Identification of MnS was confirmed using EDS measurements, although typical size (about 1 μm) and morphology made this verification virtually superfluous. These inclusions were present in all materials in quantities typical of the manufacturing method, and will therefore not be further mentioned. For the 400 V material, smaller precipitates were identified and further examined (see Figure 4). For such precipitates, an average diameter of 41 nm was estimated over approximately 15 precipitates. EDS measurement (4 nm probe) indicated a typical stœchiometry matching Ti\(_{0.6}\)V\(_{0.4}\). Precipitates were also confirmed to be nitrogen-rich using electron energy loss spectroscopy.
Weight percent of vanadium in solution in austenite as a function of temperature for alloy 200 and 400 V, calculated with Thermocalc and TCFE3 (continuous lines). Dots are for experimental values obtained either on air-cooled 40-mm bars (200 and 400 V, austenitizing temperature 1273 K (1000 °C)) or dilatometry specimens austenitized for 3 min at 1423 K (1150 °C) and quenched at 50 K/s. The slope change in calculated data is due to a shift from Ti-rich precipitates with small amounts of vanadium at high temperature to vanadium-rich precipitates at lower temperatures
Results detailed earlier (calculation and ED-ICP) however suggested that virtually all vanadium should be in solid solution. To confirm whether the presence of such precipitates was consistent with this expectation, the expected average spacing between precipitates was evaluated from the following parameters: average measured diameter (TEM), approximative volume fraction based on a density for vanadium carbides of 4.4 kg/m3 and ED-ICP results for the mass fraction of vanadium present as precipitates, and hypothesis of a homogeneous distribution of precipitates. This calculation yielded an estimated average spacing of 375 nm, i.e., a precipitate number density of \(2\times 10^{19}\) m\(^{-3}\). Micrographs such as the one shown in Figure 4 correspond to an average volume of \(2.25\times 10^{-19}\) m\(^{3}\), so that typically 4 to 5 precipitates are expected. This order of magnitude is in good agreement with observation. According to a similar calculation, approximately 30 precipitates would be expected in a micrograph such as Figure 4 if 25 pct of the vanadium had precipitated. Thus, the observation of a few precipitates in 400 V is consistent with the ED-ICP estimation that most of the vanadium is in solid solution.
To explore the question further, atom probe tomography was carried out on three different specimens of 400 V material heat-treated in a slightly different manner. The specimens were austenitized in similar conditions [1423 K (1150 °C), 3 minutes], then quenched to 723 K (450 °C), and held at this temperature for 100 seconds before further quenching to room temperature. Such heat-treatments were designed to produce a small fraction of bainite in a mostly martensitic matrix. In all three specimens, a homogeneous distribution of vanadium was observed as per Figure 5, with typical average content 0.41 to 0.43 at. pct, in very good agreement with the expected bulk composition if the few precipitates identified earlier are taken into account. It should also be noted that no evidence of vanadium clustering was found using standard statistical tests.[28] While the specimens were not examined after direct quenching to room temperature, it is evident that the observations apply to this condition, as introduction of the short isothermal holding could only have favored further precipitation.
Specimens from 200 V taken from air-cooled bars were also investigated using APT to establish whether slower cooling could affect precipitation or clustering in austenite. As per design of the steel grades being investigated, the microstructure in this condition consisted of a mixture of bainitic ferrite and retained austenite. Thus, for this particular case, APT specimens were prepared using focused ion beam extraction at an interface between austenite and ferrite. As shown in Figure 6, the results obtained through statistical analysis[28] also support the absence of precipitation or clustering.
(a) Carbon and vanadium (shifted upward by 15 pct for clarity) composition profiles perpendicular to an austenite/bainitic ferrite interface in 200 V (air-cooled bars), average over a circular surface as per (b). Carbon shows the expected redistribution between ferrite and austenite (on the left), with noticeable enrichment in the austenite. On the contrary, there is no indication of significant local variations of vanadium content, in either phase or at the interface
Taken together, all of the obtained data sustain the conclusion that none or very little of the vanadium is precipitated at the end of austenitizing, whether the latter is carried out at 1273 K or 1423 K (1000 °C or 1150 °C). In the case of air-cooled bars, this result not only indicates that vanadium was in solution after austenitizing, but also that it did not precipitate during subsequent cooling [the cooling rate for the 40 mm bars was measured to be 1 K/s between 1273 K and 873 K (1000 °C and 600 °C)]. The absence of any small Ti-rich precipitate in the 0 V material is worth mentioning. Indeed, all materials contain similar amounts of Ti, though small Ti-rich precipitates were only identified in the vanadium-bearing materials. The origin of this difference is, however, out of the scope of this investigation.
4 Influence of Vanadium on Ferrite Formation
4.1 Continuous Cooling
The impact of vanadium content on ferrite formation was evaluated using dilatometry. As detailed earlier, specimens of the different materials were austenitized at 1423 K (1150 °C) for 3 minutes before cooling at a controlled rate (CCT diagrams), or quenching to a defined isothermal transformation temperature (isothermal kinetics). An example of a CCT diagram is shown for material 200 V in Figure 7. From such diagrams, the critical cooling rate was determined, above which ferrite-pearlite microstructures are no longer present; this value is hereafter denoted CCR.
As shown in Table III, the critical cooling rate for ferrite-pearlite formation varies significantly between the different materials investigated. More surprisingly, prior austenite grain size (PAGS) was found to increase with increasing vanadium content, even for identical austenitizing conditions. These changes in average grain size were largely unexpected, as it is commonly accepted that vanadium helps control austenitic grain size, at least for defined compositions and temperature ranges.[29] For this reason, PAGS measurements were carried out using not only conventional chemical etching, but also thermal etching and EBSD reconstruction. All methods yielded identical results within 0.5 ASTM. An indirect influence on the formation of titanium-based precipitates thus appears as a likely cause for the observation of larger grains in higher V steels. However, as this issue falls outside of the scope of the paper, it will not be further discussed.
Nevertheless, the increase in grain size is another factor that may explain the decrease in critical cooling rate. It is therefore not straightforward to estimate the specific influence of vanadium. Attempts to alter the grain size by high-temperature heat-treatment were unsuccessful, not only for the 0 V material (due to equipment limitations in terms of maximum temperature), but also for the 400 V, as reducing significantly the austenitizing temperature would have caused a significant fraction of the vanadium to no longer be in solid solution.
Therefore, in order to estimate the contribution of vanadium to the decrease in critical cooling rate, CCT diagrams were also determined for the 35MnCrMoV5 steel grade (Table II), for two austenitizing conditions leading to two different average grain sizes, while ensuring that vanadium remained fully in solution in both cases. As shown in Table III, a change in PAGS from 9 to 5 ASTM in 35MnCrMoV5 led to a significantly less pronounced change in the critical cooling rate than that observed for a lesser change in PAGS, in the alloys with varying vanadium content. A crude estimate is thus that the change in critical cooling rate between the 0 and 400 V materials is 90 pct related to vanadium content and 10 pct to a change in grain size, as illustrated in Figure 8.
As discussed earlier, the influence of vanadium is often investigated through hardness measurements (e.g., Jominy tests or similar hardness vs depth measurements), and thus for its impact on hardenability. This property has deliberately not been mentioned as it initially refers to the capacity of a materials to harden during cooling rather than to its propensity to form a particular microstructure. In the present case, the significant variation in critical cooling rate may have strong technological implications for applications where the objective is to achieve a homogeneous microstructure, as is the case for high-performance hot-forged components (for example, automotive powertrain components). Indeed, the change in critical cooling rate measured between the 0 and 400 V suggests the possibility of avoiding ferrite formation on air-cooled forged parts of 25 mm in the case of 0 V, and over 80 mm in the case of 400 V.
4.2 Isothermal Transformation
Isothermal transformation kinetics to ferrite-pearlite in 0 and 400 V for transformations at (a) 873 (600 °C) and (b) 973 K (700 °C). Results for 923 K (650 °C) are not shown as they exhibited the same trend as for 873 K (summarized in Table IV). Austenitizing conditions were the same as for CCT diagrams
Evidence for the influence of vanadium in solid solution was also obtained through isothermal transformation kinetics. The addition of 0.2 pct vanadium was observed to significantly delay the ferrite-pearlite reaction at 873 K (600 °C) (Figure 9 left; Table IV), at 923 K (650 °C, Table IV), and at 973 K (700 °C, Figure 9 right). However, contrary to the observations reported above for continuous cooling, the addition of 0.4 wt pct vanadium, whilst retarding ferrite-pearlite formation when compared to the 0 V material, appears to be less effective than the addition of 0.2 wt pct.
At 973 K (700 °C) (Figure 9 right), addition of 0.2 wt pct vanadium led to slower transformation kinetics, but the result was less straightforward for the 400 V material. Indeed, the onset of transformation was found to be delayed when compared to the 0 V steel, though not as much as for 200 V (as for lower transformation temperatures); after the onset phase however, transformation in the 400 V eventually proceeded faster than in the 0 and 200 V.
5 Influence of Increasing Vanadium Content on Bainite Formation Kinetics
Measurements of the bainite start (\(B_{\rm s}\)) temperature were carried out for a cooling rate of 2 K/s using data obtained from the continuous cooling transformation diagrams mentioned earlier. This was compared to the estimated values as per the Steven-Haynes formula.[30] As expected from the very similar compositions (except for V), the calculated results are virtually identical, as the Steven-Haynes formula does not account for the influence of vanadium. Although there is a relatively large uncertainty associated with such measurements (typically \(\pm 10\) K), the results clearly show a trend of decreasing measured \(B_{\rm s}\) temperature with increased vanadium content (Table V).
Bainite formation kinetics were investigated using dilatometry, as detailed earlier. Measurements were carried out for transformation temperatures of 648 K, 673 K, 698 K, 723 K, 748 K, and 773 K (375 °C, 400 °C, 425 °C, 450 °C, 475 °C, and 500 °C). To reduce the uncertainty of measurement associated with the asymptotic nature of the bainite reaction steady state, materials were compared using the time required to reach 90 pct of the maximum strain.
As shown in Table VI (and illustrated as an example in Figure 10 left), the effect of V additions on bainite formation kinetics for temperatures from 648 K to 698 K (375 °C to 425 °C) is different from that observed for the ferrite-pearlite transformation at higher temperatures. Indeed, while ferrite-pearlite formation is significantly slower for vanadium-bearing alloys (Table IV), bainite formation is largely unaffected or even accelerated at temperatures between 648 K and 723 K (375 °C and 450 °C) (Table VI).
At 748 K (475 °C) (not shown) and 773 K (500 °C), increasing the vanadium content resulted in a dramatic reduction in the bainite formation kinetics (Figure 10, right). Because a steady state was not reached after 2 hours of isothermal holding, it is difficult to conclude as to a possible reduction of the maximum fraction of bainite formed at these temperatures (which remains limited to 10 to 20 pct). Furthermore, from these data, it is not possible to define a reliable time to reach 90 pct transformation.
6 Discussion
6.1 Influence of Vanadium on Ferrite-Pearlite Formation
As discussed earlier, the influence of vanadium on hardenability is relatively well established.[12,13,14,15,17,18,19,21] However, as pointed out by Woodhead,[16] contradictory results may stem from insufficient accounting for the effects of austenitizing temperature and grain size.
The present results confirm the significant influence of the addition of vanadium on the kinetics of ferrite-pearlite formation, whether measured during continuous cooling or isothermal transformation. In this context, vanadium has previously been compared to niobium and found, for example, to retard to a lesser degree[20] the ferrite-pearlite formation during isothermal holding. However, isothermal investigations of transformation kinetics are often carried out after rapid cooling from the austenitizing temperature, as is the case here (20 K/s) or in Enomoto’s work,[20] in which salt bath quenching was used. Thus, the thermal history is not representative of that experienced during continuous cooling.
Indeed, some of the present authors have investigated the influence of 0.030 wt pct Nb in a 0.23C-1.25Mn-1.25Cr-0.1Mo steel[31] and concluded that, whilst Nb had indeed a strong retarding influence on ferrite-pearlite formation kinetics during isothermal holding (after rapid cooling from austenitizing), there was no measureable difference between the CCT diagrams of the material with or without Nb. This was shown to be caused by the precipitation of Nb between the austenitizing temperature and the temperature at which ferrite-pearlite formation initiated, when cooling at the relatively slow cooling rate of approx. 0.8 K/s between those temperatures. The effect of Nb precipitation during cooling between austenitizing and the temperature of transformation was also a notable result of recent work by Jia and Militzer.[32]
In the present study, vanadium was shown to influence transformation kinetics during both continuous cooling (3) and isothermal transformation (4). This presumably implies that, for cooling rates in the vicinity of the critical cooling rate (0.5 to 1 K/s), there is no significant precipitation of vanadium between the austenitizing temperature and the temperature range in which ferrite-pearlite formation may occur. This is supported by ED-ICP measurements carried out on 40-mm air-cooled bars (Figure 3), which showed negligible amounts of vanadium present as precipitates.
Thus, although it is less potent than niobium for the retardation of ferrite-pearlite formation, vanadium is of greater interest for increasing the hardenability of special bar steels such as quenched and tempered steel grades (AISI 4140, 4340, etc.) or forging steels. Indeed, hardenability improvements through vanadium or niobium additions will only be effective if these elements do not precipitate between the austenitizing temperature and the ferrite-pearlite transformation temperature range, when cooling at a rate in the vicinity of the base material critical cooling rate. As the materials mentioned earlier (AISI 4140, etc.) tend to have a critical cooling rate for ferrite-pearlite formation in the range 0.1-5 K/s, and medium carbon content (0.2 to 0.4 wt pct), any niobium left in solution after austenitizing is typically precipitated out when cooling at rates close to the critical cooling rate, thus rendering Nb additions ineffective.
This limitation is also known to exist for vanadium through studies which have explored the influence of nitrogen content or austenitizing conditions (e.g., Reference 19), although these variables are analyzed as to their effect on the content of vanadium in solution at the end of austenitizing. To our knowledge, there is no clear report of a phenomenon similar to that reported earlier for Nb where vanadium is concerned; in other words, the sluggishness of vanadium precipitation in austenite implies that in many cases, the amount of vanadium in solution can be estimated at the end of austenitizing without risk of it varying significantly during cooling and before transformation. This is indeed the approach used by Adrian.[19]
The results obtained during isothermal transformation of the 400 V material may point to the limitations of the above hypothesis. As mentioned earlier, all isothermal ferrite-pearlite formation kinetics for the 400 V were found to be faster than those for 200 V (Table IV), in spite of the larger prior austenite grain size in the 400 V material (Table III). Thus, contrary to continuous cooling results (Table III), a vanadium addition of 0.4 pct was found to be less effective than that of 0.2 pct. During heat-treatments for isothermal transformations, specimens are rapidly quenched to the transformation temperature, and transformation typically does not occur before approximately 100 seconds. It is possible that the nucleation of vanadium carbonitrides is considerably faster during this time than at the higher temperatures experienced during continuous cooling experiments, so that a stimulating influence[13] is obtained instead of an inhibiting one. This can be understood through consideration of two alternative thermal paths to reach the same temperature of 923 K (650 °C) after 100 seconds. The first would consist in continuous cooling from the austenitizing temperature at a constant rate, and the second would consist in ‘instantaneous’ cooling to 923 K (650 °C) followed by isothermal holding for 100 seconds at this temperature. The second path would clearly be more favorable to nucleation if the maximum nucleation rate were in the vicinity of 923 K, since all of the time is then spent at this maximum nucleation rate rather than at higher temperatures where nucleation may be more sluggish.
Thus, as for niobium, an optimum value of vanadium addition is likely to exist for maximum enhancement of hardenability. This would be defined as a value beyond which it is not possible to maintain vanadium in full solid solution for cooling rates at which the enhancing influence is sought. In turn, this value should strongly depend on nitrogen content, which is known to have a large influence on the driving force for V(C,N) precipitation.[1] It is clear that the materials presently investigated, in which solute nitrogen is rendered virtually zero through addition of Ti, are optimized from this point of view, since the driving force for V(C,N) precipitation is considerably reduced in the absence of N.
A consequence is that most empirical modeling methods, which have so far consisted in modifying multilinear estimations of the critical diameter or other hardenability parameter, are unlikely to correctly describe the influence of vanadium, even if, as in the work of Adrian,[19] the vanadium content used as a parameter is that in solution (at the end of austenitizing). Indeed, assuming all vanadium remains in solution after austenitizing, a steel with a high driving force for V(C,N) precipitation (high V content and/or high N content) will probably only exhibit hardenability enhancement from V if the base hardenability is low; this, because the cooling rates at which the hardenability improvements may be measured are sufficiently fast to avoid V(C,N) precipitation during cooling. Conversely, the lower the driving force for precipitation, the higher the range of base hardenability over which vanadium influence will be measurable.
6.2 Influence on Bainite Formation
In contrast with observations made for the formation for ferrite-pearlite, the present results indicate that vanadium has no retarding influence on the formation of bainite at temperatures as high as approximately 723 K (450 °C), with even a small accelerating effect being measured (Table VI).
These observations need, however, to be corrected for the prior austenite grain (PAG) size variations reported earlier (Table III). Unlike for ferrite-pearlite formation, there are conflicting data regarding the influence of prior austenite grain size on bainite formation kinetics.[23] This lack of agreement has been rationalized by Matsuzaki and Bhadeshia[33] as the consequence of transformation kinetics being either predominantly controlled by growth rate or by nucleation rate. These authors have proposed to describe the role of PAGS on transformation kinetics using Eq. [1].
where C, m, and n are constant; R is the mean grain diameter; t is the time, and X is the transformation progress, that is, the fraction at a given time of the maximum volume fraction of bainite that may form at the transformation temperature.
To estimate whether changes in kinetics observed in the present case could be attributed to grain size variations, data from Matsuzaki and Bhadeshia, and from the present work, were fitted to Eq. [1]. Values for \(n=2.09\) and \(m=1\) were used throughout, as per published values for the hypo-eutectoid steel A (0.12C-2.03Si-2.96Mn) in Reference 33. The constant C was considered to be material-dependent and thus fitted to data published for steel A with a PAGS of 80 μm and data for 0 V (PAGS 12 μm). Transformation kinetics were then calculated for steel A with a PAGS of 280 μm and for 400 V (PAGS 31 μm) without modifications to the identified values of C.
Comparison of calculated and measured transformation kinetics. Square symbols are for data from Ref. [33], steel A with PAGS 80 μm (filled) and 280 μm, and transforming at 723 K (450 °C). Circles are for 0 V (filled; PAGS 12 μm) and 400 V (hollow, PAGS 31 μm), both transforming at 723 K (450 °C). Solid lines are for the corresponding calculated kinetics
As shown in Figure 11, there is an excellent agreement between calculated and experimental values for steel A when the PAGS is 280 μm (the constant C having been fitted on data for PAGS 80 μm). This result support the idea that Eq. [1] adequately captures the influence of prior austenite grain size. In turn, the calculated kinetics for 400 V at 723 K (450 °C), based on parameters fitted on 0 V and accounting for changes in PAGS, are in good agreement with experimental values. This observation therefore suggests that grain size changes alone probably explain the slight acceleration measured for increasing vanadium content, and that the vanadium content has no direct influence.
Results discussed earlier have pointed to the absence of either precipitation (TEM) or even clustering (APT) of vanadium during cooling to transformation temperatures of 723 K (450 °C) or less, even when a short isothermal holding was carried out. A clear conclusion is therefore that, in the range of temperatures 648 K to 723 K (375 °C to 450 °C) approximately, vanadium in solid solution has no measurable influence on bainite formation kinetics. This is consistent with accelerating effects reported in the literature, which have all have been attributed to the pre-existence of precipitates[34,35] or vanadium clusters,[36] but never to vanadium in solid solution.
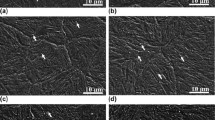
For higher transformation temperatures [748 K, 773 K (475 °C, 500 °C)] however, the addition of vanadium was found to have a dramatic impact on bainite kinetics, virtually suppressing bainite formation in the higher vanadium material (Figure 10). It is not clear whether precipitation occurs at these temperatures, and thus may impede growth. The absence of precipitates or clusters noted in air-cooled microstructures suggests that precipitation is unlikely under these conditions. Furthermore, comparison of microstructures indicates that nucleation rather than growth is limited. As shown in Figure 12, bainite is found that has grown through prior austenite grains in both 0 V (Figure 12 left) and 400 V (Figure 12 left), though nucleation on grain boundaries is significantly reduced in the 400 V specimen.
Optical micrographs (4 pct nital) of specimens of alloy (a) 0 V and (b) 400 V isothermally transformed at 773 K (500 °C) for 2 h. Note that while the 400 V micrograph was selected for illustration, many observed fields did not exhibit any bainite. On the contrary, the micrograph of 0 V is fully representative of the overall sample
The influence of vanadium on ferrite-pearlite retardation has been attributed to grain boundary segregation of this element, with confirmed enrichment factors in the vicinity of 3 to 4.[20] It is thus acting primarily through a reduction of the nucleation rate on grain boundaries. That vanadium does not impact bainite formation in the lower temperature range which is therefore not unexpected. Indeed, in this temperature range, large driving forces for nucleation are to be expected, and thus reduced influence of the changes in surface energy imparted by grain boundary segregation (in a manner analogous to the action of boron, for example). This proposal is supported by the discussion above, which has shown that bainite formation at those temperatures had a PAGS dependency consistent with a transformation principally controlled by growth. At higher temperatures, where driving force for nucleation is limited, the reduction of grain boundary energy would have a greater influence, as observed through kinetics measurements and metallography.
The overall impact of vanadium addition is thus limited, as far as bainite formation is concerned, to transformation products formed in the upper temperature range of the domain. Given that the fractions that may form in this range remain limited (<10 pct) and form over an extended duration, the influence on transformation during continuous cooling is likely to be limited. In fact, although this result falls outside the scope of the present paper, tensile properties of all three materials (0, 200, and 400 V) were found to be identical for bars of diameter 40 mm austenitized at 1273 K (1000 °C) and air cooled to room temperature.
7 Conclusions
The influence of vanadium content on the ferrite-pearlite and bainite formation was investigated in a medium carbon forging steel. It was shown that austenitization at either 1273 K or 1423 K (1000 °C or 1150 °C) led to a very large majority of the vanadium being in solid solution. This was also true after relatively slow cooling (1 K/s). Using TEM, only a small fraction of (Ti,V) carbonitrides could be identified in vanadium-bearing materials. No evidence was found for vanadium clustering in any of the materials (APT).
The increase in vanadium content was correlated with a significant reduction in the critical cooling rate for ferrite-pearlite formation (from 1 to 0.3 K/s for a vanadium increase from 0 to 0.40 wt pct). This result points to an advantage of vanadium for increasing hardenability in heavy section products, because the relatively sluggish precipitation of V(C,N) in austenite means that this element remains in solid solution even during relatively slow cooling. This is not the case for Nb, which, although a potent retardant of ferrite-pearlite formation, precipitates more readily during cooling.
Bainite formation was on the contrary shown not to be affected in the temperature range 648 K to 723 K (375 °C to 450 °C), with a slight acceleration explained by the small differences in prior austenite grain size. For bainite formation at higher temperatures 748 K to 773 K (475 °C to 500 °C), vanadium appeared to significantly impede the formation of bainite. This could possibly be explained by vanadium segregation to grain boundaries and the consequent reduction in nucleation rate when the driving force for bainite nucleation is limited.
References
R. Lagneborg, T. Siwecki, S. Zajac, and B. Hutchinson: Scand J Metall 1999, vol. 28, pp. 186–241.
G. Tweedale: Sheffield Steel and America: 1st Edition: Cambridge University Press, 1987.
J. Bellus, P. Dierickx, V. Jacot, and M. Robelet: Steel and Method for Making Cleavable Parts, European Patent ep1051531b1, 1998.
J. Bellus, P. Joly, C. Pichard, V. Jacot, C. Tomme, and D. Robat: Process for Manufacturing Steel Forging, European Patent ep0787812b1, 1996.
G. Gomez, T. Perez, H. K. D. H. Bhadeshia: Mater Sci Technol 2009, vol. 25, pp. 1502–1507.
G. Gomez, T. Perez, H. K. D. H. Bhadeshia: Mater Sci Technol 2009, vol. 25, pp. 1508–1512.
T. Sourmail, H. Michaud, E. d’Eramo, and G. Baudry: in: 2nd International Conf. Super High Strength Steels, D.I. di Metallurgia, eds., AIM, Verona, Italy, 2016.
S. Engineer, H. Justinger, P. Janssen, M. Härtel, C. Hampel, and F. Randelhoff: in: 3rd International Conference on Steels for Cars and Trucks, H.-J. Wieland, ed., TEMA Technologie Marketing AG, Salzburg, 2011, pp. 404–11.
K.-I. Sugimoto and N. Yoshikawa: in: 3rd International Conference on Steels for Cars and Trucks, H.-J. Wieland, ed., TEMA Technologie Marketing AG, Salzburg, 2011, pp. 460–67.
S. Hasler, H. Roelofs, M. Lembke, and F.G. Caballero: in: 3rd International Conference on Steels for Cars and Trucks, H.-J. Wieland, ed., TEMA Technologie Marketing AG, Salzburg, 2011, pp. 330–37.
C. Keul, L. Moseker, W. Bleck, T. Rekersdrees, A. Stuber, H. Schliephake, C. Beyer, and H.-W. Raedt: in: 3rd International Conference on Steels for Cars and Trucks, H.-J. Wieland, ed., TEMA Technologie Marketing AG, Salzburg, 2011, pp. 208–17.
M.I. Goldshtein and S.G. Guterman: Metal Sci. Heat Treat. 1964, vol. 6(7), pp. 440–42. http://www.springerlink.com/index/Q1664785027W1271.pdf.
Woodhead, J.H.: in: Proceedings: Metals Society, Chicago, 1979, pp. 3–10.
P. Mangonon: Journal of Heat Treating 1980, vol. 1 (4), pp. 47–60. doi:10.1007/BF02833255
P. Mangonon: Metall Trans A 1982, vol. 13 (2), pp. 319–320. 10.1007/BF02643323
J.W. Woodhead: in: Vanadium in High Strength Steel, Proc. Vanitec Seminar, Chicago, 1983, pp. 3–10.
B. Garbarz, F. B. Pickering: Materials Science and Technology Feb. 1988, vol. 4 (2), pp. 117–126. 10.1179/mst.1988.4.2.117
K. A. Taylor, S. S. Hansen: Metall Trans A 1991, vol. 22 (10), pp. 2359–2374. 10.1007/BF02665002
H. Adrian: Mater Sci Technol 1999, vol. 15 (4), pp. 366–378. 10.1179/026708399101505987
M. Enomoto, N. Nojiri, Y. Sato: Materials Transactions, JIM 1994, vol. 35 (12), pp. 859–867.
C. Chen, F. Zhang, Z. Yang, C. Zheng: Materials & Design Oct. 2015, vol. 83, pp. 422–430.
S. Preston: J. Heat Treat. 1990, vol. 8 (2), pp. 93–99. 10.1007/BF02831629
C. Garcia-Mateo, L. Morales-Rivas, F. Caballero, D. Milbourn, and T. Sourmail: Metals, 2016, vol. 6(6), pp. 130. http://www.mdpi.com/2075-4701/6/6/130.
S. Bechet, L. Beaujard: Rev Métall 1955, vol. 10, pp. 830–836.
C. G. de Andrés, F. Caballero, C. Capdevila, D. S. Martin: Materials Characterization 2003, vol. 49, pp. 121–127.
L. Germain, N. Gey, R. Mercier, P. Blaineau, M. Humbert: Acta Mater 2012, vol. 60, pp. 4551–4562.
D. Acevedo-Reyes: Evolution de l’état de précipitation au cours de l’austénitisation d’aciers microalliés au vanadium et au niobium: Ph.D. thesis: INSA de Lyon 2007. https://hal.archives-ouvertes.fr/tel-00511332/.
F. Danoix, G. Grancher, A. Bostel, and D. Blavette: Ultramicroscopy, 2007, vol. 107(9), pp. 739–43.
R. Stasko, H. Adrian, A. Adrian: Mater Charact 2006, vol. 56, pp. 340–347.
W. Steven, A. G. Haynes: J. I. S. I. 1983, vol. 1965, pp. 349–359.
T. Sourmail and H. Rouyer: Effect of niobium on the hardenability of 23MnCrMo5-5, unpublished work.
T. Jia, M. Militzer: Metall Mater Trans A Feb. 2015, vol. 46A (2), pp. 614–621. 10.1007/s11661-014-2659-5
A. Matsuzaki, H. Bhadeshia: Mater Sci Technol 1999, vol. 15 (5), pp. 518–522. 10.1179/026708399101506210
C. Garcia-Mateo, C. Capdevilla, F.G. Caballero, and C.G. de Andrés: ISIJ Int., 2008, vol. 48(9), pp. 1270–75. http://jlc.jst.go.jp/JST.JSTAGE/isijinternational/48.1270?from=Google.
C. Garcia-Mateo, J. Cornide, C. Capdevilla, F.G. Caballero, and C.G. de Andrés: ISIJ Int., 2008, vol. 48(9), pp. 1276–79. http://jlc.jst.go.jp/JST.JSTAGE/isijinternational/48.1276?from=Google.
K. He and D.V. Edmonds: Mater. Sci. Technol., 2002, vol. 18(3), pp. 289–96, DOI:10.1179/026708301225000743.
Acknowledgments
The authors are grateful to Vanitec for financial support of the present work and to Kira J. Weissman for careful proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted October 8, 2016.
Rights and permissions
About this article
Cite this article
Sourmail, T., Garcia-Mateo, C., Caballero, F.G. et al. The Influence of Vanadium on Ferrite and Bainite Formation in a Medium Carbon Steel. Metall Mater Trans A 48, 3985–3996 (2017). https://doi.org/10.1007/s11661-017-4188-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-017-4188-5